Abstract
Highly stable and monodispersed silver nanoparticles with uniform morphology have been successfully prepared by microwave (MW) irradiation within a few seconds from the mixture of silver nitrate, ethanol and latex copolymer. The aqueous emulsion of latex copolymer acts as both reducing and stabilizing agent. To the best of our knowledge, it was the first time that the effect of MW irradiation time and latex concentration on the silver nanoparticle preparation and properties was analyzed. The formation of silver nanoparticles was confirmed by Ultraviolet–visible spectroscopy and transmission electron microscopy (TEM). The UV–Vis spectra are marked by the characteristic surface plasmon absorption band in the range 410–420 nm. From TEM images, silver nanoparticles were observed to be spherical with sizes ranging from 4 to 10 nm. Electron diffraction patterns on selected area, indicated that the silver nanoparticles are crystalline with face centered cubic structure.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Noble metal nanoparticles have attracted a great interest in recent years because of their unique optical, electronic and chemical properties, which depend strongly on their size and shape. Thus, it would be very useful to control both of these parameters in the synthesis process of metal nanoparticles in order to fine tune the optical properties for new applications. Among the noble metal nanoparticles, silver has got much recognition because of its potential applications in various fields such as photonics [1], microelectronics [2], photo catalysis [3–5], lithography [6], biosensor material [7] and optoelectronics [8].
One characteristic of noble metal nanoparticles consists in a localized surface plasmon resonance (LSPR) frequency in the near UV, visible or infrared part of the spectrum. The LSPR frequency depends strongly on the particle geometry (size and shape), crystallinity, assembly and on the surrounding medium of the nanostructures [9–11].
During the last two decades, silver nanoparticles were synthesized using various methods such as MW-polyol process, electrochemical [12], laser ablation [13], photochemical [14], sonochemical [15] and chemical reduction [16]. Among these procedures, the Microwave-polyol method may be promising for rapid and efficient preparation of silver metallic nanoparticles; it allows a good control of average particle size and shape.
For the microwave synthesis process, heating is created by the interaction of the permanent dipole moment of the molecule with high frequency (2.45 GHz) electromagnetic radiation. This technique has been increasingly applied in material sciences due to its unique reaction effects such as very rapid internal volumetric heating of precursor solutions. Under microwave (MW) heating, the temperature is raised uniformly throughout the whole liquid volume by direct coupling of MW energy to the molecules present in the reaction mixture [17–32] leading to drastic increase in the reaction rate [33].
A reaction medium with a high dielectric loss at the standard operating frequency of a MW reactor (2.45 GHz) is required for efficient absorption and, consequently, for rapid heating [34]. Water and alcohols have high dielectric losses and a high reducing ability. Therefore they are the ideal solvents for MW heating.
For the preparation of silver nanoparticles, the MW heating which is generally fast, simple and efficient in energy has been reported and is widely used in various fields of chemistry [33]. Komarneni et al. [35] produced the nanophase platinum (Pt) and silver (Ag) metal powders by microwave-polyol (M-P) process using ethylene glycol as reducing agent of silver ions with poly(N-vinyl-2-pyrrolidone) (PVP) as a protective agent in the presence of NaOH. Nghia et al. [36] synthesized Nanowire-shaped silver by the microwave-polyol process, using ethylene glycol as a reductant and polyvinyl pyrrolidone (PVP) as a stabilizer. Abargues et al. [34] reported the preparation of colloidal spherical silver and gold nanoparticles with average diameters of 10.4 and 11 nm, respectively from the reduction of the metal salts of AgNO3 and HAuCl4 in methanol assisted by microwave irradiation, in the presence of polyvinyl alcohol (PVA) as reducing and capping agent. Patel et al. [37] studied preparation of nanosized metallic silver particles (15–30 nm) from silver nitrate (AgNO3) both in the presence and absence of stabilizer poly(vinylpyrolidone) (PVP), using a microwave technique. Tsuji et al. [38] investigated the synthesis of a mixture of anisotropic silver nanostructures (with average diameters between 20 and 188 nm) as nanorods, nanowires, nanosheets, triangular and hexagonal nanoplates, spheres and cubes within a few minutes in the presence of platinum (Pt) seeds, using a microwave (MW)-polyol method.
Jiang et al. [39] prepared silver nanoparticles sized from 14 to 25 nm in ethylene glycol by polyol process and variable frequency microwave (VFM) and have made a comparative study with conventional heating method. Karimipour et al. [40] prepared silver nanoparticles with sizes ranging from 10 to 20 nm by microwave irradiation, using oleylamine (OA) as stabilizer and dimethylformamide (DMF) as reducing agent for silver ions. Dzido et al. [41] realized the synthesis of spherical silver nanoparticles with a mean size of 10–20 nm, within a few seconds (3–24 s), using the continuous-flow microwave-assisted polyol method. Pal et al. [42] synthesized spherical silver nanoparticles with an average diameter of 10 nm within 5 s of heating time and 800 W of microwave power by dissolving polyvinylpyrrolidone (PVP) and silver nitrate (AgNO3) in ethanolic solution, under microwave heating, using ethanol as a reducing agent and PVP as a stabilizer. Baghbanzadeh and co-workers [43] have reviewed the preparation of Ag nanoparticles by reduction of the diaminesilver(I) precursor complex ([Ag(NH3)2]+) in aqueous carboxymethylcellulose (CMC) solutions under both MW heating and conventional oil-bath heating. Joseph and Mathew [44] studied the catalytic activity of spherical silver nanoparticles with an average diameter of 18.84 nm prepared by microwave irradiation route from an aqueous solution of silver nitrate (AgNO3) in the presence of hexamine as reducing agent and the biopolymer pectin as the capping agent. Helmlinger et al. [45] reported the fabrication of spherical silver nanoparticles from the reduction of silver nitrate in diethylene glycol and ethylene glycol with the presence of poly(N-vinyl pyrrolidone) (PVP) as capping agent by using the microwave assisted polyol synthesis procedure within a 20 min of heating time. They used the reduction of silver nitrate with glucose in aqueous solution for 60 min at 90 °C for comparison. Pal and Deb [46] have used microwave irradiation process at a power of 300 W and 4 min to produce spherical silver nanoparticles with average size of 11 nm from the reduction of silver nitrate by glucose in the presence of PVP as stabilizer. Aswathy et al. [47] employed the microwave heating method to form silver nanoparticles with spherical and hexagonal morphologies within 2 min of microwave irradiation at 180 W of microwave power, from the reduction of silver nitrate (AgNO3) with vanillin (C8H8O3) in aqueous surfactant media (Sodium bis (2-ethyl hexyl) sulfosuccinate (AOT) and Sodium dodecyl sulphate (SDS)). Sreeram et al. [48] yielded silver nanoparticles from silver nitrate using starch as both reductant and stabilizing agent. They employed three methods of preparation of silver nanoparticles such as direct heating at 80 °C for 30 min, controlled heating from 45–75 °C and microwave irradiation (30–120 s), in order to study the influence of the synthetic strategy on the size and shape of the obtained products. Shao-Peng et al. [49] investigated the preparation of spherical silver nanoparticles with an average size of 20 nm from ammoniacal silver nitrate Ag(NH3)2NO3 solution by microwave irradiation with a power of 700 W for 7 min. They performed a comparative study with conventional heating by using PVP to reduce the precursor Ag(NH3)+2 in the ammoniacal silver nitrate Ag(NH3)2NO3 solution at 80 °C for 1 h. Noroozi et al. [50] studied the green formation of spherical and dendritic silver nanostructures by reduction of silver nitrate (AgNO3) in the presence of PVP as both reducing and stabilizing agent under microwave irradiation, and were compared with those synthesized by conventional heating method. In the present study, we succeeded to prepare uniform small monodispersed silver nanoparticles with spherical geometry sized from 4 to 10 nm under different microwave heating times (from only 30 to 120 s) and latex copolymer concentrations. The microwave heating time used in the almost works cited above for the preparation of silver nanoparticles is too long despite of the large dielectric loss tangent (tan δ = 1.350) and a high boiling point (198 °C) of the solvent ethylene glycol, in comparison with the maximum irradiation time (2 min) used in our work when we study the effect of the heating time on the properties of the silver nanoparticles prepared using only the mixed solution of the silver precursor (AgNO3) and latex copolymer in ethanol without additional reagents and without stirring or sonication of the reaction mixture. The prepared samples were not filtered and were found to be extremely stable during 12 months in the dark under ambient conditions without any visible change, confirming the high quality and uniform diameter of the nanoparticles obtained by the microwave synthesis approach. The originality in our synthesis method is based essentially on the use of an aqueous emulsion of latex copolymer of vinyl versatate and vinyl acetate (widely used in industry). So, our challenge is to functionalize this industrial product, giving it new optical and thermal properties. Furthermore, the use of aqueous emulsion avoids the use of solvents that may have a negative impact on the environment.
Experimental
Materials and Experimental Setup
Aqueous emulsion from commercial latex copolymer and silver nitrate as the source of silver (AgNO3, 99 % purity) were used as received without any further purification. In this study, the ethanol was used as solvent as well as for dilution. The microwave setup used for the preparation of silver nanoparticles consists in microwave oven (Mars5-System from company CEM) working at frequency 2.45 GHz and maximum power of 1200 W.
The silver nanoparticles were synthesized by reduction of silver nitrate in the presence of latex copolymer under MW irradiation. Latex concentration and microwave heating time were varied in order to determine the optimum experimental conditions leading to monodispersed silver nanoparticles with controlled size and shape.
In a typical synthesis procedure, a colourless transparent latex solution was obtained by dissolving 26 mg of latex in 20 ml of ethanol. Different quantities of this solution (2, 3, 4 and 5 ml) were taken and diluted with 8, 7, 6 and 5 ml of ethanol, respectively.
2 ml of each diluted sample was taken as blank solution for optical measurements before adding 2 mg of AgNO3 leading to a latex-Ag nanocomposites. The mixtures were then placed in a microwave oven and irradiated for 50 s under a microwave power of 300 W.
In the second set of experiments, silver nitrate (1.2 mg) was dissolved in ethanol (25 ml) and separated to five identical samples with 5 ml each. Then, 13 mg of latex copolymer was added to each sample. The samples were heated under MW irradiation at 300 W with different MW irradiation times ranging from 30 to 120 s.
During the heating process, the colorless solution of the reaction mixture turned gradually faint yellow to the golden yellow color, characteristic of the silver nanoparticle formation.
All the samples were cooled at 20–25 °C temperature just after the reaction with the aim to stabilize the silver-latex colloid structure.
Characterization Techniques
FEI TECNAI G2 version of TEM operating at an accelerating voltage of 120 kV was used to characterize the shape, size and particle size distribution of the prepared nanoparticles at different MW irradiation times.
TEM micrographs, SAED patterns, and high resolution transmission electron micrographs (HRTEM) were taken for morphological analysis of silver nanoparticles synthesized at different latex concentrations using a JEM 2100 HT operated at an accelerating voltage of 200 kV.
For TEM analysis, the samples with different MW irradiation times were prepared by depositing a few drops of the silver colloidal suspensions on a copper grid covered with a holey carbon film using a fine pipette, while the samples synthesized at different latex concentrations were prepared by casting a drop of each solution onto a carbon coated copper grid. The all obtained samples were left to dry for 10 min at room temperature before being transferred to the TEM chamber.
The chemical composition of the prepared samples was examined through energy dispersive X-ray spectroscopy (EDXS) using EDAX instrument attached to the JEM 2100 TEM.
The crystal structure of the nanoparticles has been obtained from HRTEM experiments and from the corresponding fast Fourier transform (FFT) plots. The particle size distribution analysis was done on the TEM images, using Image-Pro Plus 6.0.
Environmental scanning electron microscope (ESEM) (FEI Quanta 200 Brand) operated at high vacuum mode (with resolution of 20 kV) was used to characterize the silver–latex microstructures synthesized at different MW irradiation times. For SEM analysis, some drops of the colloidal solution were deposited on a sample holder disc-shaped which was coated with an adhesive carbon film.
UV–Vis spectroscopy was used to characterize the optical properties of the prepared colloidal silver dispersions and to follow the reaction process. Absorption spectra were recorded in the range of 300–800 nm using an UV-3100 selecta spectrophotometer with quartz cuvette. The solutions prepared at different MW irradiation times were diluted (1:3 ml colloidal solution: ethanol) before spectral measurements. The blank solution was prepared by dissolving 13 mg of latex copolymer in 5 ml of ethanol.
For samples prepared at different latex concentrations, we took 2 ml of each diluted sample before adding silver nitrate (AgNO3) as a blank solution. These solutions were used as the reference samples to take the blank spectra for all measurements.
Results and Discussion
UV–Vis Absorption Spectra
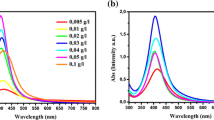
The formation of silver nanoparticles can be monitored both by visual color change and quantitatively by UV–Vis spectra measurement. The plasmonic band position, shape, and full width at half maximum (FWHM) depend strongly on the size and shape of the nanoparticles. The position of the plasmon band correlates with the average particle size of the metal nanoparticles while the FWHM traduces the particle size distribution. It was found that the colorless solution turned yellow even after 30 s of microwave irradiation. This clearly suggests that the silver nanoparticles were successfully synthesized with AgNO3-latex copolymer mixture under microwave heating. Typically, small spherical silver nanoparticles give a surface plasmon resonance (SPR) band at 350–500 nm with a peak position at about 410 nm [51–53]. Figure 1 shows the UV–Vis absorption spectra of silver nanoparticles produced under different latex concentrations and microwave heating times. A symmetric LSPR absorption bands were observed. The absorption spectra show strong dependence on the latex content (Fig. 1a). With increasing the latex concentration from 0.26 to 0.65 g/l, the absorbance of the LSPR band increases significantly due to an increase in the yield of silver nanoparticles. The LSPR peak is weak and broad at low latex concentration of 0.26 g/l, due probably to nanoparticle coalescence during the MW irradiation and the broadening of the peak decreases significantly with increase in the latex copolymer concentration. The highest LSPR intensity was obtained by using the latex concentration of 0.65 g/l, where significant amounts of silver nanoparticles are formed. The SPR bands become sharper with increasing of the latex concentration. This is probably correlated with small sizes and narrow distribution of the nanoparticles. The fast transfer of energy through microwave irradiation obviously induces more localized growth of the nanoparticles in the presence of latex molecules than in the conventional heating process.
Figure 1(b) shows the UV–Vis absorption spectra of silver nanoparticles prepared from 2.6 g/l concentration of latex copolymer with silver nitrate (AgNO3) concentration of 0.048 g/l in a microwave oven at 100 % power of 300 W and frequency of 2.45 GHz for different microwave time durations (30, 40, 70, 90 and 120 s). In the microwave oven, the reaction took place in closed CEM-HP 500 vessels. Therefore, the pressure increases drastically inside the vessel upon microwave exposure, which obviously contributes to accelerate the reaction kinetics. The silver particle formation started only after 30 s of microwave irradiation as observed from the color change of the reaction solution as well as from the surface plasmon resonance (SPR) band. This later increased in intensity with further irradiation until 40 s where the absorption reach a maximum indicating that the reduction reaction of silver ions was greatly proceeded. This increase in the absorption intensity with the reaction time could be associated to an increase in the size and concentration of silver nanoparticles [54]. Furthermore, the SPR band decreases in intensity with longer microwave exposure times (>40 s). This may be explained by the ethanol solvent being heated primarily by the dipolar polarization mechanism. Hence, its ability to absorb MW irradiation at the microwave frequency of 2.45 GHz decreases with increasing temperature as the reduced bulk viscosity leads to reduced molecular friction [55].
Figure 2a reports the maximum absorbance changes with microwave irradiation time. The net tendency shows a decrease in the absorbance with increasing of the reaction time. The maximum absorbance of silver nanoparticles can be accounted by the amount of silver ions reduced and incorporated into the particles [64].
On the other hand, Fig. 2b shows the absorption maximum of silver nanoparticles versus the latex concertation. The absorption goes up sharply as the latex concentration increases up to 0.65 g/l, indicating that the latex content in the network promotes higher absorption probably due to higher density of synthesized particles.
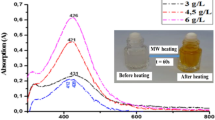
It has been reported that the intensity of the absorption is correlated to the amount and size of silver nanoparticles present in the solution [56–58]. The longer reaction time promotes the particle aggregation, leading to a red shift of the absorption peak [59]. In the Fig. 3b, the SPR peak position is slightly red-shifted from 410 to 420 nm with increasing of the reaction time from 30 to 70 s. This can be attributed to an increase in the particle size. However, such slight red-shift about ∆λ = 10 nm can occurs also from the adsorption of the silver ions at the nanoparticle-solution interfaces [60, 61]. The corresponding values of λmax of the surface plasmon absorption bands of the silver nanoparticles irradiated during 70 and 90 s were 420 and 415 nm, respectively. As a result, the sizes of the latex-capped Ag nanoparticles decreased slightly, may be due to digestive ripening of the silver nanoparticles occurring to form smaller particles upon increasing the MW irradiation treatment time. No change on the SPR position was noticed for higher heating duration up to 120 s indicating that the reduction of silver ions didn’t affect significantly the particle sizes at least in this duration time range.
The position of the SPAB peak of silver nanoparticles as a function of latex copolymer concentration is shown in Fig. 3a. The resonance peak was slightly blue shifted to lower wavelength from 420 to 410 nm with increasing of the latex concentration from 0.26 to 0.39 g/l indicating the reduction in the size of the silver nanoparticles. This blue shift in surface plasmon resonance band may be due to an increase in the density of free electrons in the colloidal silver particles [62, 63].
However, when the latex copolymer concentration was increased from 0.39 to 0.52 g/l, the SPR peak remains at 410 nm and returns again to its initial position (420 nm) at a latex copolymer concentration of 0.65 g/l. In the region between 335 and 350 nm, the SPAB is broader with an important absorption effect for lower latex concentration of 0.26 g/l, indicating that the nanoparticle formation requires a minimum latex concentration.
The curve giving the FWHM as a function of the latex concentration exhibits two different regions (Fig. 4a). The FWHM falls dramatically with increasing of the latex concentration up to 0.39 g/l, indication of the decrease in the particle size. Then, the FWHM begins to increase as the latex concentration continues to rise up to 0.65 g/l.
As observed in Fig. 4b, the FWHM increases with increasing of the reaction time till 90 s from which the FWHM decreases as the reaction time continues to increase up to 120 s.
SEM and TEM Analysis
Effects of MW Irradiation Treatment Time on the Latex-Capped Silver Nanoparticles
SEM Analysis
The scanning electron microscopy (SEM) micrographs of the silver–latex microstructures synthesized at different MW irradiation times are shown in Fig. 5a, b, c. These images clearly show the spherical shaped particles that are in the dispersed state and the phase water–ethanol occupies interstitial spaces between the latex particles.
These images were taken at a micron scale (20–100 µm), so we observe only the latex particles, but the Ag nanosized particles does not appear because the resolution of the microscope is low and the concentration used of the silver nitrate (AgNO3) precursor is quite small (0.048 g/l).
The average size of the latex microparticles increases rapidly with increasing heating time, leading to inhomogeneous particle size distribution. At 40 s of exposure to MW irradiation (Fig. 5a), individual latex microparticles homogeneously distributed with size about 2.5 µm were observed which confirms that the repulsive electrostatic forces are dominant. However, with increasing the reaction time from 40 to 90 s (Fig. 5a, b), the latex microparticles became larger in size (6 µm). Moreover, when the polymer chains inter-diffuse between neighboring latex particles, the adhesion between latex–latex contacts is greatly enhanced [66]. Consequently, the voids shrink between neighboring latex particles which coalesce together to form chains of adherent latex particles. After 120 s of MW irradiation (Fig. 5c), this adherent microparticles that combine in chains finally fuse together to form much larger microparticles (50 µm) with a little deformation in shape (the particles turns from spherical to oval shape).
Energy Dispersive X-ray (EDX) Analysis
The EDAX results (Fig. 6) confirm the formation of silver nanoparticles. The vertical axis displays the number of X-ray counts whilst the horizontal one presents the energy (KeV).
The spectrum showed that peaks of the metallic Ag are located at 2.984 and 2.806 keV. A peak at 3.151 keV is attributed to a multi-electron process in the Ag atom, such as electron repulsion. Another peak of silver atoms at a lower energy position of 2.634 keV was also detected. The signal of Cu is observed which is originated from the Cu TEM grid while the signal of the C atoms results from the latex copolymer and also from the supporting film on the copper grid used for TEM observations.
Role of the Latex Copolymer on the Morphology and Size Distribution of the Silver Nanoparticles
In order to examine the latex concentration role on the nanoparticle features, microwave was irradiated on AgNO3/latex copolymer/ethanol solution for 50 s at four different latex concentrations. TEM measurements were used to study the morphology and size of the Ag nanoparticles in the nanocomposites.
The obtained TEM images using the latex concentrations about 0.52 and 0.65 g/l, and their corresponding size distributions are presented in Fig. 7a, b.
A prominent feature of the preparation of silver nanoparticles by the MW-polyol method is that isotropic spherical Ag nanoparticles with a very narrow size distribution can be synthesized within a few seconds, demonstrating the fast reduction reaction.
During the microwave synthesis, the reduction of Ag(I) to Ag(0) leads to the formation of small spherical nuclei, which subsequently grow to form Ag atoms and then aggregate to form small crystalline Ag nanoparticles. Indeed, as can be seen from the Fig. 7a, with a latex copolymer concentration of 0.52 g/l, uniform and well-isolated spherical silver nanoparticles (shown as black spots) with a small mean diameter of 4.36 nm with a standard deviation of 0.02 nm, are homogeneously dispersed in the medium. The size distribution of the as prepared silver nanoparticles is very narrow proving that the latex copolymer controls efficiently the environment for the growth of the metallic silver nanoparticles. The copolymer not only acts as a stabilizer for the formation of silver nanoparticles but also serves as a ligand to form complexes with the silver metal ions. This co-ordination effect can substantially control the particle size.
The latex copolymer encapsulation of silver nanoparticles can provide the capping effect leading to a stable protection layer on the surface of the silver nanoparticles to prevent them from aggregation. The smaller size of the Ag nanoparticles is due to the effective stabilization of the nanoparticles by latex in solution.
The sizes of products depend strongly on the latex concentration. When the concentration of latex copolymer increased to 0.65 g/l, the silver nanoparticles had a broad size distribution with an average diameter of 9.81 nm with a standard deviation of 0.11 nm, as shown in Fig. 7b. Most of the obtained particles were spherical, but there still existed some nanosheets. For comparison, the size and size distribution of the silver nanoparticles prepared with a lower concentration of latex copolymer (0.52 g/l) were remarkably small and narrower than to those of sample with a concentration of 0.65 g/l. For Fig. 7b, both small and large particles were observed. A possible reason was that during the microwave process, smaller particles transformed with subsequent crystallization into larger particles, which involved the nucleation and growth processes of larger particles from smaller ones [65]. The latex molecules tend to aggregate in the solution when its concentration increases more than 0.52 g/l. Thus; it could not cover silver surfaces completely, resulting in the formation of larger nanoparticles. Besides of spherical silver nanoparticles, the silver nanosheets are also present in this TEM image, their size varies from 101 to 680 nm. The silver nanosheets are composed of some triangular silver nanostructures. They are extremely thin and transparent. So, we can clearly observe the spherical silver nanoparticles within them.
The high-resolution TEM image of the silver nanoparticles prepared at 0.65 g/l (Fig. 7b) reveals the crystalline character and ordered orientations of the lattice fringes.
In order to obtain information on the structure and the crystalline nature of the nanoparticles, the SAED patterns were measured from the sample containing 0.65 g/l of latex copolymer, as shown in Fig. 7b. It exhibits monocrystalline nature and have been indexed on the basis of face centered cubic (fcc) structure of silver. The diffraction spots are indexed to be (111) and (200) reflections.
The above results suggest that the silver nanoparticles produced through the MW approach exhibit a very high purity and a good crystallinity.
Effect of Microwave Heating Time on the Size Distribution of the Silver Nanoparticles
TEM was employed to obtain a more accurate view of the particle size distribution and shape of the silver nanoparticles in different samples.
Figure 8 shows the typical TEM images (a, b, c) and the corresponding size distribution histograms (d, e, f) of the silver nanoparticles obtained after MW irradiation for 40, 90 and 120 s respectively. These images clearly reveal that the Ag nanoparticles were not well dispersed and predominantly spherical in shape with variable diameters.
With the MW irradiation time of 40 s (Fig. 8a), the silver particles have a mean diameter of 5.73 nm with a standard deviation of 0.06 nm. As the MW heating time increases, large spherical particles are the major products. This result may be attributed to the fact that the silver nanoparticles have tendency to agglomerate in order to reduce their surface energy [67].
During the growth stage, the free silver ions in solution are adsorbed over the growing nuclei previously formed and reduced to silver atoms to produce larger particles with time.
The mean diameter of silver nanoparticles increased from 5.73 nm at 40 s to (8.35 ± 0.47) nm at 90 s of reaction time and no significant changes in the morphology or particle size were observed at 120 s of MW irradiation duration.
Our observations are consistent with those of Silvert et al. [68] who reported that the formation process of Ag particles stabilized in PVP using ethylene glycol as solvent and reducing agent. The mechanism of growth could be separated into three regions. At the initial stage, the majority of particles are small sized clusters. As the reaction proceeded, the small particles show coalescence and increased sizes. At the end of the reaction, the system consisted mainly of larger particles.
Conclusions
The synthesis of silver nanoparticles has been carried out by using the microwave energy as a heating source. We studied the effect of the latex concentration and microwave irradiation time on the morphology and size distribution as well as the yield of the formed silver nanoparticles.
The carried out work determines the optimum conditions to synthesize spherical silver nanoparticles with homogenous distribution in the latex copolymer which is a good environment to control the growth of the silver nanoparticles. The UV–Vis results showed an intense and narrow plasmon band at 40 s of reaction time. However, the increasing of the microwave heating time up to 120 s leads to a noticeable decrease of the SPAB intensity, possibly attributed to the aggregation of the formed silver nanoparticles, as confirmed by TEM imaging.
The proposed method is extremely time efficient, simple and cost effective, may be suitable for the synthesis of other metallic and semiconducting nanomaterials. It provides a new challenge to modulate the optical and thermal properties of latex.
The achievement of Ag nanoparticles synthesis by MW process open the way for an application of related plasmonic effects in photocatalytic reactions.
References
Y. Wang and N. Toshima (1997). J. Phys. Chem. 101, 5301.
G. Schmid (1992). Chem. Rev. 92, 1709.
M. G. Bawendi, M. L. Steigerwald, and L. E. Brus (1990). Annu. Rev. Phys. Chem. 41, 477.
W. A. Deheer (1993). Rev. Mod. Phys. 65, 611.
S. Joseph and B. Mathew (2014). J. Rec. Sc. Res. 3, 185.
A. N. Shipway, M. Lahav, and I. Willner (2000). Adv. Mater. 12, 993.
H. Q. Jiang, S. Manolache, A. C. Wong, and F. S. Denes (2004). J. Appl. Polym. Sci. 93, 1411.
Y. Cui and C. M. Lieber (2001). Science 291, 851.
Y. Sun and Y. Xia (2002). Science 298, 2176.
H. Wang, Y. Wu, B. Lassiter, C. L. Nehl, J. H. Hafner, P. Nordlander, and N. J. Halas (2006). Proc. Natl. Acad. Sci. USA. 103, 10856.
K. L. Kelly, E. Coronado, L. L. Zhao, and G. C. Schatz (2003). J. Phys. Chem. B 107, 668.
M. T. Reetz and W. Helbig (1994). J. Am. Chem. Soc. 116, 7401.
H. Willwohl, J. Wolfrum, V. Zumbach, P. Albers, and K. Seibold (1994). J. Phys. Chem. 98, 2242.
Z. Li, Y. Li, X. F. Qian, J. Yin, and Z. K. Zhu (2005). Appl. Surf. Sci. 250, 109.
K. S. Suslick, S. B. Choe, and A. A. Cichowlas (1991). Nature 353, 414.
A. Pal, S. Shah, and S. Devi (2007). Colloids Surf. A 51, 302.
C. O. Kappe and D. Dallinger (2009). Mol. Divers. 13, 71.
S. Caddick and R. Fitzmaurice (2009). Tetrahedron. 65, 3325.
J. M. Collins and N. E. Leadbeater (2007). Org. Biomol. Chem. 5, 1141.
R. Hoogenboom and U. S. Schubert (2007). Macromol. Rapid Commun. 28, 368.
C. Ebner, T. Bodner, F. Stelzer, and F. Wiesbrock (2011). Macromol. Rapid Commun. 32, 254.
S. Barlow and S. R. Marder (2003). Adv. Funct. Mater. 13, 517.
J. R. Lill, E. S. Ingle, P. S. Liu, V. Pham, and W. N. Sandoval (2007). Mass Spectrom. Rev. 26, 657.
S. L. Sçderholm, M. Damm, and C. O. Kappe (2010). Mol. Divers. 14, 869.
J. D. Moseley and C. O. Kappe (2011). Green Chem. 13, 794.
A. Loupy (ed.) Microwaves in Organic Synthesis, 2nd ed (Wiley, Weinheim, 2006).
M. Larhed and K. Olofsson (eds.) Microwave Methods in Organic Synthesis (Springer, Berlin, 2006).
C. O. Kappe and A. Stadler Microwaves in Organic and Medicinal Chemistry (Wiley, Weinheim, 2005).
D. Bogdal and A. Prociak Microwave- Enhanced Polymer Chemistry and Technology (Blackwell Publishing, Oxford, 2007).
J. R. Lill Microwave Assisted Proteomics (RSC Publishing, Cambridge, 2009).
N. E. Leadbeater (ed.) Microwave Heating as a Tool for Sustainable Chemistry (CRC, Boca Raton, 2011).
C. O. Kappe, D. Dallinger, and S. S. Murphree Practical Microwave Synthesis for Organic Chemists-Strategies, Instruments, and Protocols (Wiley, Weinheim, 2009).
A. G. Saskia (1997). Chem. Soc. Rev. 26, 233.
R. Abargues, R. Gradess, J. Canet-Ferrer, K. Abderrafi, J. L. Valdés, and J. Martinez-Pastor (2009). New J. Chem. 33, 913.
S. Komarneni, D. Li, B. Newalkar, H. Katsuki, and A. S. Bhalla (2002). Langmuir 18, 5959.
N. V. Nghia, N. N. Truong, N. M. Thong, and N. P. Hung (2012). IJMC 2, 75.
K. Patel, S. Kapoor, D. P. Dave, and T. Mukherjee (2005). J. Chem. Sci. 117, 53.
M. Tsuji, Y. Nishizawa, K. Matsumoto, N. Miyamae, T. Takeshi, and X. Zhang (2007). Colloids Surf. A 293, 185.
H. Jiang, K. Moon, Z. Zhang, S. Pothukuchi, and C. P. Wong (2006). J. Nanopart. Res. 8, 117.
M. Karimipour, E. Shabani, and M. Molaei (2015). Mater. Sci. (MEDŽIAGOTYRA) 21, 182.
G. Dzido, P. Markowski, A. Małachowska-Jutsz, K. Prusik, and A. B. Jarzebski (2015). J. Nanopart. Res 17, 1.
A. Pal, S. Shah, and S. Devi (2009). Mater. Chem. Phys. 114, 530.
M. Baghbanzadeh, L. Carbone, P. D. Cozzoli, and C. O. Kappe (2011). Angew. Chem. Int. Ed. 50, 11312.
S. Joseph and B. Mathew (2014). Res. J. Recent. Sci. 3, 185.
J. Helmlinger, M. Heise, M. Heggen, M. Ruck, and M. Epple (2015). RSC Adv. 5, 92144.
J. Pal and M. K. Deb (2012). Ind. J. Chem. 51, 821.
B. Aswathy, G. S. Avadhani, I. S. Sumithra, S. Suji, and G. Sony (2011). J. Mol. Liq. 159, 165.
K. J. Sreeram, M. Nidhin, and B. U. Nair (2008). Bull. Mater. Sci. 31, 937.
Z. Shao-Peng, T. Shao-Chun, and M. Xiang-Kang (2009). Chin. Phys. Lett. 26, 078101.
M. Noroozi, A. Zakaria, M. M. Moksin, Z. A. Wahab, and A. Abedin (2012). Int. J. Mol. Sci. 13, 8086.
T. Tsuji, K. Iryo, N. Watanabe, and M. Tsuji (2002). Appl. Surf. Sci. 85, 202.
C. Murphy and N. R. Jana (2002). Adv. Mater. 14, 80.
O. Wilson, G. J. Wilson, and P. Mulvaney (2002). Adv. Mater. 14, 1000.
A. Slistan-Grijalva, R. Herrera-Urbina, J. F. Rivas-Silva, M. Avalos-Borja, F. F. Castillon-Barraza, and A. Posada-Amarillas (2005). Physica E 25, 438.
M. Baghbanzadeh, L. Carbone, P. D. Cozzoli, and C. O. Kappe (2011). Angew. Chem. Int. 50, 11312.
P. Y. Silvert, R. H. Urbina, and K. T. Elhsissen (1997). J. Mater. Chem. 7, 293.
L. M. Qi, Y. Y. Gao, and J. M. Ma (1999). Colloids Surf. A 157, 285.
M. P. Zheng, M. Y. Gu, Y. P. Jin, and G. L. Jin (2001). Mater. Res. Bull. 36, 853.
I. Lisiecki and M. P. Pileni (1993). J. Am. Chem. Soc. 115, 3887.
P. Mulvaney (1996). Langmuir 12, 788.
A. Henglein (1998). Chem. Mater. 10, 444.
S. Kapoor (1998). Langmuir 14, 1021.
P. Mulvaney, T. Linnert, and A. Henglein (1991). J. Phys. Chem. 95, 7843.
A. Slistan-Grijalva, R. Herrera-Urbina, J. F. Rivas-Silva, M. Avalos-Borja, F. F. Castillon-Barraza, and A. Posada-Amarillas (2005). Physica E 25, 438.
L. Castro, M. L. Blázquez, J. A. Muñoz, F. González, C. García-Balboa, and A. Ballester (2011). Process Biochem. 46, 1076.
H. Luo, L. E. Scriven, and L. F. Francis (2007). J. Col. Int. Sc. 316, 500.
A. Henglein (1998). Chem. Mater. 10, 444.
P. Y. Silvert, R. H. Urbina, and K. T. Elhsissen (1997). J. Mater. Chem. 7, 293.
Acknowledgments
We wish to thank the national centre of scientific and technical research (Rabat-Morocco) for financial supports through the project “URAC10” and we gratefully appreciate Universitat de València for the use of TEM. M. Ider benefited from scholarship from France government “Eiffel grant” and financial support from the doctoral school ED3MPL of University of Maine-Le Mans, France.
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Ider, M., Abderrafi, K., Eddahbi, A. et al. Rapid Synthesis of Silver Nanoparticles by Microwave-Polyol Method with the Assistance of Latex Copolymer. J Clust Sci 28, 1025–1040 (2017). https://doi.org/10.1007/s10876-016-1096-6
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10876-016-1096-6