Abstract
The inclusion of behavioral components in the analysis of a community can be of paramount importance in marine ecology. Diel (i.e., 24-h based), seasonal activity rhythms, or longer durational in behavioral responses can result in shifts in populations, and therefore on measurable abundances. Here, we review the value of developing cabled video observatory technology for the remote, long-term, and high-frequency monitoring of fish and their environments in coastal temperate areas. We provide details on the methodological requirements and constraints for the appropriate measurement of fish behavior over various seasonal scales (24 h, seasonal, annual) with camera systems mounted at fixed observatory locations. We highlight the importance of using marine sensors to simultaneously collect relevant environmental data in parallel to image data acquisition. Here we present multiparametric video, oceanographic, and meteorological data collected from the Mediterranean observatory platform, OBSEA (www.obsea.es; 20 m water depth). These data are reviewed in relation to ongoing and future developments of cabled observatory science. Two key approaches for the future improvement of cabled observatory technology are: (1) the application of Artificial Intelligence to aid in the analysis of increasingly large, complex, and highly interrelated biological and environmental data sets, and (2) the development of geographical observational networks to enable the reliable spatial analysis of observed populations over extended distances.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Persistent climatic or human-induced environmental changes can produce long-lasting modifications in species behavior, with pervasive effects on population distributions and abundances (Peer and Miller 2014). Such environmental changes may be observed in time series data recorded at a changing location as fauna respond to modifications in key habitat parameters (Holyoak et al. 2008). Changes in the distribution of organisms (leading to observed shifts in community structure) are among the most readily detectable and emerging biotic effects of global warming (Parmesan and Yohe 2003; Parmesan 2006; Ehrlén and Morris 2015).
The linkage between observed population distributions and the behavioral response of individuals is particularly apparent in fish, due to their high mobility, which can allow entire populations to respond rapidly to environmental changes (Cheung et al. 2013). Fish species have been observed to shift their geographic and depth ranges of distribution in response to local changes in salinity, temperature, or marine productivity over short timescales (e.g., Attrill and Power 2002; Perry et al. 2005; Dulvy et al. 2008; Azzurro et al. 2011; Jørgensen et al. 2012). Population behavior can also be influenced by environmental change. Growth regulating swimming behavior within the water column of juvenile gadoid fishes has been observed to change in response to temperature change (Sogard and Olla 1996). The high behavioral plasticity of many fish species has been further highlighted by their response to fishing pressure, with certain behavioral traits being enhanced as fishing pressure changes (Sih et al. 2004; Cooke et al. 2007; Conrad et al. 2011).
Change in observed fish assemblages and population distributions may not only be the result of the behavioral response of individuals to long-term environmental changes; such changes may also be the product of day-night and/or seasonal rhythms in behavior. These changes may be linked to day-night swimming activity rhythms, or seasonal migrations related to growth or reproduction (Reebs 2002; Aguzzi et al. 2011a). Behavioral rhythms are a pervasive phenomenon in the animal kingdom. The majority of marine fauna display a variable use of habitat niches through the modification of behavior in response to tidal, day–night, and other seasonal frequency environmental changes (Kronfeld-Schor and Dayan 2003; Hut et al. 2012). When considered across all species within a community, these behavioral changes determine the overall temporal functioning of ecosystems (Tan et al. 2013).
Rhythmic behavior is the product of animal adaptation to predictable environmental changes. The astronomical motion of the Earth in relation to the Sun and the Moon produces geophysical cycles within the biosphere and therefore determine variations in key habitat parameters such as the tidal movement, light intensity, and photoperiod duration. Early in Earth’s history, these cycles favored the development of time-keeping mechanisms commonly known as “biological clocks” in animal and plant species (Loudon 2012). These are responsible, at least in part, for the temporal regulation of behavior and underlying physiology (Refinetti 2006). Geophysical cycles can act as selective agents, with the fitness of species highly dependent on the capacity of animals to tune their behavioral activity to a particular temporal window. Therefore, the adaptive value of biological clocks rests on the capacity of organisms to anticipate the onset of unfavorable environmental conditions while continuously maintaining such anticipation over their lifespan (i.e., entrainment process; Naylor 2010).
Many marine organisms studied to date (including many fish), display patterns of activity and inactivity at a diel (i.e., 24-h based) and seasonal scale at all depths associated with continental margins (Aguzzi et al. 2010; Naylor 2010). In temperate coastal areas, where both day-night illumination cycles and hydrodynamic tidal-related forces are active, fish assemblage composition changes over the 24-h period in response to the activity rhythms of the various individual species within the community. These changes have been detected either via direct fishing methods (e.g., beach seines: Dulcic et al. 2004; Harmelin-Vivien and Francour 2008; Tutman et al. 2010) or from visual census surveys (Fischer et al. 2007; Azzurro et al. 2007, 2013; Irigoyen et al. 2012). In recent years complex changes in behavioral patterns have also been characterized by hydro-acoustic techniques (Willis et al. 2006; March et al. 2010; Alós et al. 2011; Koeck et al. 2013) and the deployment of various tracking devices (Sims et al. 2009; Walli et al. 2009; Block et al. 2011).
Light intensity represents an ancient evolutionary factor driving the temporal structuring of marine ecosystems via visual predation (Paterson et al. 2011). For most fishes, light is a key factor in the trade-off between maximizing feeding activity and minimizing predation risk (Werner and Anholt 1993; Antonucci et al. 2009). Light has profound effects on the functioning of biological clocks of many fish (Pauers et al. 2012; Esteban et al. 2013; López-Olmeda and Sánchez-Vázquez 2010; López-Olmeda et al. 2012). Unfortunately, light is not often considered in marine ecology and fishery studies, with the importance of either intensity or spectral distribution in influencing behavioral rhythms is still largely unknown (Aguzzi et al. 2009a; Aguzzi and Company 2010).
A major challenge for marine ecologists is to unravel the various temporal scales of behavioral responses to environmental change or variability determining the observed species abundances in any given area (Sardà and Aguzzi 2012). Short duration population changes are often the result of activity rhythms, whereas longer duration changes may well be the result of species’ responses to monotonic environmental modifications, such as temperature increase or nutrient delivery changes resulting from global warming (Navarro et al. 2013).
Objectives of the review
Limited access to the ocean for the observation of marine organisms, communities, and the interconnectivity of these with the surrounding physical environment presents difficulties for the advancement of marine ecology not present in the field of terrestrial ecology (Underwood 2005; Menge et al. 2009; Webb 2012). To fully investigate the behavior of marine species at different temporal and spatial scales sampling design strategies, which take into account the most relevant sampling frequencies to best monitor these behaviors, is required. Marine sampling programs often fall short of this aim, with temporal frequencies required to observe behavioral changes over short temporal scales being difficult to achieve with traditional methodologies (Langlois et al. 2010; Watson et al. 2010). Sampling campaigns aimed at observing animal movements should ideally employ sampling frequencies that match both the activity rhythms (i.e., short time scale) and long-term behavioral changes of animals (Naylor 2005). This type of methodological approach is required to best achieve an understanding of how behavior influences the abundance and composition of species assemblages and therefore the local biodiversity.
The main objective of this review is to provide insight regarding the potential value of cabled video observatories as a new and reliable technology for the remote, long-term, and high-frequency monitoring of fishes in coastal habitats. We provide details of methodological requirements required to appropriately measure fish behavior at various temporal scales from fixed camera stations and we highlight the importance of concurrent monitoring of key environmental parameters, to aid in determining the causes of observed population and behavioral change.
We focus this review on fish as they comprise the base of important aquatic ecosystem services of societal strategic relevance (Pikitch et al. 2014). According to the United Nations Food and Agriculture Organization, 1 billion people, a large percentage of which are from developing countries, rely on fish as their primary source of protein (FAO 2014). The demand for marine fisheries is projected to increase by 43 % by 2030 (Delgado et al. 2003). Moreover, many fish species are considered reliable biological indicators of environmental change, as they show rapid species–specific changes in behavior in response to environmental forcing (Azzurro et al. 2011). Additionally, in comparison to some other marine fauna of commercial significance, such as crabs and other crustacean decapods, fishes are relatively easy to identify from video imagery (Templado 2014). Here we focus on coastal fish communities, which are typically subjected to variable levels of anthropogenic disturbances. Coastal zones have historically been the primary location for the settlement of human civilizations due to easy access to food resources, transportation, and commercial routes (Becker et al. 2013).
Cabled observatories for the monitoring of fish assemblages at different temporal scales
Cabled seafloor video observatories represent a substantial innovation for marine ecological research by allowing the measurement of environmental data at high-frequency (i.e. seconds to hours) and over long, continuous duration (i.e. weeks to years; Ruhl et al. 2011). These observatory platforms are deployed on the seabed and connected to shore by power supply/data transfer optic fiber cables, which allow a continuous data flow and sufficient power to operate environmental sensors regularly (Favali et al. 2006, 2010). The growing socio-economic concerns over the best practices for the exploration and sustainable management of marine commercial ecosystems and for catastrophic event prevention (e.g., tsunami waves; Kasaya et al. 2009; Chierici et al. 2012) has over the last decade driven the implementation of highly interdisciplinary, fixed monitoring platforms that integrate biological, geological, and oceanographic sensors (Favali and Beranzoli 2006; Favali et al. 2006, 2010; Lampitt et al. 2010). As a result of these endeavors, there is a global increase in research using cabled observatories; however, the majority of such research is conducted within the deep sea (Ruhl et al. 2011). Many cabled infrastructures are being installed in relatively unexplored deep seafloor regions with comparatively few being deployed in shallow coastal zones (Favali et al. 2010; Aguzzi et al. 2012a). To the best of our knowledge, the majority of these platforms have yet to produce published data on systematic faunal monitoring for methodological evaluation and discussion.
Cabled observatory science was initially developed for purposes other than ecology (e.g., for oceanographic and geophysical studies; Favali et al. 2013; Monna et al. 2014), though an increasing number of deployed platforms are currently being updated to mount video cameras, in addition to the initial sensor systems. Cameras can provide data at different levels of ecological complexity (from the individual, through population, species, and community levels; Barans et al. 2005; Kross and Nelson 2011; Pelletier et al. 2012). Cameras can be used to measure the response of fish and other fauna to environmental changes, as characterized by platform sensors measuring in parallel. Imaging is therefore a central component of environmental monitoring, and the protocols for best use and data analysis of image data will likely be a driving theme within future developments of cabled observatory technology (Vardaro et al. 2013).
Observatory imaging procedures at different temporal scales
The chance of observing an individual of a species using a fixed camera fluctuates in response to changes in local population abundance and the activity rates of individuals. In time-lapse photography, fluctuations in visual counts of fish can be considered as proxies of the animals’ behavioral rhythms (Costa et al. 2009; Aguzzi et al. 2010; Chabanet et al. 2012). Time-lapse image capture should ideally be programmed at temporal frequencies that are high enough to capture all aspects of species behavior. When data is collected through consecutive years, it is possible to quantitatively study animal and population responses to ongoing local and global changes (Glover et al. 2010). Such extended study aids in disentangling short-term variations in abundance and behavior from the progressive adjustment of biotic communities to a shifting climate.
The spatial limitations of video image collection from fixed points (i.e. the observatory platforms) produce uncertainties in the evaluation of the diurnal, nocturnal or crepuscular characteristics in species behavior from animal count time series. A researcher cannot always determine if peaks in visual counts of fish at specific locations correspond to peaks in activity without having additional supporting data (e.g., laboratory tests defining the diurnal or nocturnal character of species behavior and specific field studies on overall habitat use). For example, an increase in visual counts for a certain species may be a proxy of a decrease in the rate of swimming rather than an increase in species activity. This often occurs in species that aggregate close to observatory structures (and therefore observatory cameras) during daytime. A sheltering (and hence resting) behavior naturally occurs at artificial reefs in coastal zones (Wehkamp and Fischer 2013), where visual predators are very active, with this predation pressure of evolutionary importance in constraining the timing of prey activity to nocturnal or crepuscular hours (Horodysky et al. 2010).
In contrast to time-lapse photography, continuous video recording may be used to study important interspecific interactions occurring on shorter timescales, such as territorialism, predation or scavenging (Aguzzi et al. 2012b). Unfortunately, continuous video recording has important methodological and technological limitations, hindering its use as a reliable behavioral monitoring strategy. A major methodological limitation is related to the requirement for artificial illumination whilst filming. As reported in previous deep-sea studies (Doya et al. 2013a), fishes may be attracted to such light sources, biasing abundance observation results (Longcore and Rich 2004). Therefore in coastal areas continuous video filming can be used only during daylight hours (when artificial illumination is not required) or at night with the use of a strict time-lapse illumination/video capture shooting routine (illumination only used during short duration video recording). Collection of short duration video clips durations of less than 1 min) taken at consecutive intervals of approximately 30 min may avoid light attraction as the majority of fauna do not appear to retain a memory of previous illumination events (Doya et al. 2013a; Matabos et al. 2013). Alternatively, infrared light is not detectable by most marine organisms, and can be used to illuminate filming, though rapid attenuation of this wavelength within saltwater imposes strict distance constraints on fish counts (Widder et al. 2005). Continuous filming also introduces the problem of recounting an individual repeatedly as it swims in and out of the field of view, or lingers within the field of view, being counted as anew individual per analyzed video frame (Mazzei et al. 2014). Tracking to prevent the recounting of the same moving individual is a major issue (Han et al. 2009). Time-lapse image acquisition, at minute and hourly frequencies mitigates this problem somewhat, though for species prone to resident behaviour (e.g., scorpaenids) observations my still produce erroneously high estimations of abundance. The technical constraints in time-lapse image monitoring, combined with the bias caused by attraction of some fish species to the artificial observatory structures, reduces the applicability of using cabled observatories to record unbiased abundance data, but does not wholly prevent their use in the study of activity rhythms (and community changes). Recounting of individuals may be proportional to the differential rate of swimming, and therefor correlate with activity and passivity behavioral phases (Aguzzi et al. 2010).
Spatial coverage limitations of seafloor observatory imaging systems
Fixed cameras installed on cabled observatories are only capable of imaging a relatively small area of the marine ecosystem, usually a few square meters of seafloor and overlying water column (Aguzzi et al. 2011b). This spatial coverage limitation of cabled observatory video-imaging systems should be carefully considered when extrapolating abundance data from such restricted locations to larger spatial scales (Assis et al. 2013).
Downward-oriented cameras provide a more uniform field of view for animal counting than oblique imaging (Smith et al. 1993). Such an orientation, though well suited for the counting of benthic walking and crawling animals is less successful in capturing and identifying swimming fishes (Matabos et al. 2013). With fish, counts should ideally be performed with cameras oriented at a 45º degree angle to the seabed, or by aiming the camera parallel to the seabed, in order to maximize the observed area laterally (Aguzzi et al. 2011c). Abundances observed within the water column with a fixed camera may be influenced by the ability of a particular observer to identify fish at different distances from the camera, i.e. in distant, poorly illuminated areas of the imaged area (Davis et al. 2014). The clarity of collected images may vary over time with change in volume and composition of suspended particles in the water column, and current flow velocity (Unsworth et al. 2014). Users of image data collected from shallow sites should take into account how the field of view changes over the day-night cycle, under natural (cloudiness-related) and artificial lighting conditions. The field of view recorded during night time image acquisition is restricted to the artificially illuminated areas. When comparing daytime counts with nighttime observations, areas compared should be delineated to ensure there is comparable coverage (Aguzzi et al. 2013).
A strong variability in overall fish abundance in coastal reef areas may also be the result of habitat heterogeneity (Harvey et al. 2012). At a local scale of meters, different fish counts with the same camera, depending on the field of view to which the camera is aimed (e.g., toward a rocky zone rather than to the adjacent water column; Condal et al. 2012; Purser et al. 2013a, b). Even in more homogenous, muddy slope areas, the presence of geomorphological heterogeneity produces variability in fish counts with cameras deployed on a separation of tens of meters (Doya et al. 2013a). Therefore, the careful selection of the appropriate field of view and consideration of potential over or under abundance estimations are required. The utilization of seafloor mounted calibration panels—homogenous backdrops—can reduce this variation (Del Río et al. 2013b). Though such panels reduce the imaged area (i.e. by reducing the depth axis), this drawback could be part compensated for by the increase in the frequency (every few minutes) and duration (over multiple days) of time-lapse image acquisition. Other solutions, such as the use of cameras equipped with pan/tilt solutions, require the programming of operational routines for rotatory image acquisition and the standardization of counts across local habitat heterogeneity (Pelletier et al. 2012; Mallet et al. 2014).
The use of crawlers as hybrids between mobile ROVs and benthic observatories
Calibrating visual censuses or ROV-video transects should ideally be conducted around observatory still camera systems to evaluate the effect of habitat heterogeneity on locally detected species assemblages. ROV surveys focus on spatial rather than temporal abundance variability, allowing large areas of the seabed to be investigated though the surveys are often short and sporadic (De Leo et al. 2010; Grange and Smith 2013). Despite the clear advantage in terms of spatial coverage provided by moving imaging techniques, such photographic or video censuses are not necessarily more reliable for estimation of fish population densities than observations from fixed camera systems. For example, if the sampling design of a moving imaging system (e.g., ROV or towed camera) does not account for either the day-night or seasonal variability in the fish abundance in the survey area, the resulting abundance estimations will most likely be biased.
Mobile Internet Operated Vehicles (IOVs) such as the Deep Sea Crawler Wally (http://www.jacobs-university.de/ses/research/oceanlab/crawler; Purser et al. 2013b), are now available for conducting seabed surveys from cabled infrastructure nodes (Fig. 1). These mobile systems are potentially useful for the cross-calibration of still and ROV imaging data. Crawler platforms can mount HD still and video cameras, plus a diverse payload of multiparametric sensors to locations of interest within the reach of an umbilical power and data cable (Thomsen et al. 2012; Tessier et al. 2013). The cabled design facilitates both a high power supply to the vehicle and a high data flow from it, allowing HD video capture and high sensor temporal resolution. Crawlers may mount other sensors capable of the autonomous collection of oceanographic, biogeochemical, and ecological data to allow comparison with those mounted on nearby cabled or fixed location observatories (e.g., Sherman and Smith 2009; Smith et al. 2013).
The internet-operated seafloor crawler Wally is presently conducting video transects within the NEPTUNE node of Barkley Canyon (1000 m depth; Vancouver Island). a the study areas with details on the node positioning and transect observing stations; b an image of the crawler (pictures courtesy of NEPTUNE Canada); c video-observed fish species (from above: 13-the Sablefish, Anoplopoma fimbria, 15-the Thornyhead, Sebastolobus sp. and the Dover sole, Microstomus pacificus; classified according to the NEPTUNE Marine Life Field Guide; http://www.neptunecanada.ca/dotAsset/34625.pdf) within the framework of Wally driving web application
In coastal areas, crawlers should be used to calibrate the imaging faunistic outputs (i.e. species lists and animals counts) of cabled observatory fixed cameras, by delivering high frequency day-night and seasonal data from visual census surveys conducted along nearby transects. These transects can be established by encompassing the different key environments for the targeted fish species (e.g., seagrass meadows, sandy banks or rocky areas). Such a sampling strategy can address two objectives: (1) to establish the level of representation of faunistic observations at fixed imaging observatory locations and (2) to establish where individuals go when imaging counts drop at the fixed observatory camera. Over the last 2 years, the ‘Wally I’ crawler has been deployed on the NEPTUNE Ocean Networks Canada (ONC; Table 1) cabled infrastructure within the Barkley Canyon (Pacific Canada at ~900 m water depth) to spatially and temporally quantify fish species abundances, as well as the environmental drivers regulating species behavior. Results indicate the occurrence of seasonal variations in fish counts of many species (Doya et al. 2013b), the relationship between these changes and with growth and reproductive behaviors is still under analysis.
Cabled observatories integrated into geographical networks and data integration
Sampling results from traditional methods (e.g., trawling, beach seines or visual census) are biased by the activity rhythms of fish species, when the frequency of measurement repetition (i.e., the time factor) is not carefully scheduled to run at a statistical frequency encompassing the different temporal scales of rhythmic behaviors. Diel and seasonal repetition in sampling at one location is required in order to detect population displacements into and out of targeted areas. A conceptual scheme is presented in Fig. 2. Different species rhythmically enter and then leave the sampling windows, producing high variability in the observed species assemblage (Aguzzi and Bahamon 2009; Bahamon et al. 2009). When sampling is temporally scattered, the interpretation of species abundance and overall biodiversity is limited and below satisfactory levels, and may well bias the description and interpretation of ecosystem functioning. These errors will continue to be made if we fail to implement a suitable technology for repeat sampling at a much higher frequencies and over extended periods. Cabled observatories provide the desired high temporal frequency sampling, although they do not, individually, allow the addressing of spatial analysis questions. A group of observational nodes (observatories) operating together and comprising a large geographical network could potentially perform coordinated monitoring of fish abundances. When such a network of cabled observatories is dispersed across different marine areas or regions of the world new possibilities to track large-scale changes in the marine environment will be possible. Observatory networks such as EMSO (European Multidisciplinary Seafloor and water-column Observatory, www.emso-eu-org) and ONC (Ocean Networks Canada; www.oceannetworks.ca) will increase the number of connected camera nodes, providing progressively higher geographic and bathymetric coverages.
Conceptual scheme comparing temporal and spatial sampling biases in assessment of marine populations and overall biodiversity, as respectively produced by traditional sampling/censing methods (the repetition of which is too scattered over the time) and cabled observatory imaging surveys (i.e. too locally limited). Activity rhythms of all species within a community produce an overall temporal dynamism in marine ecosystems. The temporally variable presence of species in our sampling windows biases outcomes from our low-frequency sampling. That bias has still unknown repercussions on the quality of data used for the integrated ecosystem approaches to fishery and biodiversity management. Networks integrating local independent observations nodes within larger geographic areas may increase the level of accuracy of video-observations, when these can be compared altogether. This would facilitate the estimation of abundances for species in a fashion similar to other mobile sampling methods applied over much larger seabed surfaces. The strategic monitoring advantage of such a network could be its temporal permanence and monitoring autonomy
In the case of highly mobile organisms such as coastal fishes, the geometry of the geographical network of observatories must be carefully planned to embrace the full range of diel and seasonal displacements of species of interest (Aguzzi et al. 2013). Cabled observatory networks for coordinated faunal and environmental monitoring will eventually require the development of common protocols for analysis, as is currently underway in operational oceanography. In this area of research, standardization in data acquisition for diverse pelagic and benthic multiparametric fixed and mobile platforms is to a large extent achieved, with web portals available for access to archival data stores and for online processing (Proctor and Howarth 2008). EMODNET (http://www.emodnet.eu/) and EUROGOOS (http://eurogoos.eu/) web infrastructures are being created to further homogenize standards in data acquisition, and for centralization of data storage for common preprocessing and analysis routines. Multiparametric data are organized into data banks as input matrices that can be easily handled by the chosen statistical procedures. These developments are core to the transformation of current platforms into smart ecological surveillance instruments, delivering synthetic information on community changes based on species response to statistically identified key environmental drivers. The rules of acquisition and the choice of best routines for statistic treatment of data will likely represent in the near future the core of a newly developing ‘operational ecology’.
Automated video imaging and Citizen Science tuning
The current capacity for analysis of large image and environmental parameter image sets is limited by the lack of automation in processing (MacLeod et al. 2010). Artificial Intelligence and learning algorithms procedures for the statistical treatment of observatory data is needed to process data swiftly and reduce human time costs (Costa et al. 2011).
Automated species classification and animal counting from video imaging are dependent on system training of a limited number of locally occurring species (Aguzzi et al. 2012a, b). Universal application of a single automated video-imaging system is highly improbable (Aguzzi et al. 2009a, b, c). In addition to species determination, object recognition is largely dependent upon the contingent background (e.g., homogenous sandy or heterogeneous rocky seafloors, turbidity levels, and etc.) and overall illumination (e.g., day-night cycles in shallow waters; Beauxis-Aussalet et al. 2013). Images automatically acquired by cameras are very different from images acquired by humans, in that they are arbitrary taken in a particular orientation with no selection of specific subjects of interest.
Though universal automation is unlikely to be achieved for all observatory systems, classification procedures can be consistently implemented via comparable human-supervised computer learning approaches (Aguzzi et al. 2009b, 2011b; Phoenix et al. 2012; Schoening et al. 2012; Corgnati et al. 2014). Operators manually classify animals within sets of images by extracting descriptors of relevance on morphology (e.g., Fourier Descriptors) and colorimetry (e.g., Red–Green–Blue mean content) for the creation of reference models for classification comparisons. These sets of images can then be used as training libraries that can be used to automatically identify animals from different viewing angles in further image sets, with the animal category profiles and color content defined within certain degrees of variability. The greater the volume of reference images within a library, the more capability there is to build precise morphological models of reference that fit the true aspects of the different species at the observation site.
In spite of the potentially extensive video material that may be acquired by cabled observatories, supervised approaches in classification are most effective when a large number of operators are involved (i.e. labelers of features of interest in training images). Citizen Science represents an innovative approach for ecological and behavioral research based on centralized monitoring efforts. Citizen Science refers to the participation of the public in scientific programs and involves volunteers who collect and/or process data (Silvertown 2009; Dickinson et al. 2010). This emerging discipline is now taking advantage of new technologies, such as the Internet and mobile phones with recording capabilities, for easy data collection and sharing (Del Río et al. 2013a). In the context of marine imaging, public participatory approaches could be adopted to create and continually expand training libraries of images by involving a progressively larger number of operators into manual classification routines (Fig. 3). Specific groups of observers, such as those involved in recreational SCUBA diving, could be enrolled in remote species video-recognition endeavors as trained volunteers (e.g., Azzurro et al. 2013). These operators may propose animal images according to certain imposed criteria (e.g., best visibility). After experts evaluate their input, these images may be introduced into the library, and their morphological indicators extracted and used along with those already present for morphological classification. Routines for validation of classification choices by public users could also be automated to a certain extent using consensus criteria (i.e., when multiple annotators are used, the most frequent choice is taken as valid; see Zoonivers procedures; https://www.zooniverse.org/).
Conceptual scheme of Artificial Intelligence tuning for species classification in imaging products obtained by cabled observatories cameras thanks to Citizen Science approaches. Supervised approaches (i.e. a trained scientific operator classifies animals in subset of images) are required to create a reference library of images to be used as core for Artificial intelligence classification procedure. Citizens as Public Observers can screen stored or on-line real-time (trough web) image material proposing new classification entries, which will be incorporated in the library of images only after scientific validation. The reiterative repetition of that processing will tune automation in species classification. The example was drawn from images acquired and processed at the deep-sea observatory in Sagami bay (1150 m depth in Japan; Aguzzi et al. 2010)
Examples of highly integrated environmental monitoring at the OBSEA observatory
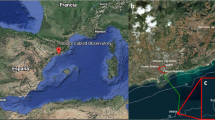
We here present a couple of examples of temporal monitoring of a coastal fish assemblage at the Observatory of the Sea (OBSEA), since this multiparametric, cabled video platform is a testing-site of the EMSO network and represents one of the few coastal observatories currently active (Table 1). OBSEA was launched in 2009 off the Catalan Coast in Spain (western Mediterranean: 41°10′54.87″N and 1°45′8.43″E) and deployed at a depth of 20 m (Aguzzi et al. 2011c) (Fig. 4). The strategic interest in using OBSEA for coastal fish monitoring is its location within a marine reserve (Colls Miralpeix Marine Reserve) in proximity of the commercial harbor entrance. The platform is located at the center of a system made by 3 concentric rings of artificial reefs, placed to prevent illegal trawling. Undoubtedly, some ecological results observed by any platform are the result of local characteristics of the surveyed area, in this case, the fact that the observatory is situated within a maritime reserve experiencing anthropic impact related to maritime activities. This factor may have skewed observed density estimates of inhabiting species, video-biodiversity estimates or potentially even prey-predator relationships.
A scheme depicting the OBSEA coastal cabled observatory infrastructure within the Northwester Mediterranean area. The potential expansion with the new crawler mobile technology, to be used for cross-calibration of faunistic data from fixed and mobile camera sources, is also presented. The surface buoy and different instruments are also reported as indication of potential spatial expansion with complex multiparametric seafloor oceanographic (instruments from 1 to 3, that will be cameras in the next future) and atmospheric sensors (the buoy)
The OBSEA is has for the last few years conducted a highly integrated monitoring of the local biosphere, encompassing meteorological, oceanographic and biological indicators. The platform is endowed with a 360° rotating Ocean Optic HD camera delivering high-value ecological data linking fish community behavior) with several environmental variables. Additionally atmospheric column measurements are performed through sensors mounted on surface buoys (see Fig. 4). Currently available data consists of: (1) visual counts (at hourly frequencies from 2009 to 2014) for all bony fishes and other, rarer species (cartilaginous fishes, cephalopods and sea birds) in images taken during daytime (nighttime images excluded since a drastic community change was observed to occur at night, with the disappearance of the majority of species in darkness; Aguzzi et al. 2013); (2) oceanographic variables in temperature, salinity, and water pressure; and, (3) meteorological variables such as temperature, wind speed, rain, and solar irradiance (as measured at a nearby land station, with buoy data acquired only from 2013). The examples of time series and multivariate data treatments refer only to bony fishes as these species are the most abundant taxonomic group in the OBSEA area.
Example 1
Faunal list and video-biodiversity estimates
A primary target of marine observational technology is the implementation of systems capable of delivering exhaustive species lists for ecosystems exploration (Bouchet 2006). With small spatial observational windows of observatory cameras, the monitoring of community changes over time should be conducted over extended periods to record changes in abundances of both common and rare species (Matabos et al. 2011). An example of a species list is presented in Fig. 5 from July 2009 to June 2010. An important methodological question concerns the minimum number of pictures required to obtain a reliable sampling of the species assemblage at a cabled observatory. This minimum number can be derived from classic diversity accumulation curves (see Fig. 5), where the plateau is indicative of a saturation in new detections despite the progressive increase in captured image numbers. As an example, we focused on the observations described above and used bony fishes (discarding other rare species), counted in images taken at 30 min frequency. Analysis indicates that only 875 h (approx. 36 days, equivalent to 1750 snapshots) are required to reach the maximum number of detectable species (i.e., the plateau on the accumulation curve). This indicates that after a cabled observatory is established in the OBSEA area, approximately 1 month would be needed to obtain a first recompilation of the fish species occurring in the area. A conclusive species list can be obtained only by repeating the video survey on a seasonal basis, given the monthly fluctuations in observable fish according to the environmental control of species life cycles (see example below).
Above, list of all species portrayed with different fields of view of the 360° rotating Ocean Optic HD camera installed on the OBSEA platform during 3 years (2009–2012) of time-lapse photographic acquisition. Below, Fish species accumulation curve at OBSEA, as calculated by permutation-tests (1000 permutations; mean ± standard deviation). The vertical grey line coincides with the threshold at which the observation reaches the maximum number of species observed (N = 21). FISHES in images are—Apogonidae: Apogon imberbis (a); Carangidae: Seriola dumerili (b); Trachurus sp. (c); Centracanthidae: Spicara maena (d); Congridae: Conger conger (e); Gobiidae: Gobius vittatus (f); Labridae: Coris julis (g); Symphodus mediterraneus (h); Symphodus melanocercus (i); Molidae: Mola mola (j); Mullidae: Mullus surmuletus (k); Pomacentridae: Chromis chromis (l); Sciaenidae: Sciaena umbra (m); Scorpaenidae: Scorpaena sp. (n); Serranidae: Epinephelus marginatus (o); Serranus cabrilla (p); Sparidae: Dentex dentex (q); Diplodus puntazzo (r); Diplodus sargus (s); Diplodus vulgaris (t); Diplodus annularis (u); Diplodus cervinus (v); Oblada melanura (w); Pagellus erythrinus (x); Sarpa salpa (y); Sparus aurata (z); Spondyliosoma cantharus (aa); Myliobatidae: Myliobatis aquila (ab). OTHER OCCASIONALLY RECORDED SPECIES: Loligo vulgaris (ac); Octopus vulgaris (ad); Sepia officinalis (ae); Phalacrocorax aristotelis (af)
Example 2
Seasonal variations in fish assemblages
To address the relationship between seasonal variability in fish assemblages and environmental drivers, the cabled observatory image acquisition was continuously performed from 1st July 2009 to 1st June 2010. We considered images taken at a 1-h frequency on alternate days within the two central weeks of each month. The following four different camera fields of view were selected: two that portrayed the left and right upper sides of the artificial reef and two that were aimed at 45° angles toward the water column. Here, we summed all counts for the four images (one per position) for each time point observation. Following this, the resulting time series were averaged for each month. In addition, oceanographic and atmospheric parameters were added to the analysis to identify those that may directly or indirectly influence the physiology and ecology of local fishes. The water temperature, salinity, wind speed and rain were measured at each image acquisition as potential proxies of water and air column conditioning on fish behavior according to species specific tolerances and sensitivities (Albouy et al. 2012; Jørgensen et al. 2012).
The presence of seasonal rhythms was evaluated by averaging the number of detected fish per month. In doing so, we considered all images for each for each imaging orientation at once. The number of available images varied month by month, with photo-sampling constancy disrupted by water turbidity over the extended monitoring period. The visual count data were then plotted with MESOR (i.e., as yearly threshold means) to identify the months when significant increases occurred (see Univariate methods section). The mean counts presented seasonal fluctuations for all the considered species (Fig. 6). Many species showed a single and compact seasonal increase that lasted several months (e.g., D. annularis and D. cervinus), whereas others showed a more punctual increase during 1 month (e.g., S. mediterraneus and O. melanura). Further species showed a scattered seasonal pattern with sparse peaks occuring throughout the year (e.g., S. cabrilla and S. cantharus). It is important to note that the seasonal changes in the behavior of coastal fish were evaluated by computing the averages of each month and not by simply summing all the counted fishes. Nevertheless, the temporal gaps in photo-acquisition did not impair the detection of a seasonal variation in fish counts, which demonstrates the efficiency of the applied monitoring method (Fig. 7a).
Seasonal visual counts fluctuations for different fish species as recorded during 2010 at the OBSEA. Species are (taxonomically listed as in Fig. 2): Seriola dumerili (a); Spicara maena (b); Coris julis (c); Symphodus mediterraneus (d); Symphodus melanocercus (e); Chromis chromis (f); Scorpaena sp. (g); Epinephelus marginatus (h); Serranus cabrilla (i); Dentex dentex (l); Diplodus puntazzo (m); Diplodus sargus (n); Diplodus vulgaris (o); Diplodus annularis (p); Diplodus cervinus (q); Oblada melanura (r); Spondyliosoma cantharus (s). Dashed horizontal lines are the MESOR
Significant seasonal increases in visual counts for 17 fish species (taxonomically listed as in Fig. 5; see data sets Fig. 6) in relation to monthly variable quantity of images. a the comparison between the total number of detected fishes according to the available number of images, which varied at each month (i.e. due to contingent turbidity and transient loss of visibility); b phase comparison integrating significant mean monthly increases in visual counts for each species (horizontal black bars as values above the MESOR) and significant increases in other oceanographic and atmospheric parameters (grey-scale rectangles), also sampled contemporarily to image acquisition). The environmental variables within square brackets are significantly (p < 0.05) related with species abundances in the Canonical Correlation Analysis
The relationships between seasonal count rhythms, oceanographic and atmospheric data were then studied using integrated comparisons of phases (i.e., as significant increases above the MESOR) (Fig. 7b). The comparison showed which species significantly increased in correlation with particular environmental parameters, thus indicating potential cause-effect relationships. Significant monthly increases in the visual counts of D. cervinus, D. vulgaris, D. dentex, and S. cabrilla occurred in conjunction with increases in temperature and salinity. By contrast, for S. porcus, O. melanura, S. maena, and C. julis., higher abundances were observed 1 or 2 months prior to this temperature increase.
We quantified the cause-effect relationship between peaks in the counts of species and increases in the state of fluctuation of selected environmental parameters by multivariate analysis. Canonical Correlation Analysis (CCorA) is an appropriate tool to analyze datasets (including multivariate time-series) that show no clear dependency between biological (i.e., species visual counts) and environmental parameters (Van der Meer 1991). The method identifies two bases, one for each variable, that are optimal with respect to correlations whilst simultaneously identifying the corresponding correlations (Sherry and Henson 2005). CCorA has been previously used in a pilot acoustic study at the OBSEA platform, tracking locomotor behavior in freely moving crustacean decapods in relation to the changing environment, as monitored by oceanographic sensors (Rotllant et al. 2014). Several species responded to temperature (S. maena, D. dentex, D. vulgaris, D. annularis, and D. cervinus) and salinity (S. mediterraneus, S. melanocercus, C. chromis, D. dentex, and D. annularis), showing count increases with an increase in the measured parameters.
This type of combined data treatment (i.e. monthly averaging of fish counts and CCorA screening) is of relevance because the technique can be used to project expected species abundances in coastal areas in a climate change scenario where an increase in water temperature may be accompanied by a potential increase in salinity. In particular, temperature is a fundamental variable that directly affects fish metabolism and behavior, and consequently, plays a role in community functioning (Hawkins et al. 2003; Munday et al. 2009; Cheung et al. 2013). Our 1-year data acquisition do not indicate conclusively the role of climate forcing on species behavior and derived community composition. A more prudent approach would be to undertake an analysis of multiannual data before inferring seasonal and climatic links. In this sense, multiparametric data acquisition should be historicized, when the length of data sets exceeding the repletion of a minimum unit (1 year) by at least three multiplications. This would allow minimum reliability in time series analysis aiming to determine recurrent seasonal periodicities, other than naturally occurring inter annual variability. Multiannual observations must be collected to eliminate inter annual variations related to seasonal rhythms. OBSEA data acquisition represents a promising step toward the creation of high frequency (i.e. hours) decadal time series which may be used for creating realistic projections of climate change effects on fish coastal communities.
Conclusions
In the present review, we synthesized and provided clear examples on the utility of cabled observatory video technology for monitoring fish behaviour in coastal marine systems. The quality of long-term datasets, generated by this relatively novel approach, provides new opportunities for ecological research and the improvement of our capability for tracking and understanding temporal variability in coastal environments. Indeed, the continuous high frequency monitoring of biotic and abiotic variables performed by a single observatory allows the investigation of biotic responses at different biological scales, ranging from individuals to entire assemblages. This information contributes to better define the characteristics that shape the behaviour and the ecological niche of a species and helps us to measure and predict the effects of both natural and anthropogenic drivers. With respect to traditional monitoring programs, based on periodical surveys, cabled observatory video technology register data at finer temporal scales providing complementary information. Both environmental and biotic data generated by cabled observatories should be available to end users, which may include a range of stakeholders, including fishermen, other ‘sea users’, marine biologists and fisheries managers. Hopefully, future developments of this technology will provide appropriate support to calibrate existing network nodes worldwide and to interconnect information supplied by each cabled observatory station. This would be certainly desirable to better meet the need for improved protection and management of the marine environment raised by ongoing globally changing environmental conditions.
References
Aguzzi J, Bahamon N (2009) Modelled day-night biases in decapod assessment by bottom trawling survey. Fish Res 100:274–280
Aguzzi J, Company JB (2010) Chronobiology of deep-water decapod crustaceans on continental margins. Adv Mar Biol 58:155–225
Aguzzi J, Bahamon N, Marotta L (2009a) The influence of light availability and predatory behaviour of the decapod crustacean Nephrops norvegicus on the activity rhythms of continental margin prey decapods. Mar Ecol 30:366–375
Aguzzi J, Costa C, Fujiwara Y, Iwase R, Ramirez-Llorda E, Menesatti P (2009b) A novel morphometry-based protocol of automated video-image analysis for species recognition and activity rhythms monitoring in deep-sea fauna. Sensors Basel 9:8438–8455
Aguzzi J, Costa C, Menesatti P, Fujiwara Y, Iwase R, Ramirez-Llodra E (2009c) A novel morphometry-based protocol of automated video-image analysis for species recognition and activity rhythms monitoring in deep-sea fauna. Sensors Basel 9:8438–8455
Aguzzi J, Costa C, Furushima Y, Chiesa JJ, Company JB, Menesatti P, Iwase R, Fujiwara Y (2010) Behavioural rhythms of hydrocarbon seep fauna in relation to internal tides. Mar Ecol Prog Ser 418:47–56
Aguzzi J, Company JB, Costa C, Menesatti P, Garcia JA, Bahamon N, Puig P, Sardà F (2011a) Activity rhythms in the deep-sea: a chronobiological approach. Front Biosci 16:131–150
Aguzzi J, Costa C, Robert K, Matabos M, Antonucci F, Juniper K, Menesatti P (2011b) Automated image analysis for the detection of benthic crustaceans and bacterial mat coverage using the VENUS undersea cabled network. Sensors Basel 11:10534–10556
Aguzzi J, Manuél A, Condal F, Guillén J, Nogueras M, Del Río J, Costa C, Menesatti P, Puig P, Sardà F, Toma D, Palanques A (2011c) The new SEAfloor OBservatory (OBSEA) for remote and long-term coastal ecosystem monitoring. Sensors Basel 11:5850–5872
Aguzzi J, Company JB, Costa C, Matabos M, Azzurro E, Mànuel A, Menesatti P, Sardà F, Canals M, Delory E, Cline D, Favali P, Juniper SK, Furushima Y, Fujiwara Y, Chiesa JJ, Marotta L, Priede IM (2012a) Challenges to assessment of benthic populations and biodiversity as a result of rhythmic behaviour: video solutions from cabled observatories. Ocean Mar Biol Ann Rev 50:235–286
Aguzzi J, Jamieson AJ, Fujii T, Sbragaglia V, Costa C, Menesatti P, Fujiwara Y (2012b) Shifting feeding behaviour of deep-sea Buccinid gastropods at natural and simulated food falls. Mar Ecol Prog Ser 458:247–253
Aguzzi J, Sbragaglia V, Santamaría G, Del Río J, Sardà F, Nogueras M, Manuel A (2013) Daily activity rhythms in temperate coastal fishes: insights from cabled observatory video monitoring. Mar Ecol Prog Ser 486:223–236
Albouy C, Guilhaumoun F, Aráujo MB, Mouillot D, Leprieur F (2012) Combining projected changes in species richness and composition reveals climate change impacts on coastal Mediterranean fish assemblages. Glob Change Biol 18:2995–3003
Alós J, March D, Palmer M, Grau A, Morales-Nin B (2011) Spatial and temporal patterns in Serranus cabrilla habitat use in the NW Mediterranean revealed by acoustic telemetry. Mar Ecol Prog Ser 427:173–186
Antonucci F, Costa C, Aguzzi J, Cataudella S (2009) Ecomorphology of morpho-functional relationships in the family of Sparidae: a quantitative statistic approach. J Morphol 270:843–855
Assis J, Claro B, Ramos A, Boavida J, Serrão EA (2013) Performing fish counts with a wide-angle camera, a promising approach reducing divers’ limitations. J Exp Mar Biol Ecol 445:93–98
Attrill MJ, Power M (2002) Climatic influence on a marine fish assemblage. Nature 417:275–278
Azzurro E, Pais A, Consoli P, Andaloro F (2007) Evaluating day-night changes in shallow Mediterranean rocky reef fish assemblages by visual census. Mar Biol 151:2245–2253
Azzurro E, Moschella P, Maynou F (2011) Tracking signals of change in Mediterranean fish diversity based on Local Ecological Knowledge. PLoS ONE 6:e24885
Azzurro E, Aguzzi J, Maynou F, Chiesa JJ, Savini D (2013) Diel rhythms in shallow Mediterranean rocky-reef fishes: a chronobiological approach with the help of trained volunteers. J Mar Biol Ass UK 93:461–470
Bahamon N, Aguzzi J, Sardà F (2009) Fuzzy diel pattern in commercial catchability of deep water continental margin species. ICES J Mar Sci 66:2211–2218
Barans CA, Arendt MD, Moore T, Schmidt D (2005) Remote video revisited: a visual technique for conducting long-term monitoring of reef fishes on the continental shelf. Mar Technol Soc J 39:110–118
Beauxis-Aussalet E, Palazzo S, Nadarajan G, Arslanova E, Spampinato C, Hardman L (2013) A video processing and data retrieval framework for fish population monitoring. In: Proceedings of the 2nd ACM international workshop on Multimedia analysis for ecological data (MAED ‘13). ACM, New York, pp 15–20. doi: 10.1145/2509896.2509906
Becker A, Whitfield AK, Cowley PD, Järnegren J, Næsje TF (2013) Potential effects of artificial light associated with anthropogenic infrastructure on the abundance and foraging behaviour of estuary-associated fishes. J Appl Ecol 50:43–50
Block BA, Jonsen ID, Jorgensen SJ, Winship AJ, Shaffer SA, Bograd SJ, Hazen EL, Foley DG, Breed GA, Harrison A-L, Ganong JE, Swithenbank A, Castleton M, Dewar H, Mate BR, Shillinger GL, Schaefer KK, Benson SR, Weise MJ, Herny RW, Costa DP (2011) Tracking apex marine predator movements in a dynamic ocean. Nature 475:86–90
Bouchet P (2006) The exploration of marine biodiversity. Fundación BBVA Madrid
Chabanet P, Loiseau N, Join J-L, Ponton D (2012) VideoSolo, an autonomous video system for high-frequency monitoring of aquatic biota, applied to coral reef fishes in the Glorioso Islands (SWIO). J Exp Mar Biol Ecol 430:10–16
Cheung WWL, Watson R, Pauly D (2013) Signature of ocean warming in global fisheries catch. Nature 497:365–369
Chierici F, Favali P, Beranzoli L, De Santis A, Embriaco D, Giovanetti G, Marinaro G, Monna S, Pignagnoli L, Riccobene G, Bruni F, Gasparoni F (2012) NEMO-SN1 (Western Ionian Sea, off Eastern Sicily): a cabled abyssal observatory with tsunami early warning capability, ISOPE-Int Soc. Offshore and Polar Engineers, Proc 22nd Int. Offshore and Polar Engineering Conf., Rhodes, July 17-22, ISBN:978-1-880653-94-4, ISSN:1098-6189
Condal F, Aguzzi J, Sardà F, Nogueras M, Cadena J, Costa C, Del Río J, Mànuel A (2012) Seasonal rhythm in a Mediterranean coastal fish community as monitored by a cabled observatory. Mar Biol 159:2809–2817
Conrad JL, Weinersmith KL, Brodin T, Saltz JB, Sih A (2011) Behavioural syndromes in fishes: a review with implications for ecology and fisheries management. J Fish Biol 78:395–435
Cooke SJ, Suski CD, Ostrand KG, Wahl DH, Philipp DP (2007) Physiological and behavioral consequences of long-term artificial selection for vulnerability to recreational angling in a teleost fish. Physiol Biochem Zool 80:480–490
Corgnati L, Mazzei L, Marini S, Stefano A, Alessandra C, Annalisa G, Bruno I, Ennio O (2014) Automated gelatinous zooplankton acquisition and recognition. Proceedings of the Computer Vision for Analysis of Underwater Imagery (CVAUI):1–8
Costa C, Scardi M, Vitalini V, Cataudella S (2009) A dual camera system for counting and sizing Northern Bluefin Tuna (Thunnus thynnus; Linnaeus, 1758) stock, during transfer to aquaculture cages, with a semi-automatic Artificial Neural Network tool. Aquacult 291:161–167
Costa C, Antonucci F, Pallottino F, Aguzzi J, Sun DW, Menesatti P (2011) Shape analysis of agricultural products: a review of recent research advances and potential application to computer vision. Food Bioproc Technol 4:673–692
Davis T, Harasti D, Smith SDA (2014) Compensating for length biases in underwater visual census of fishes using stereo video measurements. Mar Freshwat Res (in press). doi 10.1071/MF14076
De Leo FC, Smith CR, Rowden AA, Bowden D, Clark M (2010) Submarine canyons: hotspots of benthic biomass and productivity in the deep-sea. Proc Roy Soc B 277:2783–2792
Del Río J, Aguzzi J, Hidalgo A, Bghiel I, Manuel A, Sardà F (2013a) Citizen Science and marine community monitoring by video-cabled observatories: The OBSEA Citizen Science Project. Proc UT-13 IEEE-Tokyo 1-3:doi:10.1109/UT.2013.6519842
Del Río J, Aguzzi J, Costa C, Menesatti P, Sbragaglia V, Nogueras M, Sardà F, Manuèl A (2013b) A new colorimetrically-calibrated automated video-imaging protocol for the day-night fish counting at the OBSEA coastal cabled observatory. Sensors 13:14740–14750
Delgado C, Wada N, Rosegrant M, Meijer S, Ahmed M (2003) Outlook for fish to 2020: meeting global demand. Food Policy Report, International Food Policy Research Institute, Washington
Dickinson JL, Zuckerberg B, Bonter DN (2010) Citizen science as an ecological research tool: challenges and benefits. Ann Rev Ecol Evol Syst 41:149–172
Doya C, Aguzzi J, Pardo M, Matabos M, Company JB, Costa C, Milhaly S (2013a) Diel behavioral rhythms in the sablefish (Anoplopoma fimbria) and other benthic species, as recorded by deep-sea cabled observatories in Barkley canyon (NEPTUNE-Canada). J Mar Syst 130:69–78
Doya C, Aguzzi J, Purser A, Thompsen L, Company JB, Matabos M (2013b) A diel and seasonal deep-sea faunal monitoring by crawler within the Barkely canyon (NEPTUNE-Canada). 5th International symposium on chemosynthesis-based ecosystems (CBE-5). 18-23 August 2013. University of Victoria, Victoria (Canada)
Dulcic J, Fencil M, Matic-Skoko S, Kraljevic M, Glamuzina B (2004) Diel catch variations in a shallow-water fish assemblage at Duce Glava, eastern Adriatic (Croatian Coast). J Mar Biol Ass UK 84:659–664
Dulvy NK, Rogers SL, Jennings S, Stelzenmüller V, Dye SR, Skjoldsl HR (2008) Climate change and deepening of the North Sea fish assemblage: a biotic indicator of warming seas. J App Ecol 45:1029–1039
Ehrlén J, Morris WF (2015) Predicting changes in the distribution and abundance of species under environmental change. Ecol Lett (in press)
Esteban M, Cuesta A, Chaves-Pozo E, Meseguer J (2013) Influence of melatonin on the immune system of fish: a review. Int J Mol Sci 14:7979–7999
Favali P, Beranzoli L (2006) Seafloor observatory science: a review. Ann Geoph 49:515–567
Favali P, Beranzoli L, D’Anna G, Gasparoni F, Marvaldi J, Clauss G, Gerber HW, Nicot M, Marani MP, Gamberi F, Millot C, Flueh ER (2006) A fleet of multiparameter observatories for geophysical and environmental monitoring at seafloor. Ann Geoph 49:659–680
Favali P, Person R, Barnes CR, Kaneda Y, Delaney JR, Hsu S-K (2010) Seafloor observatory science. In: Hall J, Harrison D.E, Stammer D (eds.), Proceedings of the OceanObs’09: Sustained Ocean. Observations and Information for Society conference 2, Venice, Italy, 21–25 September 2009, ESA Publication. WPP-306 ISSN:1609-042X. doi: 10.5270/OceanObs09.cwp28
Favali P, Chierici F, Marinaro G, Giovanetti G, Azzarone A, Beranzoli L, De Santis A, Embriaco D, Monna S, Lo Bue N, Sgroi T, Cianchini G, Badiali L, Qamili E, De Caro M, Falcone G, Montuori C, Frugoni F, Riccobene G, Sedita M, Barbagallo G, Cacopardo G, Calì C, Cocimano R, Coniglione R, Costa M, D’Amico A, Del Tevere F, Distefano C, Ferrera F, Giordano V, Imbesi M, Lattuada D, Migneco E, Musumeci M, Orlando A, Papaleo R, Piattelli P, Raia G, Rovelli A, Sapienza P, Speziale F, Trovato A, Viola S, Ameli F, Bonori M, Capone A, Masullo R, Simeone F, Pignagnoli L, Zitellini N, Bruni F, Gasparoni F, Pavan G (2013) NEMO-SN1 abyssal cabled observatory in the western ionian sea. IEEE J Ocean Eng 38(2):358–374
Fischer S, Patzner RA, Müller CH, Winkler HM (2007) Studies on the ichthyofauna of the coastal waters of Ibiza (Balearic Islands, Spain). Rostocker Meer Beiträge 18:30–62
Food and Agriculture Organization of the United Nations—FAO (2014) The state of worlds fisheries and aquaculture, Rome
Glover AG, Gooday AJ, Bailey DM, Billet DSM, Chevaldonné P, Colaço A, Copley J, Cuvelier D, Desbruyères D, Kalogeropoulou V, Klages M, Lampadariuou N, Lejeusne C, Mestre NC, Paterson GLJ, Perez T, Ruhl HA, Sarrazin J, Soltwedel T, Soto EH, Thatje S, Tselepides A, Van Gaever S, Vanreusel A (2010) Temporal changes in deep-sea benthic ecosystems: a review of the evidence from recent time-series studies. Adv Mar Biol 58:1–95
Grange LJ, Smith CR (2013) Megafaunal communities in rapidly warming fjords along the West Antarctic peninsula: hotspots of abundance and Beta diversity. PlosOne 8:e77917
Han J, Honda N, Asada A, Shibata K (2009) Automated acoustic method for counting and sizing farmed fish during transfer using DIDSON. Fish Sci 75:1359–1367
Harmelin-Vivien ML, Francour P (2008) Trawling or visual censuses? Methodological bias in the assessment of fish populations in seagrass beds. Ecology 3:41–51
Harvey ES, Newman SJ, McLean DL, Cappo M, Meeuwig JJ, Skepper CL (2012) Comparison of the relative efficiencies of stereo-BRUVs and traps for sampling tropical continental shelf demersal fishes. Fish Res 125–126:108–120
Hawkins SJ, Southward AJ, Genner MJ (2003) Detection of environmental change in a marine ecosystem-evidence from the western English Channel. Sci Tot Env 310:245–256
Holyoak M, Casagrandi R, Nathan R, Revilla E, Speigel O (2008) Trends and missing parts in the study of movement ecology. Proc Nat Acad Sci 105:19060–19065
Horodysky AZ, Brill RW, Warrant EJ, Musick JA, Latour RJ (2010) Comparative visual function in four piscivorous fishes inhabiting Chesapeake Bay. J Exp Biol 213:1751–1761
Hut RA, Kronfeld-Schor N, van der Vinne V, De la Iglesia H (2012) In search of a temporal niche: environmental factors. Progr Brain Res 199:281–304
Irigoyen AJ, Galván DE, Venerus LA, Parma AM (2012) Variability in abundance of temperate reef fishes estimated by visual census. PLoS ONE 8:e61072
Jørgensen C, Peck ME, Antognarelli F, Azzurro E, Burrows MT, Cheung WW, Cucco A, Holt R, Hueber KB, Marras S, Mckeinz D, Metcalf J, Perez-Ruzafa A, Sinerchia M, Sfeffensen JF, Teal LR, Domenici P (2012) Conservation physiology of marine fishes: advancing the predictive capacity of models. Biol Lett 8:900–903
Kasaya T, Mitsuzawa K, Goto TN, Iwase R, Sayanagi K, Araki E, Nagao T (2009) Trial of multidisciplinary observation at an expandable sub-marine cabled station “off-Hatsushima Island Observatory” in Sagami Bay, Japan. Sensors 9:9241–9254
Koeck B, Alós J, Caro A, Neveu R, Crec’hriou R, Saragoni G, Lenfant P (2013) Contrasting fish behavior in artificial seascapes with implications for resources conservation. PLoS ONE 8:e69303
Kronfeld-Schor N, Dayan T (2003) Partitioning of time as an ecological resource. Ann Rev Ecol Syst 34:153–181
Kross S, Nelson X (2011) A portable low-cost remote videography system for monitoring wildlife. Met Ecol Evol 2:191–196
Lampitt RS, Favali P, Barnes CR, Church MJ, Cronin MF, Hill KL, Kaneda Y, Karl DM, Knap AH, McPhaden MJ, Nittis KA, Priede IG, Rolin J-F, Send U, Teng C-C, Trull TW, Wallace DWR, Weller RA (2010) In situ sustained Eulerian observatories. In: Hall J, Harrison DE, Stammer D (eds), Proceedings of the OceanObs’09: Sustained Ocean. Observations and Information for Society conference 2, Venice, Italy, 21-25 September 2009, ESA Publication WPP-306, ISSN:1609-042X, doi:10.5270/OceanObs09.pp.27
Langlois J, Harvey ES, Fitzpatrick B, Meeuwig JJ, Shedrawi G, Watson DL (2010) Cost-efficient sampling of fish assemblages: comparison of baited video stations and diver video transects. Aquat Biol 9:155–168
Longcore T, Rich C (2004) Ecological light pollution. Front Ecol Environ 2:191–198
López-Olmeda JF, Sánchez-Vázquez FJ (2010) Feeding Rhythms in Fish: From behavioural to molecular approach. In: Kulczykowska E, Popek W, Kapoor BG (eds) Biological clock in fish. CRC Press, Taylor and Francis, New York, pp 155–184
López-Olmeda JF, Noble C, Sánchez-Vázquez FJ (2012) Does feeding time affects fish welfare? Fish Physiol Biochem 38:143–152
Loudon ASI (2012) Circadian biology: a 2.5 billion year old clock. Curr Biol 22:570–571
MacLeod N, Benfield M, Culverhouse P (2010) Time to automate identification. Nature 467:154–155
Mallet D, Wantiez L, Lemouellic S, Vigliola L, Pelletier D (2014) Complementarity of rotating video and underwater visual census for assessing species richness, frequency and density of reef fish on coral reef slopes. PLoS 1:e84344
March D, Palmer M, Alós J, Grau A, Cardona F (2010) Short-term residence, home range size and diel patterns of the painted comber Serranus scriba in a temperate marine reserve. Mar Ecol Prog Ser 400:195–206
Matabos M, Aguzzi J, Robert K, Costa C, Menesatti P, Company JB, Juniper K (2011) Multi-parametric study of behavioural modulation in demersal decapods at the VENUS cabled observatory in Saanich Inlet, British Columbia, Canada. J Exp Mar Biol Ecol 401:89–96
Matabos M, Bui AO, Mihály S, Aguzzi J, Juniper DSK, Ajayamohan RS (2013) High-frequency study of benthic megafaunal community dynamics in Barkley canyon: a multidisciplinary approach using the NEPTUNE Canada network. J Mar Syst 130:56–68
Mazzei L, Marini S, Craig J, Aguzzi J, Fanelli E, Priede IG (2014). Automated video imaging system for counting deep-sea bioluminescence organisms events. Proceedings of the Computer Vision for Analysis of Underwater Imagery (CVAUI), pp 57–64
Menge BA, Chan F, Dudas S, Eerkes-Medrano D, Grorud-Colvert K, Heiman K, Hessing-Lewis M, Iles A, Milston-Clements R, Noble M, Page-Albins K, Richmond E, Rilov G, Rose J, Tyburczy J, Vinueza L, Zarnetske P (2009) Terrestrial ecologists ignore aquatic literature: asymmetry in citation breadth in ecological publications and implications for generality and progress in ecology. J Exp Mar Biol Ecol 377:93–100
Monna S, Falcone G, Beranzoli L, Chierici F, Cianchini G, De Caro M, De Santis A, Embriaco D, Frugoni F, Marinaro G, Montuori C, Pignagnoli L, Qamili E, Sgroi T, Favali P (2014) Underwater geophysical monitoring for European Multidisciplinary Seafloor and water-column Observatories. J Mar Syst 130:12–30
Munday PL, Crawley NE, Nilsson GE (2009) Interacting effects of elevated temperature and ocean acidification on the aerobic performance of coral reef fishes. Mar Ecol Prog Ser 388:235–242
Navarro J, Votier SC, Aguzzi J, Chiesa JJ, Forero MG, Phillips RA (2013) Ecological segregation in space, time and trophic niche of sympatric planktivorous petrels. PLoS ONE 8:e62897
Naylor E (2005) Chronobiology: implications for marine resources exploitation and management. Sci Mar 69:157–167
Naylor E (2010) Chronobiology of marine organisms. Cambridge University Press, Cambridge
Parmesan C (2006) Ecological and evolutionary responses to recent climate change. Ann Rev Ecol Evol Syst 37:637–669
Parmesan C, Yohe G (2003) A globally coherent fingerprint of climate change impacts across natural systems. Nature 421:37–42
Paterson JR, García-Bellido DC, Lee MSY, Brock GA, Jago JB, Edgecombe GD (2011) Acute vision in the giant Cambrian predator Anomalocaris and the origin of compound eyes. Nature 480:237–240
Pauers MJ, Kuchenbecker JA, Neitzm M, Neitz J (2012) Changes in the colour of light cue circadian activity. Anim Behav 83:1143–1151
Peer AC, Miller TJ (2014) Climate change, migration phenology, and fisheries management interact with unanticipated consequences. North Am J Fisher Manag 34:94–110
Pelletier D, Leleu K, Mallet D, Mou-Tham G, Hervé G, Boureau M, Guilpart N (2012) Remote high-definition rotating video enables fast spatial survey of marine underwater macrofauna and habitats. PLoS ONE 7:e30536
Perry AL, Low PJ, Ellis JR, Reynolds JD (2005) Climate change and distribution shifts in marine fishes. Science 38:1912–1915
Pikitch EK, Rountos KJ, Essington TE, Santora C, Pauly D, Watson R, Sumaila UR, Boersma PD, Boyd IL, Conover DO, Cury P, Heppell SS, Houde ED, Mangel M, Plagányi É, Sainsbury K, Steneck RS, Geers TM, Gownaris N, Munch SB (2014) The global contribution of forage fish to marine fisheries and ecosystems. Fish Fisher 15:43–64
Proctor R, Howarth J (2008) Coastal observatories and operational oceanography: a European perspective. Mar Technol Soc J 42:10–13
Purser A, Ontrup J, Schoening T, Thomsen L, Tong R, Unnithan V, Nattkemper TW (2013a) Microhabitat and shrimp abundance within a Norwegian cold-water coral ecosystem. Biogeosci 10:5779–5791
Purser A, Thomsen L, Barnes C, Best M, Chapman R, Hofbauer M, Menzel M, Wagner H (2013b) Temporal and spatial benthic collection via internetoperated Deep Sea Crawler. Met Oceanogr 5:1–18
Reebs SG (2002) Plasticity of diel and circadian activity rhythms in fishes. Rev Fish Biol Fisher 12:349–371
Refinetti R (2006) Circadian physiology. Francis and Taylor, New York
Rotllant G, Aguzzi J, Sarria D, Gisbert E, Sbragaglia V, Del Río J, Simeó CG, Mànuel A, Molino E, Costa C, Sardà F (2014) Pilot acoustic tracking study on adult spiny lobsters (Palinurus mauritanicus) and spider crabs (Maja squinado) within an artificial reef. Hydrobiol 742:27–38
Ruhl HA, André M, Beranzoli L, Çağatay NM, Colaço A, Cannat M, Dañobeitia JJ, Favali P, Géli L, Gillooly M, Greinert J, Hall POJ, Huber R, Karstensenm J, Lampitt RS, Larkin KE, Lykousis V, Mienert J, de Miranda JMA, Person R, Priede IG, Puillat I, Thomsen L, Waldmann C (2011) Societal need for improved understanding of climate change, anthropogenic impacts, and geo-hazard warning drive development of ocean observatories in European Seas. Prog Oceanogr 91:1–33
Sardà F, Aguzzi J (2012) A review of burrow counting as an alternative to other typical methods of assessment of Norway lobster populations. Rev Fish Biol Fisher 22:409–422
Schoening T, Bergmann M, Ontrup J, Taylor J, Dannheim J, Gutt J, Purser A, Nattkemper TW (2012) Semi-automatic image analysis for the assessment of megafuanal densities at the Arctic deep-sea observatory HAUSGARTEN. PLOS I:5. doi:10.1371/journal.pone.0038179
Sherman AD, Smith KL Jr (2009) Deep-sea benthic boundary layer communities and food supply: a long-term monitoring strategy. Deep-Sea Res II 56:1754–1762
Sherry A, Henson RK (2005) Conducting and interpreting canonical correlation analysis in personality research: a user-friendly primer. J Pers Assess 84:37–48
Sih A, Bell A, Johnson JC (2004) Behavioral syndromes: an ecological and evolutionary overview. Trends Ecol Evol 19:372–378
Silvertown J (2009) A new dawn for Citizen Science. Trends Ecol Evol 24:467–471
Sims DW, Queiroz N, Humphries NE, Lima F, Hays GC (2009) Long-term GPS tracking of ocean sunfish Mola mola offers a new direction in fish monitoring. PLoS ONE 4:e7351
Smith KL, Kaufmann RS, Wakefield WW (1993) Mobile megafaunal activity monitored with a time-lapse camera in the abyssal North Pacific. Deep Sea Res I 411:2307–2324
Smith KL, Ruhl HA, Kahru M, Huffard CL, Sherman AD (2013) Deep ocean communities impacted by changing climate over 24 y in the abyssal northeast Pacific Ocean. Proc Nat Acad Sci 110:19838–19841
Sogard SM, Olla BL (1996) Food deprivation affects vertical distribution and activity of a marine fish in a thermal gradient: potential energy-conserving mechanisms. Mar Ecol Prog Ser 133:43–55
Tan J, Kelly CK, Jiang L (2013) Temporal niche promotes biodiversity during adaptive radiation. Nat Comm 4:2102
Templado J (2014) Future trends of Mediterranean biodiversity. In: The Mediterranean Sea, Spinger, Berlin
Tessier A, Pastor J, Francour P, Saragoni G, Crec’hriou R, Lenfant P (2013) Video transects as a complement to underwater visual census to study reserve effect on fish assemblages. Aquat Biol 8:229–241
Thomsen L, Barnes C, Best M, Chapman R, Pirenne B, Thomson R, Vogt J (2012) Ocean circulation promotes methane release from gas hydrate outcrops at the NEPTUNE Canada Barkley Canyon node. Geophys Res Lett. doi:10.1029/2012GL052462
Tutman P, Glavic N, Kožul V, Antolovic N, Skaramuca B (2010) Diel fluctuations in juvenile dominated fish assemblages associated with shallow seagrass and bare sand in southern Adriatic Sea, Croatia. Rapp Comm Int Mere Méd 39:688
Underwood AJ (2005) Intertidal ecologists work in the ‘gap’ between marine and terrestrial ecology. Mar Ecol Prog Ser 304:297–302
Unsworth RKF, Peters JR, McCloskey RM, Hinder SL (2014) Optimising stereo baited underwater video for sampling fish and invertebrates in temperate coastal habitats. Est Coastal Shelf Sci 150:281–287
Van der Meer J (1991) Exploring macrobenthos-environment relationship by canonical correlation analysis. J Exper Mar Biol Ecol 148:105–120
Vardaro MF, Bagley PM, Bailey DM, Bett BJ, Jones DOB, Milligan RJ, Priede IG, Risien CM, Rowe GT, Ruhl HA, Sangolay BB, Smith KJ, Walls A, Clarke J (2013) A Southeast Atlantic deep-ocean observatory: first experiences and results. Limnol Oceanogr 11:304–315
Walli A, Teo SLH, Boustany A, Farwell CJ, Williams T, Dewar H, Prince E, Block BA (2009) Seasonal movements, aggregations and diving behavior of Atlantic Bluefin tuna (Thunnus thynnus) revealed with archival tags. PLoS ONE 4:e6151
Watson DL, Harvey ES, Fitzpatrick BM, Langlois TJ, Shedrawi G (2010) Assessing reef fish assemblage structure: How do different stereo-video techniques compare? Mar Biol 157:1237–1250
Webb TJ (2012) Marine and terrestrial ecology: unifying concepts, revealing differences. Trends Ecol Evol 27:535–541
Wehkamp S, Fischer P (2013) Impact of coastal defence structures (tetrapods) on a demersal hard-bottom fish community in the southern North Sea. Mar Env Res 83:82–92
Werner EE, Anholt BR (1993) Ecological consequences of the trade-offs between growth and mortality rates mediated by foraging activity. Am Nat 142:242–272
Widder EA, Robison BH, Reisenbichler KR, Haddock SDH (2005) Using red light for in situ observations of deep-sea fishes. Deep-Sea Res I 52:2077–2085
Willis TJ, Badalamenti F, Milazzo M (2006) Diel variability in counts of reef fishes and its implications for monitoring. J Exp Mar Biol Ecol 331:108–120
Acknowledgments
This research was funded by RITFIM (CTM2010-16274) and European Multidisciplinary Seafloor Observation (EMSO Preparatory Phase-FP7 Infrastructures-2007-1, Proposal 211816). Researchers from CSIC-UPC are members of the Associated Unit Tecnoterra. The paper was also partially funded by the Helmholtz Alliance “Robotic Exploration of Extreme Environments (ROBEX)” project. I.A. Catalán was partially supported by REC2 from the Spanish Government, CTM2011-23835. English revision was assisted by A. Purser and V. Radovanovic.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Aguzzi, J., Doya, C., Tecchio, S. et al. Coastal observatories for monitoring of fish behaviour and their responses to environmental changes. Rev Fish Biol Fisheries 25, 463–483 (2015). https://doi.org/10.1007/s11160-015-9387-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11160-015-9387-9