Abstract
Two new 1-D coordination polymers [M II (3-Bzpy)2(N(CN)2)2] (M = Cd (1), Cu (2), 3-Bzpy = 3-benzoylpyridine) have been synthesized and structurally characterized by single-crystal X-ray diffraction and IR. Single-crystal X-ray diffraction reveals that the complexes 1 and 2 are isostructural and all crystallize in monoclinic P21/c space group with cell dimensions: a = 6.3889(16) Å, b = 7.6496(12) Å, c = 26.767(6) Å, β = 99.131(19)°, V = 1291.6(5) Å3, Z = 2 for 1 and a = 6.4483(9) Å, b = 7.5449(10) Å, c = 25.965(4) Å, β = 98.559(10)°, V = 1249.2(3) Å3, Z = 2 for 2. Structure analysis manifest that 1 and 2 are all 1-D chains structures which parallel to the crystallographic b axis through end-to-end coordination by bidentate bridging dicyanamide ligands. Magnetic property of complex 2 was further investigated.
Graphical Abstract
The paper is about synthesis, crystal structure and magnetic property of two new 1-D coordination polymers. Perspective view down the a-axis of complex 1 with thermal ellipsoids drawn at the 50% probability level

.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Recent 10 years have seen a growing interest on dicyanamide ligand, due mainly to its remarkably versatile bridging modes [1–4] and the diverse magnetic properties [5–10]. The fascinating topological structures of dicyanamide can act as a uni-, bi-and tri-dentate ligand, which make it possible to construction various supramolecular architectures by introducing different ancillary ligand [10, 11]. Many coordination polymers of this kind have been reported. For example, one-dimensional (1-D) for [M II (pyr)2(N(CN)2)2] (M = Mn, Co, pyr = 2-pyrrolidone) [11], 2-D for b-[Cu (dca)2(pyz)]n (pyz = pyrazine) [12] and 3D for M(dca)2(bpeado) (M = Mn, Fe, Co, Ni, Cu; dca = dicyanamide; bpeado = 1,2-bis(4-pyridyl)-ethane-N,N′-dioxide) [10]. Even, there have diamond-like networks [13], a-Po type networks [14] and CdSO4-like networks [15] structures of this kind. The unusual magnetic properties displayed by these coordination polymers including long-range ferromagnetic ordering, ferromagnetism, weak ferromagnetism and paramagnetism [16]. In our previous work, we have successfully introduced 3-benzoylpyridine as ancillary ligand of dicyanamide system and obtained an interesting structure [17], Enlightened by this success, we reported here the synthesis, crystal structure of two 1D coordination polymers [M II (3-Bzpy)2(N(CN)2)2] (M = Cd (1), Cu (2), 3-Bzpy = 3-benzoylpyridine), and further investigated the magnetic property of 2.
Experimental
General Remarks
All chemicals used (reagent grade) were commercially available. Na [N(CN)2]2 was purchased from ACROS Company. Elemental analyses were performed by a Vario-EL III elemental analyzer for carbon, hydrogen, and nitrogen of the complex 1 and 2. Infrared spectra were recorded on a SHIMADZU IR prestige-21 FTIR-8400S spectrometer in the spectral range 4,000–400 cm−1, with the samples in the form of potassium bromide pellets. Temperature-dependent magnetization (M–T) of complex 2 were measured in the temperature range from 2 to 300 K using a quantum design vibrating sample magnetometer in a physical property measurement system.
X-ray Crystallographic Study
The single-crystal X-ray diffraction data of the complex 1 and 2 were collected at 298 K with graphite-monochromated Mo-Kα radiation (λ = 0.071073 nm), a Rigaku SCXmini diffractometer with the ω-scan technique for 1 and a Bruker SMART APEX diffractometer equipped with CCD area detector for 2 were used [18]. The lattice parameters were integrated using vector analysis and refined from the diffraction matrix, the absorption correction was carried out by using the Bruker SADABS program with multi-scan method. 2,954 (2,856) independent reflections and 2,448 (2,398) with I > 2.0σ(I) for 1 (2) were observed. Crystallographic data, data collection, and refinement parameters for complex 1 and 2 were given in Table 1. The structures of them were solved by full-matrix least-squares methods on all F 2 data, and used the SHELXS-97 and SHELXL-97 programs [19] for structure solution and structure refinement respectively. Reliability factors were defined as R1 = Σ(|F 0| − |F c|)/Σ|F 0| and the function minimized was R w = [Σw(F 20 − F 01 2)2/w(F 0)4]½, where in the least-squares calculation the unit weight was used. All non-hydrogen atoms were refined anisotropically, and hydrogen atoms were inserted at their calculated positions and fixed at their positions. Selected structural parameters are listed in Table 2. The molecular graphics were prepared by using the DIAMOND program [20]. CCDC-836397 and CCDC-836398 contains the supplementary crystallographic data in CIF format for the complex 1 and 2 reported in this paper. These data can be obtained free of charge from The Cambridge Crystallographic Data Centre via www.ccdc.cam.ac.uk/data_request/cif.
Synthesis of Complexes 1 and 2
Complex 1 (C28H18CdN8O2) was prepared as follows: 0.12 mmol (21.96 mg) 3-benzoylpyridine was added slowly to a 10 ml stirring aqueous solution of 0.12 mmol (30 mg) Cd(NO3)2·3H2O, the mixture was stirred for 10 min, after which, 10 ml methanol–water solution of 0.24 mmol (21.36 mg) sodium dicyanamide was added. The resulting solution was stirred for additional 30 min and then filtered. The filtrate was collected and left undisturbed. Colorless needles crystals formed after 3 days in high yield (83% based on Cd). Elemental analysis for 1, Anal. Calcd. (%): C, 55.05; N, 18.34; H, 2.97. Found: C, 55.08; N, 18.39; H, 3.00. IR (KBr pellet, cm−1): 2305, 2227, 2170, 1650, 1598, 1450, 1426, 1373, 780, 705, 690, 650.
The blue prism crystals of complex 2 (C28H18CuN8O2) was prepared in the same way as 1 by using Cu(NO3)2·3H2O (28 mg) instead of Cd(NO3)2·3H2O, the final yield is 80% (based on Cu). Elemental analysis for 2, Anal. Calcd. (%): C, 59.84; N, 19.94; H, 3.23. Found: C, 59.83; N, 19.96; H, 3.24. IR (KBr pellet, cm−1): 2308, 2226, 2170, 1650, 1595, 1450, 1428, 1370, 780, 708, 690, 655. The IR spectra of compounds 1 and 2 show the characteristic bands for 3-benzoylpyridine in the region 1,450–1,650 cm−1 and 690–780 cm−1. The bands around 2,200 cm−1 are due to dicyanamide ligands. Absorption at 650 cm−1 can be attributed to the Metal–N vibration.
Results and Discussion
Structural Description
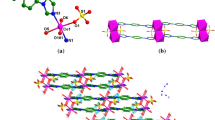
Complexes 1 and 2 are isostructural and crystallize in monoclinic P21/c space group. They are all centrosymmetric, take 1 for example, the asymmetric unit of 1 contains one 3-benzoylpyridine ligand, one 1/2 occupied CdII atom and two 1/2 occupied dicyanamide ligands, as shown in Fig. 1. The CdII atom, as shown in Fig. 2, which lies on an inversion center, is best described as ideal octahedral environment tetragonally compressed from Oh symmetry, with two N atoms from the trans arranged 3-benzoylpyridine ligands (with Cd(1)–N(1): 2.346(3) Å; Cd(1)–N(1A): 2.346(3) Å; N1–Cd1–N1: 180.00(16)°) occupy the axial positions and the four N atoms from the dicyanamide ligands (with Cd(1)–N(2): 2.333(3) Å; Cd(1)–N(3): 2.321(3) Å; N(2)–Cd(1)–N(2): 180.00(16)°; N(3)–Cd(1)–N(3): 180.0°; N(3)–Cd(1)–N(2): 92.69(12)°; N(3)–Cd(1)–N(2A): 87.31(12)°) lie in the equatorial plane. The dicyanamide ligand possesses pseudo-C2v symmetry with C≡N bond distances ranging from 1.118(5) to 1.124(5) Å, and coordination to the CdII center with C(13)–N(2)–Cd(1) and C(14)–N(3)–Cd(1) bond angles of 139.0 (3) and 161.0 (4)°, respectively.
The CdII atom and the two monodentate 3-Bzpy ligands reside on the mirror plane parallel to the crystallographic a axis through end-to-end coordination by bidentate bridging dicyanamide ligands, which act as bridges in μ-1, 5 model. A similar trend has also been observed in Mn[N(CN)2]2(2,4′-bpy)2 (2,4′-bpy = 2,4′-bipyridine) [21]. Polymeric 1-D chains are generated along the a axis with neighboring chains staggered a/2 (Fig. 2). The packing of the complex along the b axis is shown in Fig. 3. The centroid-to-centriod distances between 3-benzoylpyridine of adjacent chains are change from 4.079 to 4.164 Å, meaning that there are weak π–π stacking interactions among neighboring polymeric 1-D chains. The CdII···CdII intrachain length is 7.650 Å. For complex 2, the centroid-to-centriod distances change from 4.110 to 4.128 Å, and the CuII···CuII intrachain length is 7.545 Å. The centroid-to-centriod distances and metal···metal intrachain length are all bigger than the reported Mn[N(CN)2]2(2,4′-bpy)2 (2,4′-bpy=2,4′-bipyridine) [21].
The magnetic susceptibility of compound 2 was measured in the temperature ranging from 2 to 300 K and under 1999.985 Oe field. As shown in Fig. 4, it indicate a dominant antiferromagnetic interactions and possesses a χMT value of 0.50646 cm3 mol−1 K at 300 K [the correspond effective magnetic moment is 1.998 BM, which is close to that expected for an isolated copper (II) ion (1.73 BM)], the value decrease slowly as the temperature lowered, reaching 0.43274 cm3 mol−1 K at 50 K. Then it decreased rapidly and reaching 0.13147 cm3 mol−1 K at 2 K. The χM values of complex 2 increases from 0.00168 cm3 mol−1 at 300 K to a maximum of 0.0695 cm3 mol−1 at about 2 K. The 1/χM versus T plot for 2 could fit with Curie–Weiss equation χM = C/(T − θ) from 2 to 300 K, giving Weiss constant C = 0.51 cm3 mol−1 K and θ = −7.85 K (R = 0.9986).
Conclusions
In summary, we have successfully synthesized two coordination polymers bridged by dicyanamides. Structure analysis indicates that the frameworks of complexes 1 and 2 are 1-D chain structures which parallel to the b axis, and there are weak π–π interactions in the crystal structure. These features are the common gender of complex within this kind.
References
Shi YL, Chen XT, Li YZ, Xue ZL, You XZ (2002) New J Chem 26:1711
Batten SR, Jensen P, Moubaraki B, Murray KS, Robson R (1998) Chem Commun 439
Carranza J, Brennan C, Sletten J, Lloret F, Julve M (2002) J Chem Soc Dalton Trans 3164
Sun HL, Gao S, Maa BQ, Batten SR (2004) Cryst Eng Comm 6:579–583
Kutasi AM, Batten SR, Moubaraki B, Murray KS (2002) J Chem Soc Dalton Trans 819
Jensen P, Batten SR, Moubaraki B, Murray KS (2000) Chem Commun 793
Manson JL, Arif AM, Incarvito CD, Liable-Sands LM, Rheingold AL, Miller JS (1999) J Solid State Chem 145:369
Claramunt A, Escuer A, Mautner FA, Sanz N, Vicente R (2000) J Chem Soc Dalton Trans 2627
Kurmoo M, Kepert C (1998) New J Chem 22:1515
Kutasi AM, Harris AR, Batten SR, Moubaraki B, Murray KS (2004) Cryst Growth Des 4(3):605–610
Sun BW, Gao S, Ma BQ, Wang ZM (2001) Inorg Chem Commun 4:72–75
Jensen P, Batten SR, Fallen GD, Hockless DCR, Moubaraki B, Murray KS, Robson R (1999) J Solid State Chem 145:387
Wang ZM, Sun BW, Luo J, Gao S, Liao CS, Yan CH, Li Y (2002) Inorg Chim Acta 332:127
Martin S, Barandika MG, Ezpeleta JM, Cortes R, Larramendi JIR, Lezama L, Rojo T (2002) J Chem Soc Dalton Trans 4275
Sun HL, Gao S, Maa BQ, Batten SR (2005) Cryst Growth Des 5:1331–1333
Manson JL, Kmety CR, Palacio F, Epstein AJ, Miller JS (2001) Chem Mater 13:1068
Yu F, Li ZS, Sun BW (2008) Acta Cryst E64:993
Rigaku (2005) CrystalClear, Version 14.0. Rigaku Corporation, Tokyo
Sheldrick GM (1997) SHELXS97: programs for crystal structure analysis. University of Göttingen, Göttingen
Brandenburg K (2006) DIAMOND: crystal and molecular structure visualization, version 3.1b. Crystal impact GbR, Bonn
Liu CM, Zhang DQ, Zhu DB (2003) Transit Met Chem 28:336–338
Acknowledgments
This work was supported by the National Natural Science Foundation of China (Project 20671019).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Luo, YH., Ma, YT., Bao, QQ. et al. Syntheses, Crystal Structure and Properties of Two 1-D Coordination Polymers Bridged by Dicyanamides. J Chem Crystallogr 42, 628–632 (2012). https://doi.org/10.1007/s10870-012-0293-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10870-012-0293-x