Abstract
We here reported a series of co-crystal structure of pyrazinamide (PZA) with m-hydroxybenzoic acid (MHBA), p-hydroxybenzoic acid (PHBA) and 3,4-dihydroxy benzolic acid (3,4-DHBA) and obtained three co-crystals (co-crystal 1–3, respectively) under the same conditions. These crystal structures were characterized by single-crystal X-ray diffraction, IR spectra, thermogravimetric analyses, differential scanning calorimetry, and Raman spectrum analysis. The images of single crystal diffraction revealed that 1 was a 1:1 (PZA:MHBA) co-crystal, 2 was a 1:1 (PZA:PHBA) co-crystal, and 3 was a 1:1 (PZA:3,4-DHBA). The strong O–H···O, N–H···O hydrogen bonding interactions between PZA and hydroxyl-substituted benzoic acid primarily made the structure of co-crystals stabilized. Hirshfeld surface and fingerprint plots of the three co-crystals showed that the structures were stabilized by H···H, O–H···O, N–H···O C–H···π, and π···π intermolecular interactions.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The design of co-crystals has attracted worldwide attention, especially in the fields of pharmaceutical because they offer new opportunities for improving some relevant chemical–physical properties of a drug and not affect its therapeutic effects [1–9]. Pharmaceutical co-crystals can be defined as molecular complexes comprised of active pharmaceutical ingredients and one or more pharmaceutically acceptable co-crystal formers [10–12], while the structure of them is often controlled by the direction and selective reorganization of hydrogen or halogen bonds within the crystals [13]. Co-crystals present different characteristics because of the intermolecular interactions. For example, in the field of medicine, pharmaceutical co-crystals not only improves the physical properties and bioavailability of a drug but also have little influence on its therapeutic effects [5, 9].
In this study, we applied the co-crystal strategy to solid pyrazinamide (PZA) with hydroxyl-substituted benzoic acids (MHBA:m-hydroxybenzoic acid, PHBA:P-hydroxybenzoic acid and 3,4-DHBA:3,4-dihydroxy benzolic acid) and obtained three co-crystals (Scheme 1) [14–17]. These crystal structures were characterized by single-crystal X-ray diffraction, IR spectra, thermogravimeric analysea(TGA), and differential scanning calorimetry (DSC), while we also made use of Hirshfeld surfaces analysis to research the intermolecular interactions of PZA with hydroxyl-substituted benzoic acids.
Experimental
General remarks
M-hydroxybenzoic acid (purity: 98 %, CAS registry number: 99-06-9), p-hydroxybenzoic acid (purity: 99 %, CAS registry number: 99-96-7), 3,4-dihydroxy benzolic acid (purity: 97 %, CAS registry number: 99-50-3), PZA (purity: 99 %, CAS registry number: 5910-89-4), and all the other solvents (reagent grade) were commercially available and used as received without further purification.
Synthesis of co-crystal 1–3
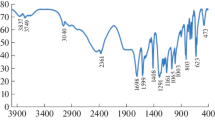
The synthesis of the co-crystals was carried out in solution crystallization experiments. Co-crystal 1 was obtained by the following procedure: stoichiometric amounts of PZA (0.5 mmol, 61.5 mg) and MHBA (0.5 mmol, 69.1 mg) were dissolved separately in a beaker with 10 ml of methanol solution, then combined and slowly evaporated at room temperature. A week later, we got club-shaped colorless crystals (yield 91 % based on MHBA). Mp: 141 °C. IR (KBr pellet, cm−1): 3,434, 3,142, 1,704, 1,648, 1,450, 1,403, 1,300, 1,244, and the Raman spectrum characteristic peak: 3,078, 1,026, 997, 816, 773, 243. The synthetic steps of crystal 2, crystal 3 and crystal 1 are similar including the solvent (yield 87 % based on PHBA). Mp: 172 °C. IR (KBr pellet, cm−1): 3,417, 3,157, 1,704, 1,601, 1,403, 1,291, 1,168 and the Raman spectrum characteristic peak: 1,608, 1,275, 1,172, 846, 830, 638, (yield 92 % based on 3,4-DHBA). Mp: 174 °C. IR (KBr pellet, cm−1): 3,520, 3,252, 1,815, 1,701, 1,500, 1,310, 1,244, and the Raman spectrum characteristic peak: 2,420, 1,100, 990, 793, 566, 475, respectively.
Thermoanalysis of co-crystals
The three co-crystals were characterized by TGA and DSC which were produced by Mettler–Toledo and conducted with the TGA/DSC STARe System at a heating rate of 10 K min−1 under an atmosphere of dry N2 flowing at 20 cm3 min−1 over a range from 40 to 300 °C.
X-Ray crystallographic study
Single-crystal X-ray diffraction analysis was performed for the three crystals 1–3 to determine their structures [18]. The crystals were selected and mounted in air onto thin glass fibers. Accurate unit cell parameters were determined by a least-squares fit of 2θ values and intensity datasets were measured on a Rigaku Raxis Rapid IP diffractometer with Mo–Kα radiation (λ = 0.71073 Å) at room temperature. The structure was solved using the SHELXS-97 package and refined with SHELXL-97 [19]. All non-hydrogen atoms were refined anisotropically. All hydrogen atom bonds to carbon, nitrogen and oxygen atoms were placed geometrically and refined isotropically. Full crystallographic details were collected in Table 1.
Hirshfeld surfaces analysis
Hirshfeld surfaces facilitate a novel method of visualizing intermolecular interactions by color-coding short or long contacts while the color intensity indicates the relative strength of the interactions. Additionally, the size and shape of a Hirshfeld surface also reflects the interplay between different atoms and intermolecular contacts in a crystal. The 2-D fingerprint plots, which are derived from the Hirshfeld surfaces, complement these. They quantitatively summarize the nature and type of intermolecular contacts experienced by the molecules in the crystal. Each point on the standard 2-D graph represents a bin formed by discrete intervals of de and di, and the points on the surfaces are colored as a function of the fraction of the surface points in the bin [20]. For instance, the blue color indicates relatively few points while the green color means moderate points and the red color suggests many points. The 2-D fingerprint plots can also be broken down to give the relative contribution to the Hirshfeld surface area of each type of interaction present, quoted as the ‘‘contact contribution’’. For a given crystal structure, the Hirshfeld surfaces as well as the fingerprint plots are all unique [21], and the number of unique Hirshfeld surfaces depends on the number of crystallographically independent molecules in the corresponding asymmetric unit [22].
Results and discussion
Crystal structures of co-crystals 1–3
We obtained three co-crystals from crystallization experiments through the reaction of PZA with hydroxyl-substituted benzoic acid at a molar of 1:1 in methanol solution; the diffraction data of the three co-crystals were collected at room temperature [23].
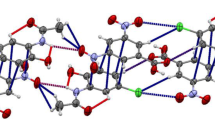
Co-crystal 1 crystallizes as colorless club-shaped crystals. The structure determination shows that 1 forms a 1:1 (PZA:MHBA) co-crystal in the orthometric Pbca space group with Z = 8. PZA and MHBA moleculars were connected by N–H···O (distance of 2.976 Å) (Fig. 2 1a) and O–H···O (distance of 2.583 Å) hydrogen bond. The PZA molecules are also stabilized by intramolecular N–H···O hydrogen bonds (distance of 2.526 Å), and the structure of PZA molecule and MHBA molecule are further stacked by π···π intermolecular interactions, with distances of 3.357 Å (Fig. 2 1b). The hydrogen bonds of the three co-crystals are summarized in Table 2 and Fig. 1. As shown in crystal 1, co-crystal 2 also crystallizes as colorless club-shaped crystals which the structure determination shows that 2 forms a 1:1 (PZA:PHBA) co-crystal in the orthometric Pca21 space group with Z = 8. PZA and PHBA moleculars were connected by N–H···O hydrogen bonds (distance of 3.063 Å). The PZA molecules are also stabilized by a pair of intramolecular N–H···O hydrogen bonds (distances of 2.839 and 2.873 Å) (Fig. 2 2a), while the PHBA molecules are also stabilized by a pair of intramolecular O–H···O hydrogen bonds (distance of 2.652 and 2.644 Å) (Fig. 2 2a). The plane separations between PZA and PHBA molecules are 3.291 and 3.365 Å, respectively (Fig. 2 2b).
Unlike co-crystals 1 and 2, co-crystal 3 crystallizes as light yellow club-shaped crystals and the structure determination shows that 3 forms a 1:1 (PZA:3,4-DHBA) co-crystal in the triclinic p–1 space group with Z = 4. PZA and 3,4-DHBA moleculars were connected by N–H···O (distance of 2.958 Å) (Fig. 2 3a) and O–H…O (distance of 2.603 Å) hydrogen bond. The structure of PZA molecule and 3,4-DHBA molecule are further stacked by π···π intermolecular interactions, with distances of 3.396 and 3.381 Å (Fig. 2 3b). The packing structures of PZA molecules and guest molecules are spaced from each other.
Differential scanning calorimetry (DSC) and thermogravimetric analysis (TGA) of cocrystal 1-3
Differentical scanning calorimetry (Fig. 3a) and thermogravimetric analysis (TGA; Fig. 3b) were used to investigate the behaviors of the three crystals. The DSC patterns of co-crystals 1–3 show the effect of guest molecules to the PZA molecule on the melting point. For co-crystal 1, we find there is only one endothermic peak at 140 °C. For co-crystals 2 and 3, they have almost the same endothermic peaks: 172 and 175 °C, respectively. TGA thermograms of the three crystals show they have mass loss only once: 93.51, 90.78, and 88.64 %, respectively, and the PZA molecule and guest molecules show sublimation at the same time.
Hirshfeld surfaces analysis
The three-dimensional Hirshfeld surfaces and two-dimensional fingerprint plots of the PZA molecules in the three co-crystals are illustrated in Fig. 4, which shows surfaces that have been mapped over d norm (−1.3 to 1.2, −0.81 to 1.4 ,and −0.83 to 1.2 Å for co-crystals 1, 2 and 3, respectively). It is clear that the information present in Table 3 is summarized effectively in these spots. The red color point in the 2-D fingerprint plots of co-crystals 1–3 on the d norm surfaces correspond to H···H, H···N, and H···N interactions. In the co-crystal 1 (Fig. 4a), the H···H interactions appear as a sharp knife which makes the most significant contribution to the total Hirshfeld surfaces (28.5 %) and the H···H interactions in the 2-D fingerprint of co-crystal 1 is different from that of co-crystal 2 and co-crystal 3, but the H···O interactions in the 2-D fingerprints of the three co-crystals are similar. The H···O intermolecular interactions look like a fork, which comprises 23.1 % of the total Hirshfeld surfaces, while N···H interactions play an important role in contributing to the total Hirshfeld surfaces (17.8 %). The C–H···π, C···C, and C···O interactions are in the corner of the 2-D fingerprint plot, and comprise 8.9, 5.1, and 7.0 %, respectively, of the total Hirshfeld surfaces. The Hirshfeld surfaces analysis for PZA in co-crystal 2 is displayed in Fig. 4b. The H···H interactions make the most significant contribution to the total Hirshfeld surfaces (32.6 %), and the H···O intermolecular interactions comprise 21.8 % of the total Hirshfeld surfaces, while H···N interactions play an important role in contributing to the total Hirshfeld surfaces (11.6 %). The C–H···π, C···C, and C···O interactions are in the corner of the 2-D fingerprint plot, and comprise 7.2, 7.8, and 4.4 %, respectively, of the total Hirshfeld surfaces.
The Hirshfeld surfaces analysis for PZA in co-crystal 3 (Fig. 4c) is similar to co-crystal 1. For example, the H···H interactions are still the main contributor to the total Hirshfeld surfaces (29.5 %), amounting to 28.5 % for co-crystal 1, while the H···O intermolecular interactions comprise 21.8 % of the total Hirshfeld surfaces. For co-crystal 2 it is 25.6 %, with the H···N interactions contributing 15.2 % to the total Hirshfeld surfaces. The C–H···π, c···c, and c···o interactions are in the corner of the 2-D fingerprint plot, and comprise 8.6, 5.7, and 8.0 % to the total Hirshfeld surfaces, respectively. The details of the main intermolecular interactions of three co-crystalsare shown Fig. 5.
Conclusions
We have investigated the detailed co-crystal formation of liquid PZA with co-crystal formers of hydroxyl-substituted benzoic acid (MHBA:m-hydroxybenzoic acid, PHBA:p-hydroxybenzoic acid, and 3,4-DHBA:3,4-dihydroxy benzolic acid). In addition, we make use of a new analysis method: the Hirshfeld surfaces and fingerprint plot analyses. With this method, we can not only detail the intermolecular interactions of pyrazinamide molecules in the three co-crystals but also know which kind of interactions contributes most to their stabilization.
References
R. Caliandroa, G.D. Profio, O. Nicolotti, J. Pharm. Biomed. Anal. 10, 78–79 (2013)
Y.H. Luo, B.W. Sun, Cryst. Growth Des. 13(5), 2098–2106 (2013)
O. Almarsson, M. J. Zaworotko, Chem. Commun. 1889–1896 (2004)
Y.H. Luo, B. Xu, B.W. Sun, J. Cryst. Growth (2013). doi:10.1016/jjcrysgro.201303007
C.B. Aakeroy, D.J. Salmon, Cryst. Eng. Commun. 7, 439–448 (2005)
Y. H. Luo, G. G. Wu, B. W. Sun, J. Chem. Eng. D. (2013) doi: http://dx.doi.org/10.1021/je3011997
S.G. Fleischman, S.S. Kuduva, J.A. McMahon, B. Moulton, R.D.B. Walsh, N. Rodr_ıguez-Hornedo, M.J. Zaworotko, Cryst. Growth Des. 3, 909–919 (2003)
Y.H. Luo, Y.R. Tu, J.L. Ge, B.W. Sun, Sep. Sci. Technol. (2013). doi:10.1080/014963952013.780256
S.L. Childs, L.J. Chyall, J.T. Dunlap, V.N. Smolenskaya, B.C. Stahly, G.P. Stahly, J. Am. Chem. Soc. 126, 13335–13342 (2004)
G.R. Desiraju, Angew. Chem. 107, 2541 (1995)
G.R. Desiraju, Angew. Chem. Int. Ed. Engl. 34, 2311 (1995)
L.R. MacGillivray, J. Org. Chem. 73, 3311 (2008)
T. Frisˇcˇic, W. Jones, Cryst. Growth Des. 9, 1621 (2009)
Y.H. Luo, Q. Zhou, B.W. Sun, J. Chem. Res. 36(9), 506–509 (2012)
Y.H. Luo, J. Xu, B.W. Sun, J. Chem. Res. 36(12), 697–700 (2012)
Y.H. Luo, G.G. Wu, S.L. Mao, B.W. Sun, Inorg. Chim. Acta 397, 1–9 (2013)
Y.H. Luo, Y.T. Ma, Q.Q. Bao, B.W. Sun, J. Chem. Crystallogr. 42, 628–632 (2012)
Y.H. Luo, C.G. Zhang, B. Xu, B.W. Sun, Cryst. Eng. Commun. 14, 6860–6868 (2012)
G.M. Sheldrick, SHELXL-97: Program for the Refinement of Crystal Structures (University of Gottingen, Germany, 1997)
M.A. Spackman, J.J. McKinnon, Cryst. Eng. Commun. 4, 378–392 (2002)
J.J. McKinnon, M.A. Spackman, A.S. Mitchell, Acta Crystallogr. Sect. B. 60, 627–668 (2004)
J.J. McKinnon, F.P.A. Fabbiani, M.A. Spackman, Cryst. Growth Des. 27, 75 (2007)
Z.C. Liu, M. Frasconi, J.Y. Lei et al., Nat. Commun. 4, 1855 (2013)
Acknowledgments
This work has been supported by the prospective joint research project of Jiangsu province (BY2012193), Fundamental Research Funds for the Central Universities (CXZZ12 _0119), Natural Science Foundation of China (21371031) and Funds for the Ministry of Science and Technology (212401025).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Lou, M., Mao, SH., Luo, YH. et al. Synthesis, co-crystal structure and characterization of pyrazinamide with m-hydroxybenzoic acid, p-hydroxybenzoic acid and 3,4-dihydroxy benzolic acid. Res Chem Intermed 41, 2939–2951 (2015). https://doi.org/10.1007/s11164-013-1402-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11164-013-1402-y