Abstract
The results of degradation efficiency of 2-sec-butyl-4,6-dinitrophenol (DNBP) in a batch system by various advanced oxidation processes revealed the order of TiO2/UV/O3 > TiO2/O3 > UV/O3 > O3 > UV/TiO2. All processes followed pseudo-first order kinetics. The influence of operational parameters such as initial pH, initial concentration of DNBP, ozone and catalyst dosage on the TiO2/UV/O3 process, which was the most significant investigated method. The ozone dosage was found to have the noticeable impact on the process; however, initial pH and TiO2 dosage were less effective. The mineralization of 40 mg/L of DNBP and petrochemical wastewater under the obtained optimal conditions was monitored by total organic carbon and chemical oxygen demand, respectively. The results demonstrated that the TiO2/UV/O3 process was a very effective method for degradation and mineralization of DNBP in aqueous solutions and industrial wastewater. The degradation intermediates were identified by GC–MS.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Discharge of persistent organic pollutants (POPs) such as phenolic compounds poses serious threats to the environment and human health. Nitro-substituted phenols are classified as one of the most toxic and carcinogenic compounds. 2-Sec-butyl-4,6-dinitrophenol (DNBP) is a typical example of this class, which is manufactured in large quantities and used as an inhibitor for vinyl aromatics polymerization in the petrochemical industry and as a herbicide in agriculture [1, 2]. During DNBP synthesis and application processes, it can be introduced into the surface water. Due to the undesirable side effects of DNBP, governments restrict its maximum concentration level (MCL) in water. The MCL of DNBP in drinking water is 7 ng/mL according to the environmental protection agency (EPA) of the USA [3]. Thus, the environmental fate of DNBP and its removal in water sources are of great public concern. DNBP is resistant to biological treatment and other processes like adsorption [4], which merely transfers the contaminant from aqueous solution to secondary waste. So, the application of more effective methods like advanced oxidation processes (AOPs) are more desirable, because they cannot only oxidize and degrade the pollutants non-selectively but also mineralize them [5]. The main oxidizing agent in these processes is hydroxyl radical (E0 = 2.8 eV), which can be produced under ultraviolet (UV) light-catalyzed reactions [6–8]. Titanium dioxide (TiO2) has been extensively utilized as a heterogeneous catalyst in AOPs. When TiO2 absorbs UV light, photo-induced TiO2 is generated and electrons are excited from the valence band of TiO2 to the exciting band, which results in electron–hole pairs. Oxygen molecules act as electron acceptors to form superoxide ions (•O2 −), and hydroxyl ions or water molecules function as electron donors to produce hydroxyl radicals [9] as follows:
Although the UV/TiO2 process can degrade contaminants in aqueous solutions, the degradation rate is not very rapid [10]. The high reactivity and disinfection of ozone (O3), which is a stronger oxidant than oxygen (E0 = 2.07 V) makes it suitable for application in various water treatment processes [11]. Hence, ozonation has been used for treatment of wastewaters containing phenolic compounds [12]. However, it has been proven that in the conventional ozonation method complete degradation of organic compounds is not achieved. The degradation efficiency of humic acid and oxalic acid was made more effective by TiO2-catalyzed ozonation (TiO2/O3) in comparison with ozone alone [13]. This can be attributed to both the enhancement of ozone dissolution and its decomposition in the presence of TiO2 particles [14]. The combination of ozone with photocatalytic oxidation elicits the advantages of all the above-mentioned processes. The production of extra hydroxyl radicals enhances not only by reduction of dissolved O3 molecules with photogenerated electrons at conduction band of TiO2 but also by absorption of UV light by ozone itself which is called the UV/O3 process and is applied to degrade organic pollutants [15] (Eqs. 6–10).
The utilization of ozone combined with the photocatalytic oxidation process for degradation of organic pollutants like monochloroacetic acid, pyridine, neonicotinoid insecticides, toluene, sulfamethoxazole and 4-chloronitrobenzene has been reported [16–21]. However, to the best of our knowledge, there is no report for the degradation of DNBP by this process. The aim of this study was to compare various AOPs processes including UV/TiO2, O3, UV/O3, TiO2/O3, and TiO2/UV/O3 to find the synergetic effect of ozonation and photocatalysis on the degradation of DNBP as a model contaminant from phenolic compounds. Thus, to investigate the effect of operational parameters such as initial DNBP concentration, initial pH, ozone and catalyst dosage in TiO2/UV/O3, process experiments were carried out. Eventually, the mineralization of DNBP in aqueous solution and in the wastewater of the Tabriz Petrochemical Complex was investigated.
Experimental
Materials
2-Sec-butyl-4,6-dinitrophenol (DNBP) was used as an alkyl dinitro phenol compound and obtained from Retell Fine Chemical (Tianjin, China). An aqueous solution of DNBP was prepared in deionized water. Titanium dioxide (Degussa P25, Germany) which has 80 % anatase and 20 % rutile, with average particle size of 21 nm and surface area of 50 ± 15 m2/g, was applied as catalyst [22]. All other chemicals were purchased from Merck, Germany.
Experimental set-up and procedure
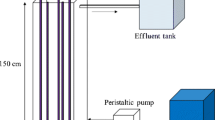
Experiments were carried out in a batch cylindrical quartz reactor with inner diameter of 50 mm and volume of 500 mL. In each experiment, 250 mL of the reaction mixture containing desired amounts of DNBP and TiO2 were poured into the reactor. The pH of the solution was adjusted by adding perchloric acid or sodium hydroxide. The solution was agitated by a magnetic stirrer. The mixture of ozone–oxygen which was supplied from the ozone generator (Donali, Iran) was fed into the reactor by a diffuser from its bottom. The concentration of ozone was adjusted by varying the voltage and measured by the KI method [23]. A UV lamp (10 W, 254 nm, GPH212T5L/4, Germany) was placed next to the quartz reactor to provide UV irradiation. The light intensity on the outer side of the reactor was 2.3 mW/cm2, which was measured by UV-Lux-IR meter (Leybold, Germany).
Analytical methods
High performance liquid chromatography (HPLC) (Shimadzu, SCL-6A, Japan) was applied to the determination of the concentration of DNBP (C) in the solution. A ZOBAX column (5 μm, C18) and mobile phase of methanol/water (7:3 v/v) with flow rate of 1.0 mL/min were employed in the HPLC analysis. To investigate the mineralization of DNBP, total organic carbon (TOC) by catalytic oxidation (Shimadzu TOC-5000, Japan) and chemical oxygen demand (COD) by the open reflux method [6], were used for the DNBP aqueous solution and petrochemical wastewater containing DNBP. The generated intermediates during DNBP degradation by TiO2/UV/O3 were identified by GC–MS analysis (Agilent Technologies, Palo Alto, CA, USA).
Results and discussion
Comparison of various AOPs for degradation of DNBP and kinetics study
The individual effect of UV irradiation and TiO2 addition is negligible for the degradation of DNBP in aqueous solution. The degradation efficiency (DE%) at a certain time is defined as the percentage ratio of the degraded amount (C0–C) to its initial concentration (C0). The decreasing order of DE% for DNBP (40 mg/L) by different AOPs processes after 15 min of treatment was TiO2/UV/O3 (99.1 %), UV/O3 (94.5 %), TiO2/O3 (92.8 %), O3 (91.9 %), and UV/TiO2 (14.2 %) (Fig. 1a).
Moreover, mineralization of DNBP during the above-mentioned AOPs was investigated by monitoring of the TOC. Although the order of the mineralization was the same as the degradation, significant differences for TOC removal were observed after 45 min of the processes (Fig. 1b). The TiO2/UV/O3 process demonstrated 71.9 % TOC elimination, which was 57, 45.9, 26.9, and 19.9 % higher than that of the UV/TiO2, O3, TiO2/O3, and UV/O3 processes, respectively. The results indicate that simultaneous application of heterogeneous photocatalysis with ozone, which has high oxidative ability, is an efficient coupled process for the degradation and mineralization of DNBP. These findings can be explained by (1) more dissolution and decomposition of O3 in water in the presence of TiO2 [13], (2) O3 acts as an electron acceptor from the conduction band of TiO2 [14], preventing recombination of electron–hole pairs, and (3) O3 absorbs UV light (O3/UV) [15]. All the mentioned reasons cause the formation of extra hydroxyl radicals resulting in the enhancement of DE% and mineralization of DNBP.
All applied AOPs in this study follow pseudo-first-order kinetics (Fig. 2):
The apparent pseudo-first-order reaction rate constant (kapp) was determined from the slope of ln (C0/C) versus process time (t) (Table 1). The high correlation coefficients obtained confirmed the assumed kinetics. The synergistic effect of O3 and UV/TiO2 is considerable and can be measured in the apparent pseudo-first-order rate constant in the following equation as 46 %:
As a result, other experiments for DNBP degradation were carried out by the coupled photocatalytic ozonation process.
Influence of operational parameters on photocatalytic ozonation and intermediates identification
The effects of experimental parameters including ozone and TiO2 dosage, initial DNBP concentration, and pH on the degradation and mineralization of DNBP were investigated. Degradation and mineralization of DNBP declines by increasing the DNBP concentration (Fig. 3), because the identical amount of oxidizing species, which are generated under the same conditions, have to react with more DNBP molecules and its degradation intermediates [24]. By increasing the O3 dosage, the degradation and mineralization of DNBP increase due to the formation of more oxidizing species like hydroxyl radicals (Fig. 4) [11]. However, as can be seen from Fig. 4, a further increase in O3 dosage does not have a remarkable effect on DE% because of unreacted O3 being released in the system [25]. The effect of TiO2 dosage on the DE% and TOC is depicted in Fig. 5. It can be concluded that neither DE% and TOC vary noticeably by increasing the TiO2 dosage. This result is consistent with the degradation of 4-chloronitrobenzene by the TiO2/UV/O3 process [21]. The DE% and TOC removal of DNBP was studied in pH of 3.5, 6.5, and 9.5 (Fig. 6). The degradation of DNBP shows approximately the same results in various pHs, but the pH of 9.5 shows the best performance for the mineralization of DNBP. In acidic medium (pH = 3.5), the predominant form of ozone molecule is in the solution, which can degrade organic pollutants by direct electrophilic attack [26]. In the basic medium (pH = 9.5), indirect attack of hydroxyl radicals, which were generated by O3 decomposition [27], can degrade DNBP more efficiently. Furthermore, the reaction of •OH with DNBP might be more efficient at high pH rather than at low pH. In order to identify intermediates in the photocatalytic ozonation, 250 mL of DNBP (40 mg/L) was treated for 15 min. Then, the intermediates were extracted and detected by the GC–MS method [28, 29]. Five intermediates, which were recognized by comparison with commercial standards when the match factor was above 90 %, are presented in Table 2. However, several intermediates could not be identified because of their rapid oxidization to other derivatives or low match factors of chromatographic peaks.
Study of photocatalytic ozonation treatment of a real wastewater
The application of the TiO2/UV/O3 process for mineralization of a real wastewater containing DNBP is essential from the practical point of view. Wastewater with a yellow color, which has approximately 40 mg/L of DNBP, was provided by the Petrochemical Complex (Tabriz, Iran). The initial COD of the wastewater was 1,053 mg/L with pH of 8.5. After 60 min of treatment, 67.2 % of the COD was eliminated, which proved the destruction of organic molecules in the wastewater (Fig. 7).
Conclusion
The photocatalytic ozonation process for degradation of DNBP, as a model pollutant from phenolic compounds, was compared with UV/TiO2, O3, UV/O3, and TiO2/O3 processes. All processes followed the pseudo-first-order kinetics. Moreover, the operational parameters including ozone and TiO2 dosage, initial DNBP concentration, and pH on the degradation and mineralization of DNBP were investigated in the TiO2/UV/O3 process, and some of the degradation intermediates were identified by GC–MS. The mineralization of DNBP in aqueous solution and petrochemical wastewater was investigated by TOC, and the COD showed the proper ability of the TiO2/UV/O3 process for the destruction of phenolic compounds.
References
J. Nakajima, S. Tazikani, H. Tomioka, Jpn. Petrol. Inst. 46, 359–367 (2003)
T.A. Gasiewicz, Handbook of Pesticide Toxicology (Academic, San Diego, 1991), pp. 1191–1269
U.S. Environmental Protection Agency. Lab Cert Bulletin, EPA-815-N-00-001a (2000)
E. Ayranci, N. Hoda, Chemosphere 60, 1600–1607 (2005)
A.R. Khataee, M. Zarei, Desalination 273, 453–460 (2011)
R. Marandi, M.E. Olya, B. Vahid, M. Khosravi, M. Hatami, Environ. Eng. Sci. 29, 957–963 (2012)
M. Kilic, G. Kocturk, N. San, Z. Cinar, Chemosphere 69, 1396–1408 (2007)
P. Pichat, L. Cermenati, A. Albini, D. Mas, H. Delprat, C. Guillard, Res. Chem. Intermed. 26, 161–170 (2000)
N. Getoff, Res. Chem. Intermed. 27, 343–358 (2001)
S. Ahmed, M.G. Rasul, W.N. Martens, R. Brown, M.A. Hashib, Water Air Soil Pollut. 215, 3–29 (2011)
Y. Yang, J. Ma, Q. Qin, X. Zhai, J. Molecul, Catal. A 267, 41–48 (2007)
C. Sonntag, Water. Sci. Technol. 55, 19–23 (2007)
H. Paillard, M. Doreand, M.M. Bourbigot, in Proceedings of the 10th World Congress of International Ozone Association, Monaco, 1991
C. Cooper, R. Burch, Water Res. 33, 3695–3700 (1999)
F.J. Beltran, F.J. Rivas, O. Gimeno, J. Chem. Technol. Biotechnol. 80, 973–984 (2005)
P. Kopf, E. Gilbert, S.H. Elbert, Photochem. Photobiol. A 136, 163–168 (2000)
U. Cernigoj, U.L. Stangar, P. Trebse, Appl. Catal. B 75, 229–238 (2007)
K.P. Yu, G.W.M. Lee, Appl. Catal. B 75, 29–38 (2007)
F.J. Beltran, A. Aguinaco, J.F. Garcia-Araya, A. Oropesa, Water Res. 42, 3799–3808 (2008)
H. Qi, D.Z. Sun, G.Q. Chi, Environ. Sci. 19, 1136–1140 (2007)
Y. Miaomiao, C. Zhonglin, L. Xiaowei, B. Yue, S. Jimin, Hazard. Mater. 167, 1021–1027 (2009)
A.R. Khataee, A.R. Amani-Ghadim, M. Rastegar Farajzade, O. Valinazhad Ourang, J. Exp. Nanosci. 6, 138–148 (2011)
M.J. Taras, A.E. Greenberg, R.D. Hoak, M.C. Rand, Standard Methods for the Examination of Water and Wastewater (American Public Health Association, Washington DC, 1974), pp. 271–274. (Section 143)
C.H. Chiou, C.Y. Wu, R.S. Juang, Sep. Purif. Technol. 62, 559–564 (2008)
L. Zeng, J.W. Mckinley, J. Hazard. Mater. 135, 218–225 (2006)
L. Ming-Chun, R. Gwo-Dong, Water Res. 30, 1670–1676 (1996)
A. Rodriguez, R. Rosal, J.A. Perdigon-Melon, M. Mezcua, A. Aguera, M.D. Hernando, P. Leton, A.R. Fernandez-Alba, Hdb. Env. Chem. 5, 127–175 (2008)
A.R. Khataee, M. Safarpour, M. Zarei, S. Aber, J. Mol. Catal. A (2012) in press
J.L. Little, J. Chromatogr. A 844, 1–22 (1999)
Acknowledgments
The authors sincerely appreciate the Water and Wastewater Company of East Azerbaijan, Tabriz, Iran. We also thank Dr. B. Vahid, Mr. Fatholahnejad and Mrs. Movahed for their valuable comments and help.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Mousanejad, T., Khosravi, M., Tabatabaii, S.M. et al. Photocatalytic ozonation for degradation of 2-sec-butyl-4,6-dinitrophenol (DNBP) using titanium dioxide: effect of operational parameters and wastewater treatment. Res Chem Intermed 40, 711–722 (2014). https://doi.org/10.1007/s11164-012-0996-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11164-012-0996-9