Abstract
Poly(N-vinyl-2-pyrrolidone) (PVP) and gelatin protected silver nanostructures are prepared in formamide by simple chemical route. Both PVP and gelatin stabilized silver nanoparticles in formamide lead to the formation of nanostructures of various definite geometric shapes and sizes. The effect of anisotropy on the surface plasmon absorption band is analyzed by monitoring the UV-Visible absorption spectra of gelatin stabilized silver nanoparticles. The particles were characterized by UV-Visible absorption spectra and TEM.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The size and shape dependent physical, optical and electronic properties of metal and semiconductor nanocrystals make them compelling components of modern material chemistry [1–3]. The intrinsic properties of metal nanostructures can be tailored by controlling their size, shape, composition, crystallinity and structure [4–7]. The interest in the synthesis of novel metal nano-structures with controlled geometries [8–10] is motivated by the size and shape tunable optical properties of the nanoparticles, which has projected application in nanoelectronics, optical filters, photon energy transport and Surface-enhanced Raman or fluorescence scattering. Metal crystallites are important due to their close-lying conduction and valence bands in which electrons move freely. The free electrons give rise to surface plasmon absorption bands which depend on the particle shape, size and chemical environment. Different methods for the synthesis of nanostructures have been developed. The techniques include chemical reduction of metal ions in aqueous or non-aqueous solvents, electrochemical pathways, thermal decomposition, etc.
Control over the growth of the primarily formed nanoclusters and their agglomeration is affected by the use of stabilizers in the form of donor ligands [11–17], synthetic polymers [18–22] surfactants, etc. The interaction of the stabilizer with the metal ions plays a very important role in controlling the size and morphology of the particles. In condensed medium it has been shown that it is possible to control the size and morphology of the particles using different ratios of the metal ions and stabilizer [16, 23]. Although many chemical routes are available for the production of size controlled or shape selective transition metal particles in aqueous solution, it is desirable to design new synthetic methods for producing them in non-aqueous solutions, which could bring about some new feature in their properties.
There is an upsurge in the research activities on the synthesis of nanoparticles in non-aqueous solvents. Since most of the reactions take place in organic solvents, synthetic approach for the formation of metal nanoparticles having different morphology in addition to the stabilization is highly desired. In addition, if one can design a simple protocol for preparation of particles having different morphologies in the same medium without the addition of any external reducing agents, this may have its own important implications. Reduction of metal ions to zero-valent metal, even at room temperature in absence and presence of any reducing agent in DMF is reported by Liz-Marzan [24–28]. Recently, our group has reported the reduction of silver ions in formamide without the addition of external reducing agent [8, 9].
In present paper, we discuss the effect of stabilizers (PVP and Gelatin) on the formation of silver nanoparticles in formamide.
Experimental section
Material
AgNO3, Gelatin (BDH, India), PVP (Mol wt. = 360,000) (Sigma), formamide (UV spectroscopy grade, Spectrochem, India) were used as received. All solutions were freshly prepared and kept in dark to avoid any photochemical reactions. Water purified through a millipore system was used.
Preparation of silver nanoparticles in presence of stabilizer
Stable dispersion of silver nanoparticles were prepared by mixing of 1% PVP (w/v) or 1% gelatin (w/v) in formamide followed by addition of required concentration of silver salt (AgNO3) at room temperature. With time, the solution gradually turned yellow and evolution of stable dispersion of silver nanoparticles was observed.
Characterization
Samples for transmission electron microscopy (TEM) were prepared by putting a drop of the colloidal solution on a copper grid coated with a thin amorphous carbon film. Samples were dried and kept under vacuum in a desiccator before putting them in a specimen holder. TEM characterization was carried out using a JEOL JEM-2000FX electron microscope. Particle sizes were measured from the TEM micrographs and calculated by taking at least 100 particles. Absorption measurements were carried out on a Chemito-Spectrascan UV 2600 spectrophotometer. The spectra were recorded at room temperature using either 0.2 cm or 1 cm quartz cuvette. However, all the spectra were normalized to 1 cm path length of the cuvette.
Results and discussion
Silver nanostructures in presence of different stabilizers
In our earlier study we have reported the direct reduction of silver ions by the solvent, formamide, in presence and absence of different stabilizers without addition of any external reducing agent [9]. Addition of stabilizer affects the nucleation and growth process of nanoparticles [29]. Addition of silver salt to formamide at room temperature in absence of any stabilizer leads to reduction of silver ions that eventually lead to the formation of metallic silver film on the inner walls of the container due to aggregation of the initially formed nanoparticles.
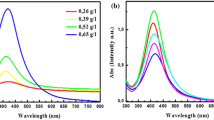
Stabilization of the nanoparticles can be achieved by incorporation of suitable stabilizer. Figure 1 shows a typical time-dependent evolution of silver nanoparticles in formamide containing 1 × 10−2 mol dm−3 AgNO3 and 1% PVP (w/v) which acts as the stabilizer.
Recently Tsuji et al. [30] have reported the control of nanostructures of different sizes and morphologies by varying the metallic salt to surfactant or polymer ratio. The control of the size and shape of silver nanostructures in DMF by changing the concentration of silver salt and stabilizer is also reported by Liz-Marzan et al. [31]. With the attempt to synthesize different silver nanostructures in formamide, PVP stabilized silver nanoparticles were formed at different silver ion concentration. It was observed that by changing the [Ag]/[PVP] ratio nanostructures of different geometric shapes were formed. The binding of PVP to metal ions can be the reason for formation of different morphologies of silver in the same reaction mixture. Figure 2 shows TEM image of particles formed by reduction of 5 × 10−3 mol dm−3 of silver ions in the presence of 1% PVP (w/v). It can be clearly seen that in addition to spherical particles, nanorods, nanoplates and thin semi-transparent films having definite geometric shapes are also obtained. Initially, nucleation results in the formation of small spherical nanoparticles. As the particles grow in size, with time at some point there is adhesion of neighbouring nanoparticles that finally leads to the formation of larger crystalline nanorods, nanoplates and nanosheets. The density of the nanorods increases with the increase in the concentration of the silver ions. However, particles formed using lower concentration of metal ions did not show the formation of larger nanostructures. This shows that slow nucleation leads to thermodynamically stable spherical particles.
Figure 3 depicts the formation of thin films. It is also observed that the films are so thin that they tear from their center leading to void space. These void spaces also have definite shape. These observations only lead to the conclusion that the particles formed have highly geometric crystalline structure. An interesting phenomenon noticed in Fig. 3 was that at certain areas there are groups of spherical particles, which eventually agglomerate to form thin films. The films are so thin that particles beneath these films are also observed. Thus the mechanism of formation of larger nanostructures is evident form the TEM image.
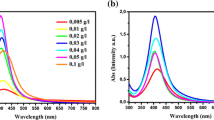
Stable dispersions of silver were also obtained in formamide by using gelatin as a stabilizer. The position and shape of the surface plasmon absorption band of noble metal nanostructures are strongly dependent on the size and shape of the particle, dielectric constant of the medium and surface-adsorbed species. The formation process and the optical properties of silver nanoparticles can also be identified from both the colour change and UV-visible absorption spectra of the solution. It is known that the colour of the metal colloid is caused by sum of effects of absorption and scattering of visible light [13]. The formation of silver nanostructure in the presence of 1% gelatin (w/v) was traced visually through a continuous change from pale yellow during the formation of initial clusters, through orange towards wine red. UV-visible absorption spectra of silver nanoparticles obtained on addition of 1 × 10−2 mol dm−3 of AgNO3 to formamide containing 1% gelatin (w/v) is shown in Fig. 4. A broad peak centered at ~440 nm was observed which is attributed to the characteristic surface plasmon excitation of spherical nanoparticles. A small peak at 410 nm was also observed which could be due to the presence of very small particles. Several weak bands at 360, 380 and ~510 nm are also observed. The presence of various absorption bands indicates the existence of silver nanoparticles of various shapes and sizes. According to Mie theory small nanocrystals should exhibit a single surface plasmon band where as anisotropic particles should exhibit multiple bands depending on their shape [4]. Absorption spectra of larger metal colloids dispersion can exhibit broad or additional bands in the UV-visible range due to the excitation of plasmon resonances or quadrupole and higher multipole plasmon excitations [32]. It is clearly seen from Fig. 4 that the UV-Visible spectra show distinct dipole and quadrupole plasmon resonances. A small peak at ~360 nm can be attributed to the out-of-plane quadrupole resonance. The second small peak at 410 nm can be due to the out-of-plane dipole resonance of small spherical nanoparticles. Silver nanorods are known to exhibit two distinct peaks, at ~440 nm due to transverse plasmon resonance which is similar to the spherical nanoparticles and at ~530 nm due to longitudinal peak. The presence of these peaks reveals the possibility of formation of nanorods in the system. It has been demonstrated both theoretically and experimentally that the long wavelength resonance are the in-plane dipole plasmon resonance and is very sensitive to the aspect (length to diameter) ratio of the particles [33]. The surface plasmon resonance spectra show the possibility of the presence of silver nanostructure of various sizes and shapes. To confirm the morphologies of the particles, TEM analysis was carried out.
A typical TEM image for a solution containing 1 × 10−2 mol dm−3 AgNO3 and 1% gelatin (w/v) is shown in Fig. 5. The image shows the presence of silver nanoparticles of different shapes and sizes. The particles have definite geometric shapes. Most of the particles are spherical in shape of size less that 10 nm. The small particles aggregate to form larger nanostructures of different shapes mainly nanorods, triangles and polygons along with spherical nanoparticles. The TEM analysis corroborates well with the results drawn from the corresponding UV-visible absorption spectra.
Probable mechanism for the reduction of silver ions by formamide
We have reported in out earlier study that reduction of silver ions by formamide leads to evolution of CO2 in presence and absence of stabilizers [8, 9]. However the rate of evolution was proportional to the rate of reduction of silver ions. The probable mechanism for reduction of silver ions with formamide is shown in Eq. 1.
It was observed that the radicals of formamide are also reducing in nature. The radicals of formamide were characterized using pulse radiolysis technique (A. Sarkar et al. under preparation).
Conclusion
In conclusion we have demonstrated successful synthesis of silver nanostructures in formamide in the presence of different stabilizers. It appears that the stabilizers play an important role in the formation of nanoparticles of different shapes and sizes. Aggregation of smaller particles leads to formation of larger thin nanosheets and nanostructure having definite geometric shapes. Void spaces in the semi-transparent nanosheets reveal the crystalline structure of the nanoparticles. The effect of anisotropy on the plasmon resonance can be clearly seen by monitoring the UV-Visible absorption spectra of the synthesized nanostructures.
References
A.P. Alivistos, J. Phys. Chem. 100, 13226 (1996)
C.B. Murray, D.J. Norris, M.G. Bawendi, J. Am. Chem. Soc. 115, 8706 (1993)
C.M. Lieber, Solid State Commun. 107, 607 (1998)
U. Kreibig, M. Vollmer, Optical Properties of Metal Clusters. (Springer-Verlag, Berlin, 1995)
M.A. El-sayed, Acc. Chem. Res. 34, 257 (2001)
J.B. Jackson, N.J. Halas, J. Phys. Chem. B 105, 2743 (2001)
S.L. Westcott, S.J. Olendenburg, T.R. Lee, N.J. Halas, Chem. Phys. Lett. 300, 651 (1999)
A. Sarkar, S. Kapoor, T. Mukherjee, J. Colloid Interface Sci. 287, 496 (2005)
A. Sarkar, S. Kapoor, T. Mukherjee, J. Phys. Chem. B 109, 7698 (2005)
Y. Wu, P. Yang, J. Am. Chem. Soc. 123, 3165 (2001)
J.L. Marignier, J. Belloni, M.V. Delcourt, J.P. Chevalier, Nature 317, 344 (1985)
C. de Cointet, M. Mostafavi, J. Khatouri, J. Belloni, J. Phys. Chem. B 101, 3512 (1997)
S. Kapoor, Langmuir 14, 1021 (1998)
S. Kapoor, Langmuir 15, 4365 (1999)
S. Kapoor, Langmuir 16, 5496 (2000)
T.S. Ahmadi, Z.L. Wang, T.C. Green, A. Henglein, M.A. El-sayed, Science 272, 1924 (1996)
B.G. Ershov, A. Henglein, J. Phys. Chem. B 102, 10663 (1998)
H. Hirai, Y. Nakao, N. Toshima, Chem. Lett. 545 (1978)
A. Henglein, Chem. Rev. 89, 1861 (1989)
P.V. Kamat, Chem. Rev. 93, 267 (1993)
J. Belloni, Radiat. Res. 150, S9 (1998)
A. Henglein, D. Meisel, J. Phys. Chem. B 102, 8364 (1998)
Y. Li, M.A. El-sayed, J. Phys. Chem. B 105, 8938 (2001)
I. Pastoriza- Santos, Langmuir 15, 948 (1999)
I. Pastoriza-Santos, Pure Appl. Chem. 72, 83 (2000)
I. Pastoriza-Santos, C. Serra-Rodriguez, L.M. Liz-Marzan, J. Colloid Interface Sci. 221, 236 (2000)
I. Pastoriza-Santos, L.M. Liz-Marzan, Langmuir 18, 2888 (2002)
I. Pastoriza-Santos, L.M. Liz-Marzan, Nano Lett. 2, 903 (2002)
T. Tojo, M.C. Blanco, F. Rivadulla, M.M. Lopez-Quintela, Langmuir 13, 1970 (1997)
M. Tsuji, M. Hashimoto, Y. Nishizawa, M. Kubokawa, T. Tsuji, Chem. Eur. J. 11, 440 (2005)
M. Giersig, I. Pastoriza-Santos, L.M. Liz-Marzan, J. Mater. Chem. 14, 607 (2004)
P.V. Kamat, M. Flumiani, G.V. Hartland, J. Phys. Chem. B 102, 3123 (1998)
R.C. Jin, Y.W. Cho, C.A. Mirkin, K.L. Kelly, G.C. Schatz, J.G. Zheng, Science 294, 1901 (2001)
Acknowledgement
The authors thank Dr. S. K. Sarkar, Head, RPCD, for his encouragement during the course of the work.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Sarkar, A., Kapoor, S. & Mukherjee, T. Effective chemical route for the synthesis of silver nanostructures in formamide. Res Chem Intermed 35, 71–78 (2009). https://doi.org/10.1007/s11164-008-0010-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11164-008-0010-8