Abstract
Four mancozeb derivatives containing 1,3,4-thiadiazole, 5a–5d, were successfully synthesized, then characterized by IR, 1H NMR, and 13C NMR spectroscopy and elemental analysis. Their antimicrobial activity against paddy fusarium, Botrytis cinerea, cucumber fusarium, tomato gibberella, and grape white rot was tested in vitro by use of the filter paper disk-diffusion technique. Compounds 5a–5d had moderate to excellent antimicrobial activity in comparison with mancozeb, 6. Among these compounds, 5a had the best inhibitory activity.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
1,3,4-Thiadiazole compounds have been of much interest in synthetic chemistry and as pesticides because of their unique biological properties. Recently, 1,3,4-thiadiazole derivatives have been shown to have a variety of biological activity, for example as fungicides [1], plant growth regulators [2, 3], insecticides [4], herbicides [5], and anticholinergic [6] and anticonvulsant [7] compounds. Therefore, these compounds attract much attention as novel pesticides. Dithiocarbamate salts are widely used in the petroleum [8], agriculture [9], pharmaceutical [10], environmental protection [11], and rubber [12] industries. However, because resistance to Zineb has been observed, development of novel potent antimicrobial agents with superior bioavailability is highly desirable. Herein, we describe the synthesis and antimicrobial activity of four novel mancozeb derivatives containing 1,3,4-thiadiazole (Scheme 1).
Results and discussion
All the reagents described in this paper were commercially available, and all solvents were freshly distilled before use. Melting points were recorded on an X-4 microscopic melting point apparatus, and are uncorrected. Elemental analysis (C, H, and N) was performed with a VarioEL III. IR spectra, as KBr pellets, were recorded on a Vector-22 instrument. 1H NMR and 13C NMR spectra were recorded on an Avance III 400 MHz instrument, with CDCl3 as solvent and SiMe4 as internal reference.
Synthesis of 2-amino-1,3,4-thiadiazole derivatives
A mixture of 20 mL concentrated hydrochloric acid, 0.026 mol carboxylic acid, and 0.022 mol thiosemicarbazide was heated under reflux for 5 h. After cooling to room temperature, the mixture was adjusted to pH 8 with 40 % sodium hydroxide solution. The white solid powder obtained was isolated by filtration, washed with water, and finally recrystallized from 30 % ethanol.
Results from characterization of 2-amino-1,3,4-thiadiazole derivatives 3a–3d are listed in Tables 1, 2, and 3.
Synthesis of mancozeb and 1,3,4-thiadiazole derivatives of mancozeb
A mixture of 0.010 mol 1,2-ethanediamine or 2-amino-1,3,4-thiadiazole derivative and 0.010 mol sodium hydroxide aqueous solution was stirred, heated to 30 °C, then 0.020 mol carbon disulfide was slowly added dropwise to the mixture. The reaction was monitored until completion then cooled to room temperature. Manganous chloride (0.01 mol) aqueous solution was slowly added dropwise, the mixture was stirred for 0.5 h, then 0.01 mol zinc chloride aqueous solution was slowly added dropwise, followed by stirring for 1.5 h. Mancozeb was obtained as a gray solid powder after filtration and drying.
Results from characterization of mancozeb, 6, and 1,3,4-thiadiazole derivatives of mancozeb, 5a–5d, are listed in Tables 4, 5, and 6.
Antimicrobial activity of 1,3,4-thiadiazole derivatives of mancozeb
Antimicrobial activity was determined by use of the filter paper disk method [13]. Paddy fusarium, Botrytis cinerea, cucumber fusarium, tomato gibberella, and grape white rot were selected for the test.
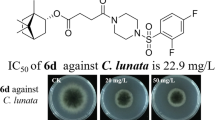
The samples (1,3,4-thiadiazole derivatives of mancozeb 5a–5d) were separately dissolved in an appropriate solvent at a concentration of 10 mg/mL. Compound 6 was used as the standard. Cultures of known inoculum size (106 cells/mL) of the tested strains were spread on nutrient agar plates. Sterile filter paper disks 6 mm in diameter was placed on the plates and the 0.5 ml of 10 mg/mL solutions of the mancozeb derivatives were applied to the filter disks. The plates were incubated for three days at 28 °C. Antimicrobial activity was evaluated by measuring the diameter of the zone of inhibition; the results are summarized in Table 7.
Investigation of the antimicrobial screening data revealed that all the novel mancozeb derivatives 5a–5d had moderate to excellent antimicrobial activity against the five strains tested. Compound 5b had the best inhibitory activity against Botrytis cinerea, besides the standard 6; its zone of inhibition reached 23.6 mm. Compounds 5c and 5d had better inhibitory activity than standard 6 against cucumber fusarium; their zones of inhibition were 15.1 mm and 13.6 mm, respectively. All these novel mancozeb derivatives had stronger inhibitory activity than the standard 6 against grape white rot. Among these mancozeb derivatives, compound 5a had the most broad spectrum and strongest inhibitory activity.
From the discussion above we can propose a speculative mechanism of the antimicrobial activity of these novel mancozeb derivatives. Mancozeb had a relatively strong inhibitory antimicrobial effect and mancozeb containing the 1,3,4-thiadiazole group had stronger antimicrobial activity than mancozeb because the 1,3,4-thiadiazole group is a fat-soluble functional group. Different 1,3,4-thiadiazole-containing derivatives of mancozeb had different antimicrobial activity against the five strains tested. Because of less steric hindrance, compound 5a had high activity against paddy fusarium, tomato gibberella, and grape white rot.
Conclusion
Novel mancozeb derivatives containing the 1,3,4-thiadiazole group were synthesized in good yield. Their antimicrobial activity was tested. Compound 5a had the best inhibitory activity. This work is an excellent basis for discovery of potent antimicrobial agents.
References
A. Niewiadomy, J. Matysiak, Pestycydy/Pesticides 1, 5 (2010)
B.P. Sadhu, K. Gupta, Geobios (IND) 24(1), 55 (1997)
F. Narum, T. Hattor, N. Matsum et al., Tetrahedron 60, 7827 (2004)
R. Wan, J.Q. Zhang, F. Han et al., Nucleosides, Nucleotides Nucleic Acids 30(4), 280 (2011)
R.M. Allan, C.F. David, S.K. Han, J. Heterocycl. Chem. 22(3), 915 (1985)
Z. Muhi-eldeen, F. Al-Jawed, S. Eldin et al., Eur. J. Med. Chem. 17, 479 (1982)
C.B. Chapleo, M. Mayers, P.L. Myers et al., J. Med. Chem. 29, 2273 (1986)
J.C. Ricardo, A.S. Verônica, G. Salvador et al., Anal. Sci. 18, 1253 (2002)
R.L. Metealf, T.R. Fukuto, M. Fredeiekson et al., J. Agric. Food Chem. 13, 473 (1965)
S. Sonia, G. Marina, P. Eduardo et al., Mutat. Res. 514, 201 (2002)
K.M. Ashok, F. Werner, Talanta 52, 341 (2000)
J. Wang, World Rubber Ind. 36(6), 8 (2009)
L. Du, F. Lu, (China Light Industry Press, 2005), p. 24
Acknowledgments
Professor Zhang Zhiwei is kindly acknowledged for antimicrobial activity tests. This work was supported by the Natural Science Foundation of Shaanxi Province, People’s Republic of China (project no: 2009JZ002).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Liu, Y., Liang, G. & Yin, D. Synthesis and antimicrobial activity of novel mancozeb derivatives containing 1,3,4-thiadiazole. Res Chem Intermed 41, 2019–2024 (2015). https://doi.org/10.1007/s11164-013-1328-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11164-013-1328-4