Abstract
Currently, the use of alternative renewable energies is broadly supported in many countries, some of which are seriously evaluating the possibility of using hydrogen as an alternative fuel in their power systems. Hydrogen production by biological processes, such as dark fermentation, is a very promising alternative. However, this process has only been studied on the laboratory scale, and there is limited experience at the pilot scale. The main reasons of non-scaling hydrogen production by dark fermentation at large scale are unpurified hydrogen production, stability of the bioprocesses, as well as their low conversion yields joined at the formation of byproducts. Improvement of energetic yields of dark fermentation requires a better knowledge of the microorganisms involved in the mixed culture and their possible interactions, as well as the use of appropriate substrates and strategies, such as solid-state fermentation, the purification of hydrogen and the coupling of dark fermentation with other biological processes as anaerobic digestion. The present work offers an overview of the current knowledge dealing with H2-production by dark fermentation and its integration into a concept of an environmental biorefinery. Several key points are addressed, such as the benefits of using local waste as substrates, the new solid-state fermentation processes, the coupling of hydrogen purification with the production process, the association of the H2-producing process with other biological processes, such as anaerobic digestion towards biohythane production (H2/CH4). Information about pilot plant experiments was added to illustrate the feasibility of producing fermentative hydrogen and methane from organic waste at a pilot scale, as developed at Feng Chia University (Taiwan).
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
Today, approximately 80 % of the energy used worldwide comes from fossil fuels and the remaining 20 % comes from nuclear and renewable energy sources (Singh and Wahid 2015). Governments support the use of alternative renewable energies, arguing that unlike fossil fuel combustion, alternative renewable energies represent a source of renewable energy (Edwards et al. 2008; Andrews and Shabani 2012; Orecchini 2006). However, there are other less-mentioned reasons that also argue for the need for an energetic change, such as reducing energy dependence on other countries, the stabilization of fossil fuel prices and an increase of employment due to renewable energy production (Hernandez Sobrino et al. 2010). Hydrogen is a promising alternative as an energetic carrier and can be from alternative renewable energies. Countries that are seriously evaluating the possibility of using hydrogen (H2) as an alternative fuel in their power systems are the United Kingdom, Denmark, the United States, Italy, Taiwan, China, India, Korea, Switzerland, Austria, Canada, Japan and Germany (Dutta 2014).
The advantage of using hydrogen as fuel depends on the type of primary energy source used for its production (Salemme et al. 2014). Currently, most hydrogen is produced from non-renewable sources, such as oil, natural gas and coal. H2 can also be produced from renewable sources, such as biomass, which makes these processes a promising avenue for the production of hydrogen as an environmentally friendly fuel (Chaubey et al. 2013).
Hydrogen can be produced from biomass using existing thermochemical methods and also by developing biological methods. At commercial levels, gasification or pyrolysis are the main thermochemical methods, which cost 60–200 % more than conventional methods (steam methane reforming; $0.75/kghydrogen). Moreover, these methods have high energetic impacts when running at 600–1200 °C, putting them at a disadvantage when considering the advantages of producing hydrogen using lower amounts of energy (Parthasarathy and Narayanan 2014; Wu et al. 2009; Show et al. 2012).
Biological processes can be divided into two major categories: photo-production and dark fermentation (Kothari et al. 2012). The photo-production of hydrogen involves the transformation of solar energy by microalgae or photosynthetic bacteria (direct or indirect bio-photolysis and photo-fermentation), but its application is challenged by its low efficiency to transfer light into chemical energy, with low yields of hydrogen and a subsequent high complexity in the reactor’s design. On the other hand, dark fermentation hydrogen yields from carbohydrates are higher than those from photo fermentation, and its operation is simpler (Elsharnouby et al. 2013).
Hydrogen production by dark fermentation has been investigated these last decades; however, researches are still at a laboratory scale and there are limited experiments with pilot scale systems. The numerous laboratory studies in regards to hydrogen production by dark fermentation, study operational conditions to enhance hydrogen production using different substrates, reactors, and inoculums with and without treatment. According to researchers, larger-scale systems of bio- hydrogen production have not been reported mainly due to the low stability of dark fermentation, hydrogen separation of biogas (unpurified), low organic matter removal (because of the formation of by-products such as organic acids and alcohols) and low energy efficiency of the process (Ghimire et al. 2015; Lin et al. 2012; Ntaikou et al. 2010; Wang et al. 2013; Show et al. 2012). The following review offers an overview of current knowledge in regards to the production of hydrogen via dark fermentation, describes the microorganisms involved in the mixed culture and their possible interactions, substrates used and the possibility of using newly developed technologies such as solid state fermentation. Also, reasons for not scaling-process at large scale are discussed such as the process-stability, hydrogen purification, upgrading hydrogen and biogas in an integrated production-separation system and coupling dark fermentation with other biological processes such as anaerobic digestion. In addition, information from pilot plant experiments describe the main problems observed and an example of a pilot scale system of fermentative hydrogen and methane production from organic wastes, their energy/economic assessments and the application of H2/CH4 biogas, which were developed at Feng Chia University, Taiwan.
2 Hydrogen by dark fermentation: current status
Generally, dark fermentation occurs in nature within a larger process called anaerobic digestion. During this process, organic matter is degraded in an anaerobic bioreactor, which contains microorganisms, such as bacteria (hydrolytic, acidogenic, acetogenic and homoacetogenic) and methanogenic archaea, to produce both methane and carbon dioxide as final products. In the anaerobic digestion process, hydrogen is produced as an intermediate product and is immediately consumed by the hydrogenotrophic methanogenic archaea. Also, can be transformed by other bacteria, such as homoacetogens (autotroph-acetogenic) and nitrate- and sulfate-reducing microorganisms (Chang et al. 2011; Traversi et al. 2012; Saady 2013).
Hydrogen production by dark fermentation can be carried out either by a pure culture or a mixed culture of acidogenic-acetogenic bacteria. The advantage of a pure culture is that metabolic changes are easier to detect/control and more information on the conditions that promote the high production of hydrogen is revealed. Nevertheless, from a technical standpoint, a mixed culture is desirable because it does not require a sterile process (substrates can use cheaper raw materials, such as industrial wastes) and may generate synergies between microorganisms, e.g., by eliminating the use of expensive reducing agents (strict and facultative anaerobes) or the metabolization of complex substrates (hydrogen producers and specialized hydrolytic microorganisms) (Niu et al. 2010; de Sá Ribeiro Vasconcelos et al. 2011; Elsharnouby et al. 2013).
Based on the number of electrons that can be generated from the complete oxidation of glucose, up to 12 molecules of H2 could be produced with a single substrate, which means that the maximal theoretical conversion yield is \(12\,{\text{mol}}_{{\text{H}}_{2}} {\text{mol}}^{-1}{}_{\text{hexose}}\) (Zhang et al. 2006; Willquist et al. 2010). However, the maximal metabolic conversion yield in dark fermentation is 33 % of this (\(4\,{\text{mol}}_{{\text{H}}_{2}} {\text{mol}}^{-1}{}_{\text{hexose}}\)) and depends on the metabolic routes for producing hydrogen (acetate, butyrate, ethanol, format decomposing, butanol etc.) (Hallenbeck et al. 2012). Furthermore, by using mixed cultures, the conversion rate is only approximately 21 %, with butyrate as the major by-product (\(2.5\,{\text{mol}}_{{\text{H}}_{2}} {\text{mol}}^{-1}{}_{\text{hexose}}\)) (Rafrafi et al. 2013; Guo et al. 2014a). Considering an adequate process yield of 60–80 %, some authors think that hydrogen production by dark fermentation has a fairly low yield, but through the use of appropriate mixed cultures and substrates an efficient purification of hydrogen is produced, and the integration of other processes that can be combined with dark fermentation can improve energetic yields, as will be discussed in the next sections (Parthasarathy and Narayanan 2014; Singh and Wahid 2015).
2.1 Microbiology of dark fermentation in a mixed culture
In general, the inoculum sources used to produce hydrogen by mixed cultures containing acetogenic and acidogenic bacteria can be used to produce hydrogen. Particularly, sludge that come from anaerobic digesters, active sludge reactors systems, compost piles, soil, cow excrement and river sediments contain microorganism with hydrogenase enzymes, which in turn dispose the excessive electrons accumulated during fermentation through hydrogen oxidation (Elsharnouby et al. 2013; Chang et al. 2011; Traversi et al. 2012; Valdez-Vazquez and Poggi-Varaldo 2009). However, by using mixed cultures, the possibility of having hydrogen consuming species or non-hydrogen producing species always exists.
Hydrogen consumers can be hydrogenotrophic archaea, homoacetogenic bacteria, or nitrate- and sulfate- reducers that utilize the electrons from hydrogen to reduce a substrate. In the absence or under low concentrations of nitrate or sulfate, the main hydrogen consumers are homoacetogenic bacteria and methanogenic archaea. (Wang et al. 2013; Chang et al. 2011).
Regarding, methanogenic archaea, there are pretreatments that reduce the existence of these microorganisms, which include interventions on the inoculum or the fermentative culture (Wong et al. 2014). Pretreatments include thermal shock or acid/base addition (due to the incapability of these to form spores), biokinetic control with a low HTR in a continuous system (for their low generation times) and the addition of oxygen. For the addition of oxygen, the effect of oxygen has not yet been clarified. The oxygen may have an effect because the methanogenic microorganisms can be considered as strict anaerobes (their ability to accept electrons from carbon dioxide and their ability to donate hydrogen electrons) and/or because oxygen can aid in the balance of oxide reduction (Vásquez et al. 2009; Ren et al. 2010 Ntaikou et al. 2010; Bakonyi et al. 2014). The thermal shock has been widely used, but its cost for the energy expenditure and its technical complexity on large scale haven’t been well studied and needs to be analysed case by case, depending of the different conditions used (Hawkes et al. 2007; Bundhoo et al. 2015).
Homoacetogenic microorganisms are a type of acetogenic microorganism that modifies its metabolism under stress conditions (e.g., when the substrate is limited) and grows with H2/Co2 as the sole source of carbon and energy (Saady 2013; Siriwongrungson et al. 2007). The most common genera of homoacetogenic bacteria correspond to Acetobacterium, Butyribacterium, Clostridium, Eubacterium, Peptostreptococcus and Sporomusa and are characterized by their ability to rapidly grow and, for some of them, to form spores, are obligate or strict, anaerobes, but have several adaptation strategies and can have equal optimum pH than hydrogen producers as C. ljungdahlii (Wang et al. 2013). However, their role and the mechanism of the syntrophic process under the absence of methanogenic microorganisms during a hydrogen producing mixed culture is unclear and because are not monophyletic group, thus, analysis of homoacetogens by 16S rRNA based approaches is problem and although in some cases their presence can be determined by the increase in acetate concentration, they do not always produce acetate (assimilation of CO2 into biomas) (Chang et al. 2011; Wang et al. 2013; Valdez-Vazquez and Poggi-Varaldo 2009). In literature, a continuous reactor operation can be studied from 14 to 700 days, and the decrease of hydrogen producers can be attributed to the development of homoacetogenic microorganisms during the reactor’s operation (Lin et al. 2006; Kim et al. 2006; Fang et al. 2002; Zhao et al. 2008; Lay et al. 2012; Kim et al. 2010; Ren et al. 2010).
In addition, non-hydrogen producing microorganisms, such as bacteria that produce reducing agents (i.e., lactate and propionate), compete for substrate with hydrogen producing microorganisms, Nevertheless, by-products, such as propionate, can also be produced by the same microorganisms that produce hydrogen when their metabolism changes due to a change in their environment (Hawkes et al. 2007). To eliminate or decrease the amount of non-hydrogen producing bacteria and favor the hydrogen production pathways, the by-products that minimize the production of hydrogen can be eliminated or decreased using operational conditions that disfavor their metabolic pathways (Saady 2013).
2.1.1 Hydrogen producing microorganisms in a mixed culture
Hydrogen producing microorganisms in dark fermentation are classified as either spore/non spore forming or as strict/facultative anaerobes. In most cases, these microorganisms are classified as spore-forming strict anaerobes and as non spore-forming facultative anaerobes in the Clostridiaceae and the Enterobacteriaceae families, respectively. Differences in metabolisms exist in both of these groups of microorganisms, especially in the by-products that can be obtained during fermentation, which depends on the respective theoretical hydrogen yield of the microorganism (Kothari et al. 2012; Mathews and Wang 2009; Das et al. 2001, 2008; Demuez et al. 2007; Show et al. 2012).
Both types of hydrogen producing microorganisms can be found in a mixed culture, although this depends on the treatment of the inoculum that is used to eliminate methanogens (as discussed in the previous section). For example, thermal shock pretreatment is favorable to the presence of the genus Clostridium species, which can represent more than 60 % of the microorganisms in a pretreated inoculum (Niu et al. 2010; Zhang et al. 2006; Zeidan and Van Niel 2010; Fang et al. 2002).
2.1.1.1 Metabolic pathways to produce hydrogen
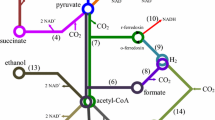
The two main biochemical pathways for the fermentative production of hydrogen from glucose under anaerobic conditions are shown in Fig. 1. Common in many organisms, the Embden–Meyerhof (EM) pathway leads to glucose degradation to form ATP and NADH. Depending on the metabolism of the microorganism, pyruvate can be converted to acetyl CoA and CO2, which generate a reduced ferredoxin molecule (Fdred) that is further reoxidized by producing H2.
Another possibility is to transform pyruvate into acetyl CoA and formate. In the former pathway, which is utilized mainly by strict anaerobic microorganisms, such as Clostridium sp, the reaction is catalyzed by the pyruvate ferredoxin oxido reductase (PFOR). The second pathway is dependent on the presence of the formate hydrogen lyase (FHL) and is utilized by facultative anaerobes, such as Escherichia coli (Cai et al. 2011). During conventional hydrogen production achieved by microorganisms with an active PLF pathway, degraded formate is converted to H2 and CO2 via catalysis by a formate hydrogen lyase. Depending on the microorganism involved, this reaction can occur through [NiFe] hydrogenase (Ech hydrogenase) or formate dependent [FeFe] hydrogenase. Then, acetyl CoA is oxidized to acetate, with the production of one ATP molecule. In these cases, the microorganisms cannot access the NADH produced during glycolysis to produce more hydrogen (H2). Thus, NADH is oxidized through the production of various reduced carbon compounds (i.e., ethanol or lactate), which places a limit on the yield of a maximum of 2 mol of H2 per mole of glucose (see Fig. 1, Hallenbeck et al. 2012). Hydrogen production in microorganisms via the PFOR pathway occurs through the oxidation of reduced ferredoxin (Fdred) with a ferredoxin-dependent hydrogenase (Fd-[FeFe]). Furthermore, under special conditions, it is possible to re-oxidate the NADH generated during glycolysis to produce additional hydrogen molecules through two other hydrogenases, i.e., NADH-dependent (NADH-[FeFe]) and NADH-Fdred dependent hydrogenase (NADH-Fdred-[FeFe]). Finally, 2–4 mol of hydrogen per mole of glucose can be obtained, depending on the metabolic pathway, which in turn is directly related to the hydrogen partial pressure inside the reactor (Angenent et al. 2004; Hallenbeck et al. 2012; see Fig. 1).
2.1.2 Substrates and the potential use of dark fermentation
Simple sugars, such as glucose, sucrose and lactose, have been generally used in the production of hydrogen via dark fermentation as model substrates, especially because of their high biodegradability and the clear understanding of the degradation pathways (Xiao et al. 2013; Show et al. 2011; Guo et al. 2010; Wang and Wan 2009; Levin 2004). However, these types of model substrates are very expensive and the costs can triple in the production of fuel at a large scale (Das 2009; Show et al. 2011; Xiao et al. 2013).
In recent years, the use of wastes or wastewaters from different industries containing highly degradable organic material has gained importance (Boboescu et al. 2014). The production of energy, along with the treatment of wastes, has been the reason behind the development of environmentally friendly and economically sustainable systems (Show et al. 2011; Lin et al. 2012; Boboescu et al. 2014; Wang and Wan 2009; Wong et al. 2014; Chong et al. 2009b). The wastewaters that are mainly investigated are from the industry (production of coffee, beer, cheese, fruit and vegetables processing) and even the renewable energy industry, such as biodiesel, where the principle by-product is glycerol, as shown in Table 1. Hydrogen yields can range from 0.46 to \(24.97\,{\text{mmol}}_{{{\text{H}}_{2}}} {{\text{g}}^{-1}{}_{\text{COD}}}\), depending on the type of wastewater, its concentration and the conditions of operation (values ranging from 2 to 112 % of the theoretical yield in dark fermentation if the water only had glucose). For example, it is possible to obtain higher yields of hydrogen from wastewaters rich in carbohydrates and, in some cases, from wastewaters that have been mixed with wastewaters with low traces of carbohydrates (Show et al. 2011; Lin et al. 2012). Biohydrogen production from solids, such as lignocellulosic residues and municipal waste, has been largely reviewed in the recent literature (Guo et al. 2010; Show et al. 2012; Ghimire et al. 2015). However, the choice of waste streams does not only depend on the hydrogen yield but also on local availability. As discussed in the next section, a solid state fermentation may present several advantages for upscale applications (Fernandes et al. 2010; Ngo et al. 2011; Mangayil et al. 2012).
2.1.3 New technologies in dark fermentation: solid state fermentation for H2 production (SS-DF)
Solid-state anaerobic digestion (SS-AD), also called dry anaerobic digestion or solid-state anaerobic digestion, has received a great deal of interest during the last decade because presented several advantages; in particular, these include lower water requirements as well as smaller reactor sizes (Kothari et al. 2014; Karthikelan and Visvanethan 2012; Jha et al. 2011).
Widely developed, SS-AD represented approximately 60 % of the total treatment capacity in Europe in 2010 (De Baere et al. 2010), corresponding to 3.5 k tons a year. Compared to conventional liquid anaerobic digestion (AD), SS-AD is carried out at high total solids (TS) contents, basically higher than 20 % TS. Solid materials, such as food wastes, agricultural wastes or organic fractions of municipal solid wastes (OFMSW) are used.
The digester size can also be reduced substantially and/or the processes can be operated at higher organic loading rates. In addition to such process intensifications, high-solid systems present operational and technological advantages, such as lower energy requirements to heat the reactor when operated at the same organic loading rate, simpler phase-separation of the digestate, and simpler pretreatment of the incoming materials (Kothari et al. 2014; Karthikelan and Visvanethan 2012; Jha et al. 2011).
SS-DF can also be attractive for process integration in a waste management scheme. Illustratively, the extraction of metabolic by-products, such as VFA, can be facilitated because of their higher concentrations in the digestate, also called fermentate.
In high-solids systems, both physical (mass transfers) and biological (microbial kinetics) processes are strongly interconnected. Due to the presence of high solids, the properties of a part of the unavailable water in the reactors differ somewhat from those containing a great amount of free water in terms of vapor pressure, enthalpy, entropy, viscosity and density (Vaxelaire 2001). Water distribution, which mainly depends on the interactions of the water with the solid matrix, determines the water bioavailability necessary for microbial activity. A recent work (Garcia-Bernet et al. 2011) was devoted to the characterization of biowaste and associated digestates sampled in industrial-scale digesters. Hydration and vicinal water fractions of biowaste and digestates were similar and represented only \(0.1\,{\text{g}}\,{\text{water}}\,{\text{g}}^{- 1}{} _{{{\text{TS}}}}\). Meanwhile, the capillary fraction changed with microbial degradation, and this latter fraction was more important in the digested media, ranging from \(2\,{\text{to}}\,2.5\,{\text{g}}\,{\text{water}}\,{\text{g}}^{- 1}{} _{{{\text{TS}}}}\). Water content is also well known to modify high-solid reactor performances.
Concerning the specific case of SS-DF, operating at high TS contents leads to lower H2 yields. In batch systems using wheat straw as a substrate, Motte et al. (2013, 2014) investigated the effect of increased TS content on H2 production and metabolic pathways, in both mesophilic and thermophilic conditions. Under thermophilic conditions, a drastic decrease in the H2 yields was reported, from \(15.3\,\pm\,1.6\,{\text{NmL}}\,{\text{H}}_{2} {\text{g}}^{- 1}{}_{\text{TS}}\) in wet conditions (10 and 14 % TS) to \(3.4\,\pm\,0.8\,{\text{NmL}}\,{\text{H}}_{2}\,{\text{g}}^{- 1}{}_{\text{TS}}\) in dry conditions (25–34 % TS) (Motte et al. 2014). This decrease was related to both metabolic shifts (i.e., towards lactic acid formation) and microbial population shifts. Such decreases in H2 production were also observed in mesophilic conditions with different shifts of metabolic pathways (Motte et al. 2013). Both wet (10 and 14 % TS) and dry (19–28 % TS) fermentations showed acetic and butyric acid metabolisms, whereas butyric acid metabolism occurred mainly in highly dry fermentation systems (TS > 28 %). Consistently, Robledo-Narváez et al. (2013) and Valdez-Vazquez and Poggi-Varaldo (2009) showed a negative impact of solid contents at even higher TS content ranges (20.9–35.1 % TS and 15–35 % TS, respectively) on H2 production.
Nonetheless, the key mechanisms involved in SS-DF limitations are still unknown and constitute an open issue. A critical factor is the availability of water, which is reduced by higher water adsorption onto the solid, leading to higher concentrations of inhibitory soluble compounds, such as fermentative organic metabolites. In addition, high TS content is related to low mass transfer rates. Under unmixed or sequentially mixed conditions, the transport of soluble compounds (VFAs, dissolved gases) is governed by the diffusion processes and diffusive transport is strongly related to the porosity and the viscosity of the media and, thus, to the total water content (Abbassi-Guendouz et al. 2012). Bollon et al. (2013) determined experimentally the diffusion coefficients in high-solid digested media, and found that the diffusion coefficient in digestates was very small when compared to water (the ratio between the diffusion coefficient in the digestate and water (fD) were 1.8 × 10−2 and 0.54 × 10−2 at 8 % TS and 25 % TS, respectively). As a consequence, this low diffusion rate can induce local chemical environments unfavorable to biological reactions. Further studies are thus required to elucidate the mechanisms involved in SS-DF.
3 Considerations and integration technologies for scaling dark fermentation at large scale
3.1 Stability of hydrogen production by dark fermentation
The “stability” of a hydrogen production process refers to the maintenance of the production of hydrogen and/or metabolites in accordance to a previous variation established by the author, for example 10 % (Kyazze et al. 2006). In literature, the hydrogen production process by dark fermentation with unsterile conditions and mixed cultures have shown to be problematic in maintaining stable processes and this “potential instability” is often considered to be one of the causes for not scaling the dark fermentation (Tenca et al. 2011; Kyazze et al. 2006).
Some reports have directly studied the improved hydrogen yield stability due to the effect of substrate concentration, organic loading rates, hydraulic residence time (HTR) and nutrients in a set range (Kyazze et al. 2006; Gómez et al. 2009; Krupp and Widmann 2009; Zhang and Wang 2013). Many also claim that to improve the stability of the process, it is necessary to know the microbial diversity in the system (Quéméneur et al. 2011; Hung et al. 2008). However, other authors have also highlighted the stability of the process under similar conditions (Hussy et al. 2005).
Works that have studied the cause of the deterioration of hydrogen production have found its connection with changes in microbial diversity, especially for non-sterile feed which could act as continuous inoculum of undesirable microorganisms as non-producing H2 acidogenic microorganisms and/or hydrogen consumers in the reactor increase (Castelló et al. 2009; Kim and Shin 2008; Jo et al. 2007). Jo et al. reported the deterioration of continuous H2 production by dark fermentation from Korean food waste due to a population shift to indigenous lactic acid bacteria and they prevented it by storing the feed at a low temperature (4 °C). Also, Xia et al. (2015); reported that under thermophilic conditions (50–80 °C), most mesophilic hydrogen consumers are inhibited, thereby improving the process’s stability and the efficiency of hydrogen fermentation.
3.2 Hydrogen purification from dark fermentation: membrane separation processes
Pure hydrogen is becoming increasingly important in many areas with consumption requirements (i.e., PEM fuel cells); therefore, the reason why it separates from different gas streams is fundamental. Currently, there are two mature technologies to separate hydrogen from different gas mixtures (i.e., hydrocarbons); pressure swing adsorption (PSA) and cryogenic distillation (Ashik et al. 2015; Ibeh et al. 2007). These technologies have been widely used in chemical and petrochemical industries, but they are energy-intensive and the cost associated with the process operation is generally high.
Alternatively, membrane separation processes have been considered as a promising technology. Low energy consumption, cost effectiveness at low gas volumes and continuous operation are some of its advantages (Ashik et al. 2015). However, the most relevant benefit of separating hydrogen using membrane technology is the ability to directly integrate the separation and production processes. In this case, membrane reactors (MR) can be designed and built, offering reduced capital costs (reduction of size) and improved selectivities and yields (Gallucci et al. 2013). Particularly, researchers have studied different configurations of these membrane reactors to improve the efficiency of the water–gas shift reaction (WGSR) process during hydrogen production at high temperatures and pressures via steam methane reforming (SMR) (Mendes et al. 2010). On the other hand, the separation of hydrogen generated during fermentation must be different because the biological process occurs close to the ambient conditions. In this case, the appropriate membranes must be compatible with the feed gas characteristics (i.e., materials resistant to impurities), cost-effective and able to be configured in a robust design.
Most of the membranes used for hydrogen separation from a H2/CO2 gas mixture during a thermo-catalytic process occurring at high temperatures (i.e., approximately 800 °C) consist of thermo stable inorganic materials (i.e., metallic membranes composed mainly of palladium) (Lukyanov et al. 2009). Although during a fermentative hydrogen production process the main gas products also correspond to CO2 and H2, the membrane systems for hydrogen separation must be different. The appropriate membrane systems could be made of materials commercially more attractive, having a low operation temperature and reasonable costs (i.e., polymers).
Recently, some hydrogen separation studies using membrane systems have been focused on developing new polymeric materials or modifying existing ones to improve the hydrogen selectivities (Qiao et al. 2015; Rabiee et al. 2014; Wang et al. 2013). However, most of the permeation tests have been carried out under ideal conditions using special modules and synthetic gas mixtures. Thus, the development of suitable membrane modules using such materials is crucial to accomplish an effective separation of a gas mixture product of a fermentative process. Two recent studies have tested commercially available membrane modules. Bakonyi et al. (2013a) demonstrated that a polyimide membrane module (UBE industries) exhibited potential for processing hydrogen containing biogas mixtures. In another study, Bakonyi et al. (2015) installed a Permselect® (PDMS) gas separation membrane to an anaerobic membrane bioreactor and tested its ability to separate hydrogen from the raw fermentation gaseous mixture. They obtained a final hydrogen composition of 67.3 vol%, corresponding to 30 % enrichment efficiency. Both contributions boost polymeric membranes to be considered as feasible options for in situ fermentative hydrogen recovery.
Most of the studies related to biological hydrogen separation/purification have been realized using polymeric membranes. Some of them have investigated the separation of synthetic mixtures composed of H2, N2 and CO2 to simulate the gas produced during biological processes. Conventional porous or non-porous membrane modules, membrane systems with moving CO2 liquid absorbents (contactors) and supported ionic liquid membranes (SILMs) have been studied (Liang et al. 2002; Gassanova et al. 2006; Bélafi-bakó et al. 2006; Bakonyi et al. 2012, 2013a, 2015; Ramírez-Morales et al. 2013). Bakonyi et al. (2013b) present a very complete overview of recent applications of these types of membranes for the separation of biological hydrogen, with an emphasis on the operational conditions affecting their performance.
Reported studies related to the integration of membrane systems directly to the production process, testing the performance under realistic gas compositions, have been rare. Bélafi-bakó et al. (2006) coupled two polymeric membrane modules to a fermentative hydrogen process carried out by Thermococcuslitoralis in a batch reactor. A final hydrogen concentration of 73 vol% was obtained after two-stage membrane modules made of polyethersulfone-polyimide (PES-PI) and highly dense polyethylene (HDPE), respectively. As mentioned above, Bakonyi et al. (2015) coupled a commercial module (PDMS) to separate the raw gas from the fermentation. Some approaches have also tried to use selective membranes to extract hydrogen from the fermentation and decrease the negative effect of the hydrogen partial pressure on the culture (Liang et al. 2002; Zheng et al. 2010). However, none of them have been carried out in a continuous mode and the final effects on fermentation have not been clarified.
Taking into consideration the gap that exists between experimental studies carried out in continuous systems, a new concept based on the integration of gas membranes and fermentation technologies has been proposed. Ramírez-Morales et al. (2013) called the new process a hydrogen-extractive membrane bioreactor (HEMB). The CO2 separated by an extractive membrane is continuously returned to the reactor, achieving a decrease in the overall hydrogen partial pressure. In addition, an enriched hydrogen stream could be obtained and further purified during the next stages (i.e., a multi-step membrane system). However, to achieve a proper implementation of both technologies, a correct selection of the membrane material, module configuration, and integrated bioprocess design (including an effective control strategy) is necessary.
Polymers can be a right choice as they can achieve significant hydrogen separation ability under non-extreme conditions (similar those happening during the bioprocess), presenting selectivities of H2/CO2 that range from 1.48 to 16 (Buonomenna and Bae 2015). However, conventional polymeric materials are limited by a trade-off between permeability and selectivity determined by an upper-bound relationship, as described by Robeson (2008). In this case, Robeson’s upper bound must be considered when selecting the membrane material because the separation factor/selectivity decreases with the increase in the permeability of the more permeable gas component. The membrane and module material also must manage with the range of impurities and chemical compositions of the feed gas. During the fermentation, non-desired substances can be produced, even at very low concentrations. Water vapor, siloxanes, H2S, CO and NH3 could cause corrosion and create resistance to the mass transfer phenomena through the membrane by providing support for biofilm formation. In the case of H2S, its presence may cause undesired changes in the polymer structure of the membrane affecting the separation ability and shortening the lifetime. In addition to CO2, at high pressures, H2S can also be a potentially plasticizing chemical in polymeric membranes, as it has a high penetrant solubility (Vaughn et al. 2012). Additionally, Scholes et al. (2010) found that the permeability of CO2 decreased when it was permeating simultaneously with H2S. The authors stated that this phenomenon was related to competitive sorption of both components into the polymeric matrix. Some solutions have been carried out to remove H2S from the raw feed gas. For example, it was proposed that membrane based biogas upgrading systems should separate simultaneously CO2/CH4 and H2S/CH4 using membranes based on glassy and rubbery polymers, respectively (Chen et al. 2015). In the case of fermentative hydrogen, a similar approach composed of two steps of membrane separation for desulfurization and upgrading can be implemented. Additional to the use of membrane modules for gas cleaning, other methods can also be used. Implementing an adsorption process using activated carbon/silica, absorption processes (using water or the proper chemicals) and condensation methods, such as cold traps, could prevent long-term drawbacks and improve the overall system operation.
Generally, there are three major module configurations for gas separation: flat sheet, spiral wound, and hollow fiber modules. For hydrogen separation applications, hollow fibers are preferable to the other two because they are easy to manufacture and provide a higher area per volume ratio. This high packing density is an advantage for membrane materials with high selectivity that present low permeability. Nevertheless, challenges to overcome when using this membrane module design involve minimizing some non-ideal effects, such as concentration polarization and pressure losses.
Finally, an integrated process control design is necessary. Coupling different equipment (i.e., compressors, pumps, sensors, condenser/cold traps) into the production process implies an increase in operational complexity. A suitable control strategy must be implemented to maintain the proper operational conditions of the production-separation system (i.e., proper pressure at the head space of the reactor, across the membrane and in the permeate/retentate streams) and address possible disturbances. In addition, multi-step and recycling designs in the membrane module configurations can be used for improving the overall efficiency and purification of the product (obtaining an acceptable purity and hydrogen recovery).
Additionally, some biological methods of biogas upgrading that are under evaluation can be applied to separate fermentative hydrogen. One of them is based on the use of the photosynthetic CO2 capture capacity of microalgae for biogas enrichment. In this case, methane and hydrogen produced during an integrated two-stage anaerobic process can be upgraded along the microalgae growth. Finally, the microalgal biomass generated can be then used as feedstock for biofuel production (Meier et al. 2015).
3.3 Dark fermentation by-products: biohydrogen and methane production by coupling dark fermentation and anaerobic digestion in two-stage anaerobic process
The microbial metabolites produced with the hydrogen in a dark fermentative process can be further converted into methane in strict anaerobic digestion bioprocesses. By producing methane from fermentative end products, the total energy recovery from the initial biomass is maximized and makes the dark fermentation process more industrially viable.
In addition, fermentative hydrogen production coupled with anaerobic digestion represents an interesting alternative to thermo-chemical processes by producing a defined mixture of H2/CH4, so-called Hythane®, that can be further used as biofuel. Hythane®, formally a mixture of hydrogen (5–20 %) and methane (80–95 %), is considered to be an environmentally friendly fuel (Fulton et al. 2010). Indeed, by adding a small percent of hydrogen to natural gas, the emission of combustion pollutants, such as carbon monoxide (CO), unburned hydrocarbons (HCs) and nitrogen oxides (NOx), is drastically reduced (Jamal and Wyszynska 1994; Varde and Frame 1984). Villante and Genovese (2012) made an exhaustive energetic and environmental sustainability analysis using a mixture of hydrogen and methane, called hydromethane. In their study, they considered all the main possible options available for its production and final applications. They concluded that hydromethane is not only efficient in terms of total energy recovery but also substantially reduces CO2 emissions and presents positive energy savings when used to fuel vehicles when compared to methane only. Moreover, using a hydrogen/methane mixture seems to be highly beneficial in high-temperature fuel cells because the overall efficiency increases and the thermal gradient decreases in the cell (Nikooyeh et al. 2007). For industrial applications, the reliability of this fuel has been already proved through the commercial exploitation of vehicle fuels in India (Das et al. 2000) and as an alternative for energy storage in Germany (De Saint Jean et al. 2014).
The integrated production of hydrogen and methane is carried out in a two-stage process, which consists of a fermentation reactor coupled with an anaerobic digestion reactor (Fig. 2). Usually, in industrial anaerobic digestion plants where hydrogen is not collected, the first step corresponds to an hydrolytic/acidogenic reactor where long chain polymers are hydrolyzed into shorter polymers and further converted to organic acids (Escamilla-Alvarado et al. 2014). The acids are then converted into methane in a second methanogenic stage (Willquist et al. 2012).
A two-stage process dedicated to the production of a mixture of hydrogen/methane may present the same advantages encountered when adding a hydrolytic step prior to methanogenesis. First, two-stage processes have been largely reported to improve the stability and the robustness of the methanogenic process and higher organic loading rates are achieved when compared to a traditional one stage methanogenic process (Ke and Shi 2005). Second, the physical separation existing between hydrogen and methane producing reactors makes possible the individual optimization of process parameters for maximizing and finely controlling the production of both gases. At the same time, the growth of hydrogenotrophic methanogenic archaea in hydrogen-producing reactors should be avoided. Consequently, yields and productivities of hydrogen and methane producing reactors vary greatly according to substrate characteristics, pH, temperature, HRT, OLR and the mode of operation (Cavinato et al. 2012).
A literature overview of the main operating conditions and the related yields and productivities obtained in two-stage hydrogen and methane processes is presented in Table 2. In dark fermentation, the hydraulic retention time (HRT) is generally maintained from 69 to 86 % lower than in the methanogenic step to prevent the development of methanogens. Using low HRT takes advantage of the slow growth of methanogens when compared to fermentative bacteria. In this context, methanogens are rapidly washed out in reactors operated continuously. Similarly, pH ranges between 4.9 and 6 are particularly adapted to hydrogen production. Such pH ranges not only favor the growth of acidogenic H2-producing microorganisms but are also unfavorable to methanogens. Interestingly, Guo et al. (2014b) reported a first stage fermenter operated at a pH of 7.5, but such high pH mainly favors methanogens contamination. The optimal pH range for methanogenic reactors is significantly higher, ranging between 6.8 and 8.3, and coupling dark fermentation and methanogenesis may require some pH adjustment. As an alternative, Liu et al. (2006) worked at a low pH of 5.5 and observed a significant and interesting methane yield (500 LCH4/kgVS) from OFMSW (Organic Fraction Municipal Solid Waste).
As shown in Table 2, the productivities in the first stage range between 10.0 and 51,324 LH2/kgVS/d while, in the second stage, productivities are ranging between 20.5 and 26,597 LCH4/kgVS/d. The best overall process performance, in terms of both H2 (147,300 L/kgVS/d) and CH4 (383,000 L/kgVS/d) yields, was obtained by Kobayashi et al. (2012) using food waste as substrate. These authors continuously operated two stirred tank reactors at a thermophilic temperature (55 °C) and with a working volume of 8 and 40 L in the first and second stage, respectively. A system of sludge recirculation from the second stage to the first one was used, including heat treatment of the sludge (100 °C for an hour) in the recycling loop, to kill methanogens before their addition into the hydrogen-producing reactor. Overall, it was concluded that sludge recirculation improved both hydrogen production and carbohydrate degradation when compared to a two-stage process with no sludge recirculation.
The third main advantage of coupling dark fermentation and anaerobic digestion concerns the total energy recovery, which is much higher than in the one-step system, regardless of the substrate and the temperature. Liu et al. (2006) showed a methane yield that was higher in a two stage process compared to a one stage process. They observed an increase of 21 % working in mesophilic conditions and using household solid waste as substrate. Luo et al. (2011) reported that their two-stage process produced 11 % more energy when compared to anaerobic digestion alone, using an industrial waste issued from the biodiesel industry (glycerol and rapeseed cake). Similarly, Nasr et al. (2012) observed an increase of 18.5 % in the total energy yield with a two-stage process using raw thin stillage as substrate in a reactor operated at mesophilic temperatures. Only one study reported no significant difference in total energy production between a two-stage process and one stage process (Schievano et al. 2012). These results were explained by the accumulation of undegraded intermediate metabolites during the methanogenic step.
In addition, Patterson et al. (2013) calculated the environmental burdens generated by single stage (CH4) and two-stage processes (H2/CH4), using a Life Cycle Assessment (LCA) approach in accordance with European guidance (Pottering and Necas 2009), and using two different feedstock (food waste and wheat feed) in comparison with fossil fuels (diesel). They found that using a two-step hydrogen-methane production process from food waste substantially reduces environmental burdens in terms of carcinogens and ecotoxicity when compared with the production of diesel. They also reported that a two-stage process using wheat straw increases energy outputs and reduces the environmental burdens compared to a single stage process (methane only).
Although the use of a two-step process presents several advantages, the main limitation of using such a system for long-term operations is the cost of maintenance and monitoring, and in particular the accumulation of nitrogen that can be detrimental to both processes. Ammonia inhibition of both hydrogen and methane production has been largely reported (Abouelenien et al. 2010; Walker et al. 2011; Rajagopal et al. 2013; Liu et al. 2014). Mainly, H2 and CH4 production can be inhibited at ammonia concentrations higher than 800 mg/L (Salerno et al. 2006; Nielsen and Angelidaki 2008). Moreover, protein degradation does not generate hydrogen in dark fermentation (Monlau et al. 2012; Guo et al. 2014a). When considering the use of protein-rich substrates, high quantities of ammonia can be released after the decomposition of proteins. Considering this, the use of sludge as an additive or as a sole substrate is not favorable to H2 production, and several other substrates should be considered with precaution, according to their ammonia content, such as food waste, OFMSW or agro-industrial waste (Kobayashi et al. 2012). Nevertheless, bacterial adaptation can occur in the presence of small and continuous amounts of ammonia. Velsen (1979) reported that after an initial adaptation of sewage sludge with ammonia concentrations of 815 mg/L, methane production was observed at high and inhibitory concentrations up to 5000 mg/L. However, in most of the cases, ammonia removal is necessary, especially when recycling a leachate or a liquid phase that tend to accumulate higher levels of ammonia. Several N removal processes can be applied, such as stripping (Serna-Maza et al. 2014), membrane separation using natural zeolites (Montalvo et al. 2012) or any microbial removal processes, i.e., Anamox (Shalini and Joseph 2013), nitrification/denitrification (Botheju et al. 2010), or nitritation/denitritation (Malamis et al. 2014). The most effective technique seems to be the stripping method, which consists of a physical separation process where ammonia is removed from the liquid phase by flushing a neutral gas. Liu et al. (2014) reported that more than 97 % of the ammonia was removed from pig manure at a temperature and pH of 36 °C and 12.4, respectively.
3.4 The energy efficiency of dark fermentation
The amount of COD in a form of H2 represents only a fraction of the total COD after dark fermentation. When considering the acetate pathway (max. 4 mol H2/mole Glc) or the ‘mixed cultures’ pathway (2.5 mol H2/mole Glc (Hawkes et al. 2007), the total amount of COD recovered in H2 from the fermentation process represents only a maximum of 33 and 21 % of the COD, respectively, as was mentioned above. By considering the calorific value of hydrogen and methane as 142 kJ/g hydrogen and 50 kJ/g methane, respectively, the total amount of energy in kJ recovered in a form of H2 counts for a maximum of 41 % (acetate pathway) and 27 % (mixed culture pathway) of the total energy for fully biodegradable substrate. Therefore, the hydrogen production efficiency as well as the downstream usage of the metabolites should be both evaluated from an energetic aspect and according to the initial substrate. Li et al. (2010) proposed to estimate the energy efficiency of the hydrogen production process with a ratio between the heat value issued from the amount of hydrogen produced and the intrinsic heat value of the substrate as expressed in Eq (1).
In this study, the authors calculated the energy efficiency recovered from different substrate (rice, potato, lettuce, lean meat, peanut oil and banyan leaves). The energy efficiency was found between 0 (banyan leaves, lean meat) up to 1.35 MJ kg−1VS (rice). However, most of the energy remains in form of microbial metabolites or undegraded substrate. Concerning the H2/CH4 production, the overall energy recovery yield achieved by the two coupled bioprocesses can be assessed by considering the H2 and CH4 calorific according to the amount of substrate added. Schievano et al. (2014) reported a partial contribution of the energy recovery of the hydrogen stage, estimated at 1.79 MJ kg−1VS added in comparison with the methane stage estimated at 12.34 MJ.kg-1VS added, i.e. 12.6 % of the total energy in form of H2, using manure and market biowaste as substrates. This study reported a total energy recovery of a single stage methane process (14.21 MJ kg−1VS added) slightly higher than the two-stage system (14.13 MJ kg−1VS added). Similarly, Monlau et al. (2015) reported no significant difference between the total energy produced in a one stage methane process (6.88 MJ kg−1VS added) and two-stage H2/CH4 process (7.09 MJ kg−1VS added) from wheat straw, when the process are coupled to an alkaline pretreatment. However, the energy recovery and, by extension, the benefit of coupling H2 and CH4 production is strongly dependent of the type of substrate. Nasr et al. (2012) compared the performance of single-stage and two-stage process in energy outcome using thin stillage as substrate. This study reported for one liter of substrate in a single-stage continuous flow anaerobic digestion generates 38.5 L of methane (1.38 MJ). In comparison, in a two-stage continuous-flow anaerobic digestion process this study observed a production of 19.5 L of hydrogen in the first stage and 38.7 L of methane which is represent to a total energy recovery of 1.64 MJ with an increase of 18.5 % in the energy yield. Luo et al. (2011) established a stable two-stage process with the organic loading rate at 4.5 gVS L−1 d−1 for increasing bioenergy production from organic wastes (stillage). The total energy recovery found in this study was 0.7 ± 0.07 MJ kg−1 for hydrogen production and 12.4 ± 0.51 MJ kg−1 for methane production which is 11 % higher than that in a single stage process (11.8 ± 0.49 MJ kg−1).
4 Pilot scale hydrogen production by dark fermentation
Although at the laboratory scale it is possible to understand the process, at the pilot scale it is necessary to define other variables that can affect the performances and to acquire as much experience as possible at this scale to solve specific problems, such as storing feedstock or maintaining anaerobic conditions, which are easily solved at the laboratory scale but are costly at the pilot scale (Lin et al. 2010, 2011). In Table 3, pilot-scale H2 fermentation experiences with conditions used and H2 production rates (HPR) of the process are arranged. As feedstock, sucrose-containing wastewaters and food waste have been mainly used, and the pilot fermenter size varies from 0.15 to 1.48 m3. To suppress the activity of indigenous non-H2-producers, such as lactic acid bacteria, food waste was sometimes fed after thermal shock (Lee and Chung 2010) or alkali-shock (Kim et al. 2010). Mostly, a completely stirred tank reactor (CSTR) mode was employed and the fermenter was operated under mesophilic conditions. The pH was controlled within the range of 4.5–6.5 by pumping an alkaline solution, directly increasing the pH of the feedstock (Ren et al. 2006), or recirculating the followed methane fermenter effluent (Cavinato et al. 2012). Compared to feeding solid-type biomass, shorter HRT (<1 d) was applied in the case of feeding liquid-type substrates. While the liquid-type substrate was continuously fed, solid-type substrate was only fed once or twice a day.
The highest HPR, 15.59 m3m−3d−1, was achieved by Lin et al. (2011) when sucrose was fed at a high organic loading rate of \(240\,{\text{kg}}_{\, \text{COD}} {\text{m}}^{ - 3} {\text{d}}^{ - 1}\). However, the obtained H2 yield was very low, less than 15 % of the theoretical value (\(4\,{\text{mol}}_{{\text{H}}_{2}} {\text{mol}}^{-1}{}_{\text{hexose}}\)). Moreover, the obtained HPR was less than one tenth of the highest lab-scale performance (Wu et al. 2006). In the pilot-scale experiment, the highest H2 yield of \(2.5{-}3.0\,{\text{mol}}_{{{\text{H}}_{2}}} / {\text{mol}}_{\text{hexose}}\) was obtained in the hyperthermophilic fermenter, which was inoculated with Clostridium saccharolyticus (Claassen and Vrije 2007) and is the only reported study that used a pure culture in a pilot-scale H2 fermenter. In other pilot-scale studies, the inoculum source was generally obtained from anaerobic digester sludge and compost.
In the case of using solid-type feedstock, the highest performance (5.4 m3m−3d−1 and \(2.4\,{\text{mol}}_{{\text{H}}_{2}} {\text{mol}}^{-1}{}_{\text{hexose}}\)) was obtained by Ueno et al. (2007). In terms of H2 yield, it seemed quite successful, which might be contributed to its operation under thermophilic conditions. Such temperatures can promote hydrolysis and simplify the microbial diversity favorable for H2 production (Shin et al. 2004). In the study of Ueno et al. (2007), the feedstock was composed of 20 % carbohydrates, which resulted in \(0.056\,{\text{L}}_{{{\text{H}}_{2}}} {\text{kg}}^{-1}{}_{\text{COD}}\). This meant that only 4 % of the energy content in the feedstock was converted to H2, considering that 1 kg COD is equivalent to \(1.4\,{\text{m}}^{3}{}_{{\text{H}}_{2}}\).
In scaling up H2 fermenters, agitation is an important issue for two reasons: (1) removing dissolved H2 from the broth and (2) and accurate pH control. The H2 in the liquid phase can inhibit hydrogenase activity (Kim et al. 2006), and pH control deviation should be minimal to warrant high H2 yields (Moon et al. 2015). In pilot-scale CSTR operations, the reported agitation speed ranged from 15 to 180 rpm, and this might enable a complete mixing up to the highest scale reported of 1.5 m3. However, complete mixing is not feasible in a real scale fermenter (>50 m3) without special design and operation. Therefore, it is absolutely necessary to focus on agitation and pH control at the pilot-scale. Interestingly, Jayalakshmi et al. (2009) invented an inclined plug-flow reactor, which has a 20° horizontal angle to facilitate the easy movement of solid waste within the fermenter. This would decrease the economic burden of the agitation, but may cause sudden drops of H2 production in the long run.
4.1 Example of a pilot-scale bio-hydrogen/methane fermentation system in Feng Chia University (Taiwan)
Recently, an advanced two-phase hydrogen and methane production system and its operational technology were established and named “Innovative Hydrogenation & Methanation Technology (HyMeTek)” by Feng Chia University (FCU), Taiwan. To commercialize this HyMeTek technology, a pilot scale system including two feedstock storage tanks for carbon sources (volume 0.75 m3 for each), two feedstock tanks for nutrient solution (0.75 m3 for each), a hydrogen production fermentor (0.4 m3) and a methane digestor (2.5 m3) were built at the FCU campus. The stainless H2 and CH4 bioreactors were designed by an up-flow anaerobic sludge blanket (UASB) model and equipped with a warm-water jacket for temperature control (35 °C). A system control panel was equipped for controlling the temperature, pH, a solenoid valve switch and the feedstock inflow rate. A maximum H2 production rate of 2.97 m3m−3d−1 with a H2 yield of \(1.5\,{\text{mol}}_{{\text{H}}_{2}} {\text{mol}}^{-1}{}_{\text{hexose}}\) were obtained at HRT 9 h from a food industrial wastewater (\(60\,{\text{g}}\,_{\text{COD}} {\text{L}}^{-1}\)). The CH4 digestor was fed with the H2 fermentor effluent and was operated at HRT 67 h with a maximum CH4 production rate of 0.86 m3m−3d−1and CH4 yield of \(27.56\,{\text{mL}}\,{\text{g}}^{-1}{}_{\text{COD}}\), using a NaOH solution for biogas purification. Hydrogen and methane biogases were mixed in a buffer tank. This buffer tank was used as a storage tank for the biogas mixture. A membrane bioreactor (2.5 m3) and a microalgae cultivation photobioreactor (1.0 m3) were combined at this pilot plant to expand the functions of cleaning the effluent to reach water quality standards and capture CO2 from hydrogen and methane tanks. Figure 3 shows the flow scheme for these zero carbon emission HyMeTek systems, including a green hydrogen gas station.
There are numerous ways to apply bioenergy bio-H2 and bio-CH4 as biogas fuels, bioelectricity and heat. Internal rate of return (IRR) had been employed with a bioH2fermentor of 50 m3 and a bioCH4 fermenter of 300 m3 to determine the economic benefit and biogas purification by chemical methods (Hsu et al. 2014). Biohydrogen and biomethane can be directly used to replace natural gas with carbon dioxide being recovered for other industries. As shown in Table 4, IRR was calculated as 32.47 %, showing that the two-phase biohydrogen and biomethane production system from condensed molasses can pay for itself within 3.19 years. Moreover, the commercialization potential (payback within 15 years) of a two-phase biogas production system was verified for sugary wastewater based on IRR analysis.
5 Future perspectives
Actually, hydrogen yield by dark fermentation and mixed cultures has a low yield (21 %), considering a process with an adequate commercial process yield (60–80 %). However, the use of waste or wastewaters improves the attractiveness of this process but the type of waste or wastewater depends on local availability. The solid state fermentation for hydrogen production is an interesting solution to upscale applications because of the lower water requirements as well as the use of smaller reactors, but this needs to be further studied.
In literature, some authors report low stability of the process and other authors report high stability. It is clear that changes of stability have a direct explanation to changes in operational parameters and these have been associated with changes in the microbial community.
Hydrogen purification of biogas is necessary for later use in fuel cells and adding other biological processes to improve the energetic efficiency of the system, such as anaerobic digestion, which generates methane, can increase the energy production of the system, energy efficiency of dark fermentation in a two-stage H2/CH4 compared to a single system of CH4 production depends on the substrate, but could increase by up to about 12 % more energy efficient hydrogen production stage. It is possible to separate the use of hydrogen and methane, but biomethane (the mixture of methane and hydrogen) could be a transit form of pure hydrogen in the near term, improving fuel efficiency.
Pilot systems needs to define other operational problems, such as agitation, that can affect the process, and focus research using this new operational parameters that can appear when producing hydrogen at a larger scale. Hydrogen- producing- Taiwan- plant, highlights that is important adding technologies as mentioned, in order to develop a sustainable system that is possible recovered the inversion in a few years (3.19 years).
6 Conclusion
Several factors that are crucial prior to scaling up the bioprocesses to improve hydrogen yields in dark fermentation processes have been discussed in this manuscript, such as (1) the use of an adequate treatment of the mix culture to remove hydrogen consumers and enrich specific hydrogen producers, which can be translated into improved stability (2) the choice substrate as a wastewaters or waste (3) the possibility of to develop a hydrogen producing solid state fermentation, (4) the integration of hydrogen purification as membrane separation and biological processes, and (5) the coupling of dark fermentation with other biological processes, such as anaerobic digestion (biothane). In conclusion, a deep knowledge about dark fermentation has been acquired in all of these domains, and to date, large-scale facilities are required to demonstrate the possibility of producing H2 stably and continuously in bioreactors operated in real industrial environments.
References
Abbassi-Guendouz A, Brockmann D, Trably E, Dumas C, Delgenes J-P, Steyer J-P, Escudie R (2012) Total solids content drives high solid anaerobic digestion via mass transfer limitation. Bioresour Technol 111:55–61. doi:10.1016/j.biortech.2012.01.174
Abouelenien F, Fujiwara W, Namba Y, Kosseva M, Nishio N, Nakashimada Y (2010) Improved methane fermentation of chicken manure via ammonia removal by biogas recycle. Bioresour Technol 101:6368–6373. doi:10.1016/j.biortech.2010.03.071
Andrews J, Shabani B (2012) Re-envisioning the role of hydrogen in a sustainable energy economy. Int J Hydrogen Energy 37:1184–1203. doi:10.1016/j.ijhydene.2011.09.137
Angenent LT, Karim K, Al-Dahhan MH, Domiguez-Espinosa R (2004) Production of bioenergy and biochemicals from industrial and agricultural wastewater. Trends Biotechnol 2:477–485. doi:10.1016/j.tibtech.2004.07.001
Antonopoulou G, Gavala HN, Skiadas IV, Angelopoulos K, Lyberatos G (2008) Biofuels generation from sweet sorghum: fermentative hydrogen production and anaerobic digestion of the remaining biomass. Bioresour Technol 99(1):110–119. doi:10.1016/j.biortech.2006.11.048
Ashik UPM, Wan Daud WMA, Abbas HF (2015) Production of greenhouse gas free hydrogen by thermocatalytic decomposition of methane: a review. Renew Sustain Energy Rev 44:221–256. doi:10.1016/j.rser.2014.12.025
Azbar N, Çetinkaya Dokgöz FT, Keskin T, Korkmaz KS, Syed HM (2009) Continuous fermentative hydrogen production from cheese whey wastewater under thermophilic anaerobic conditions. Int J Hydrogen Energy 34(17):7441–7447. doi:10.1016/j.ijhydene.2009.04.032
Bakonyi P, Nemestóthy N, Ramirez J, Ruiz-Filippi G, Bélafi-Bakó K (2012) Escherichia coli (XL1-BLUE) for continuous fermentation of bioH2 and its separation by polyimide membrane. Int J Hydrogen Energy 37:5623–5630. doi:10.1016/j.ijhydene.2012.01.009
Bakonyi P, Kumar G, Nemestóthy N, Lin CY, Bélafi-Bakó K (2013a) Biohydrogen purification using a commercial polyimide membrane module: studying the effects of some process variables. Int J Hydrogen Energy 38:15092–15099. doi:10.1016/j.ijhydene.2013.09.133
Bakonyi P, Nemestóthy N, Bélafi-Bakó K (2013b) Biohydrogen purification by membranes: an overview on the operational conditions affecting the performance of non-porous, polymeric and ionic liquid based gas separation membranes. Int J Hydrogen Energy 38:9673–9687. doi:10.1016/j.ijhydene.2013.05.158
Bakonyi P, Nemestóthy N, Simon V, Bélafi-Bakó K (2014) Review on the start-up experiences of continuous fermentative hydrogen producing bioreactors. Renew Sustain Energy Rev 40:806–813. doi:10.1016/j.rser.2014.08.014
Bakonyi P, Nemestóthy N, Lankó J, Rivera I, Buitrón G, Bélafi-Bakó K (2015) Simultaneous biohydrogen production and purification in a double-membrane bioreactor system. Int J Hydrogen Energy 40:1690–1697. doi:10.1016/j.ijhydene.2014.12.002
Bélafi-bakó K, Bucsu D, Pientka Z, Balint B, Herbel Z, Kovacs K, Wessling M (2006) Integration of biohydrogen fermentation and gas separation processes to recover and enrich hydrogen. Int J Hydrogen Energy 31:1490–1495. doi:10.1016/j.ijhydene.2006.06.022
Boboescu IZ, Ilie M, Gherman VD, Mirel I, Pap B, Negrea A, Maróti G (2014) Revealing the factors influencing a fermentative biohydrogen production process using industrial wastewater as fermentation substrate. Biotechnol Biofuels 7(1):139. doi:10.1186/s13068-014-0139-1
Bollon J, Benbelkacem H, Gourdon R, Buffiere P (2013) Measurement of diffusion coefficients in dry anaerobic digestion media. Chem Eng Sci 89:115–119. doi:10.1016/j.ces.2012.11.036
Botheju D, Svalheim Ø, Bakke R (2010) Digestate nitrification for nutrient recovery. Open Waste Manag J 3:1–12. doi:10.2174/1876400201003010001
Bundhoo MAZ, Mohee R, Hassan MA (2015) Effects of pre-treatment technologies on dark fermentative biohydrogen production: a review. J Environ Manag 157:20–48. doi:10.1016/j.jenvman.2015.04.006
Buonomenna MG, Bae J (2015) Membrane processes and renewable energies. Renew Sustain Energy Rev 43:1343–1398. doi:10.1016/j.rser.2014.11.091
Cai G, Jin B, Monis P, Saint C (2011) Metabolic flux network and analysis of fermentative hydrogen production. Biotechnol Adv 29(4):375–387. doi:10.1016/j.biotechadv.2011.02.001
Castelló E, García y Santos C, Iglesias T, Paolino G, Wenzel J, Borzacconi L, Etchebehere C (2009) Feasibility of biohydrogen production from cheese whey using a UASB reactor: links between microbial community and reactor performance. Int J Hydrogen Energy 34(14):5674–5682. doi:10.1016/j.ijhydene.2009.05.060
Cavinato C, Giuliano A, Bolzonella D, Pavan P, Cecchi F (2012) Bio-hythane production from food waste by dark fermentation coupled with anaerobic digestion process: a long-term pilot scale experience. Int J Hydrogen Energy 37:11549–11555. doi:10.1016/j.ijhydene.2012.03.065
Chang S, Li J, Liu F (2011) Evaluation of different pretreatment methods for preparing hydrogen-producing seed inocula from waste activated sludge. Renew Energy 36:1517–1522. doi:10.1016/j.renene.2010.11.023
Chaubey R, Sahu S, James OO, Maity S (2013) A review on development of industrial processes and emerging techniques for production of hydrogen from renewable and sustainable sources. Renew Sustain Energy Rev 23:443–462. doi:10.1016/j.rser.2013.02.019
Chen XY, Vinh-Thang H, Ramirez AA, Rodrigue D, Kaliaguine S (2015) Membrane gas separation technologies for biogas upgrading. RSC Adv 5:24399–24448. doi:10.1039/C5RA00666J
Cheng SS, Chao YC, Yang KH, Bai MD (2011) Process recovery of biohydrogenation in a pilot plant from methanogens invasion. Int J Hydrogen Energy 36:8779–8784. doi:10.1016/j.ijhydene.2010.09.066
Chong ML, Abdul Rahman NA, Rahim RA, Aziz SA, Shirai Y, Hassan MA (2009a) Optimization of biohydrogen production by Clostridium butyricum EB6 from palm oil mill effluent using response surface methodology. Int J Hydrogen Energy 34(17):7475–7482. doi:10.1016/j.ijhydene.2009.05.088
Chong M-L, Sabaratnam V, Shirai Y, Hassan MA (2009b) Biohydrogen production from biomass and industrial wastes by dark fermentation. Int J Hydrogen Energy 34(8):3277–3287. doi:10.1016/j.jenvman.2014.12.049
Claassen PAM, Vrije GJ (2007) Hydrogen from biomass. Agrotechnology and Food Sciences Group, member of Wageningen UR Web.www.biohydrogen.nl/…/public_report_bwpii_13030. Accessed 5 Feb 2015
Das D (2009) Advances in biohydrogen production processes: an approach towards commercialization. Int J Hydrogen Energy 34(17):7349–7357. doi:10.1016/j.ijhydene.2008.12.013
Das D, Veziroglu TN (2008) Advances in biological hydrogen production processes. Int J Hydrogen Energy 33(21):6046–6057. doi:10.1016/j.ijhydene.2008.07.098
Das D, Veziroǧlu TN (2001) Hydrogen production by biological processes: a survey of literature. Int J Hydrogen Energy 26(1):13–28. doi:10.1016/S0360-3199(00)00058-6
Das LM, Gulati R, Gupta PK (2000) A comparative evaluation of the performance characteristics of a spark ignition engine using hydrogen and compressed natural gas as alternative fuels. Int J Hydrogen Energy 25:783–793. doi:10.1016/S0360-3199(99)00103-2
De Baere L, Mattheeuws B, Velghe F (2010) State of the art of anaerobic digestion in Europe. In: Proceedings of the 12th international congress on anaerobic digestion. Guadalajara, IWA
de Sá Ribeiro Vasconcelos L, Corrêa de Oliveira T, Ferreira dos Santos T, Matos A, Christe Cammarota M, Morais Oliveira EM, Santana Ferreira-Leitão V (2011) Hydrogenase activity monitoring in the fermentative hydrogen production using heat pretreated sludge: a useful approach to evaluate bacterial communities performance. Int J Hydrogen Energy 36:7543–7549. doi:10.1016/j.ijhydene.2011.03.119
De Saint Jean M, Baurens P, Bouallou C (2014) Parametric study of an efficient renewable power-to-substitute-natural-gas process including high-temperature steam electrolysis. Int J Hydrogen Energy 39:17024–17039. doi:10.1016/j.ijhydene.2014.08.091
Demuez M, Cournac L, Guerrini O, Soucaille P, Girbal L (2007) Complete activity profile of Clostridium acetobutylicum [FeFe]-hydrogenase and kinetic parameters for endogenous redox partners. FEMS Microbiol Lett 275:113–121. doi:10.1111/j.1574-6968.2007.00868.x
Dutta S (2014) A review on production, storage of hydrogen and its utilization as an energy resource. J Ind Eng Chem 20:1148–1156. doi:10.1016/j.jiec.2013.07.037
Edwards PP, Kuznetsov VL, David WIF, Brandon NP (2008) Hydrogen and fuel cells: towards a sustainable energy future. Energy Policy 36:4356–4362. doi:10.1016/j.enpol.2008.09.036
Elsharnouby O, Hafez H, Nakhla G, El Naggar MH (2013) A critical literature review on biohydrogen production by pure cultures. Int J Hydrogen Energy 38:4945–4966. doi:10.1016/j.ijhydene.2013.02.032
Escamilla-Alvarado C, Ponce-Noyola MT, Poggi-Varaldo HM, Rios-Leal E, Garcia-Mena J, Rinderknecht-Seijas N (2014) Energy analysis of in-series biohydrogen and methane production from organic wastes. Int J Hydrogen Energy 39:16587–16594. doi:10.1016/j.ijhydene.2014.06.077
Fang HHP, Liu H (2002) Effect of pH on hydrogen production from glucose by a mixed culture. Bioresour Technol 82:87–93. doi:10.1016/S0960-8524(01)00110-9
Ferchichi M, Crabbe E, Gil GH, Hintz W, Almadidy A (2005) Influence of initial pH on hydrogen production from cheese whey. J Biotechnol 120(4):402–409. doi:10.1016/j.jbiotec.2005.05.017
Fernandes BS, Peixoto G, Albrecht FR, Saavedra del Aguila NK, Zaiat M (2010) Potential to produce biohydrogen from various wastewaters. Energy Sustain Dev 14(2):143–148. doi:10.1016/j.esd.2010.03.004
Fulton J, Marmaro R., Egan G. (2010) United States Patent system for producing a hydrogen. Patent No: US 7,721,682 B2. Date of Patent: 25 May 2010
Gallucci F, Fernandez E, Corengia P, van Sint Annaland M (2013) Recent advances on membranes and membrane reactors for hydrogen production. Chem Eng Sci 92:40–66. doi:10.1016/j.ces.2013.01.008
Garcia-Bernet D, Buffiere P, Latrille E, Steyer J-P, Escudie R (2011) Water distribution in biowastes and digestates of dry anaerobic digestion technology. Chem Eng J 172:924–928. doi:10.1016/j.cej.2011.07.003
Gassanova LG, Netrusov AI, Teplyakov VV, Modigell M (2006) Fuel gases from organic wastes using membrane bioreactors. Desalination 198:56–66. doi:10.1016/j.desal.2006.09.009
Ghimire A, Frunzoc L, Pirozzi F, Trably E, Escudie R, Lens PNL, Esposito G (2015) A review of dark fermentative biohydrogen production from organic biomass: process parameters and use of by-products. Appl Energy 144:73–95
Gómez X, Cuetos MJ, Prieto JI, Morán A (2009) Bio-hydrogen production from waste fermentation: mixing and static conditions. Renew Energy 34(4):970–975. doi:10.1016/j.renene.2008.08.011
Guo XM, Trably E, Latrille E, Carrère H, Steyer JP (2010) Hydrogen production from agricultural waste by dark fermentation: a review. Int J Hydrogen Energy 35(19):10660–10673. doi:10.1016/j.ijhydene.2010.03.008
Guo XM, Trably E, Latrille E, Carrere H, Steyer J-P (2014a) Predictive and explicative models of fermentative hydrogen production from solid organic waste: role of butyrate and lactate pathways. Int J Hydrogen Energy 39:7476–7485. doi:10.1016/j.ijhydene.2013.08.079
Guo YC, Dai Y, Bai YX, Li YH, Fan YT, Hou HW (2014b) Co-producing hydrogen and methane from higher concentration of corn stalk by combining hydrogen fermentation and anaerobic digestion. Int J Hydrogen Energy 39:14204–14211
Hallenbeck P, Abo-Hashesh M, Ghosh D (2012) Strategies for improving biological hydrogen production. Bioresour Technol 110:1–9. doi:10.1016/j.biortech.2012.01.103
Hawkes F, Hussy I, Kyazze G, Dinsdale R, Hawkes D (2007) Continuous dark fermentative hydrogen production by mesophilic microflora: principles and progress. Int J Hydrogen Energy 32(2):172–184. doi:10.1016/j.ijhydene.2006.08.014
Hernandez Sobrino F, Rodriguez Monroy C, Hernández Pérez JL (2010) Critical analysis on hydrogen as an alternative to fossil fuels and biofuels for vehicles in Europe. Renew Sustain Energy Rev 14:772–780. doi:10.1016/j.rser.2009.10.021
Hsiao CL, Chang JJ, Wu JH, Chin WC, Wen FS, Huang CC, Lin CY (2009) Clostridium strain co-cultures for biohydrogen production enhancement from condensed molasses fermentation solubles. Int J Hydrogen Energy 34(17):7173–7181. doi:10.1016/j.ijhydene.2009.06.028
Hsu CW, Li YC, Chu CY, Liu CM, Wu SY (2014) Feasibility evaluation of fermentative biomass-derived gas production from condensed molasses in a continuous two-stage system for commercialization. Int J Hydrogen Energy 39:19389–19393. doi:10.1016/j.ijhydene.2014.07.171
Hung CH, Cheng CH, Cheng LH, Liang CM, Lin CY (2008) Application of Clostridium-specific PCR primers on the analysis of dark fermentation hydrogen-producing bacterial community. Int J Hydrogen Energy 33(5):1586–1592. doi:10.1016/j.ijhydene.2007.09.037
Hussy I, Hawkes FR, Dinsdale R, Hawkes DL (2005) Continuous fermentative hydrogen production from sucrose and sugarbeet. Int J Hydrogen Energy 30(5):471–483. doi:10.1016/j.ijhydene.2004.04.003
Ibeh B, Gardner C, Ternan M (2007) Separation of hydrogen from a hydrogen/methane mixture using a PEM fuel cell. Int J Hydrogen Energy 32:908–914. doi:10.1016/j.ijhydene.2006.11.017
Jamal Y, Wyszynska M (1994) Onboard generation of hydrogen-rich gaseous fuels: a review. Int J Hydrogen Energy 19:557–572. doi:10.1016/0360-3199(94)90213-5
Jayalakshmi S, Joseph K, Sukumaran V (2009) Biohydrogen generation from kitchen waste in an inclined plug flow reactor. Int J Hydrogen Energy 34:8854–8858. doi:10.1016/j.ijhydene.2009.08.048
Jha AK, Li JZ, Nies L, Zhang LG (2011) Research advances in dry anaerobic digestion process of solid organic wastes. Afr J Biotechnol 10:14242–14253. doi:10.5897/AJB11.1277
Jo JH, Jeon CO, Lee DS, Park JM (2007) Process stability and microbial community structure in anaerobic hydrogen-producing microflora from food waste containing kimchi. J Biotechnol 131(3):300–308. doi:10.1016/j.jbiotec.2007.07.492
Jung KW, Kim DH, Shin HS (2010) Continuous fermentative hydrogen production from coffee drink manufacturing wastewater by applying UASB reactor. Int J Hydrogen Energy 35(24):13370–13378. doi:10.1016/j.ijhydene.2009.11.120
Karthikelan OP, Visvanethan C (2012) Bio-energy recovery from high-solid organic substrates by dry anaerobic bio-conversion processes: a review. Rev Environ Sci Bio/Technol 12:257–284. doi:10.1007/s11157-012-9304-9
Ke S, Shi Z (2005) Applications of two-phase anaerobic digestion for industrial wastewater treatment.pdf. Int J Environ Pollut 23:65–80. doi:10.1504/IJEP.2005.006396
Kim SH, Shin HS (2008) Effects of base-pretreatment on continuous enriched culture for hydrogen production from food waste. Int J Hydrogen Energy 33(19):5266–5274. doi:10.1016/j.ijhydene.2008.05.010
Kim DH, Han SK, Kim SH, Shin HS (2006) Effect of gas sparging on continuous fermentative hydrogen production. Int J Hydrogen Energy 31:2158–5169. doi:10.1016/j.ijhydene.2006.02.012
Kim DH, Kim SH, Kim KY, Shin HS (2010) Experience of pilot-scale hydrogen-producing anaerobic sequencing batch reactor (ASBR) treating food waste. Int J Hydrogen Energy 35:1590–1594. doi:10.1016/j.ijhydene.2009.12.041
Kobayashi T, Xu K-Q, Li Y-Y, Inamori Y (2012) Effect of sludge recirculation on characteristics of hydrogen production in a two-stage hydrogen–methane fermentation process treating food wastes. Int J Hydrogen Energy 37:5602–5611. doi:10.1016/j.ijhydene.2011.12.123
Kongjan P, Angelidaki I (2011) Performance and microbial community analysis of two-stage process with extreme thermophilic hydrogen and thermophilic methane production from hydrolysate in UASB reactors. Bioresour Technol 102(5):4028–4035. doi:10.1016/j.biortech.2010.12.009
Kothari R, Singh DP, Tyagi VV, Tyagi SK (2012) Fermentative hydrogen production: an alternative clean energy source. Renew Sustain Energy Rev 16:2337–2346. doi:10.1016/j.rser.2012.01.002
Kothari R, Pandey AK, Kumar S, Tyagi VV, Tyagi SK (2014) Different aspects of dry anaerobic digestion for bio-energy: an overview. Renew Sustain Energy Rev 39:174–195. doi:10.1016/j.rser.2014.07.011
Krupp M, Widmann R (2009) Biohydrogen production by dark fermentation: experiences of continuous operation in large lab scale. Int J Hydrogen Energy 34(10):4509–4516. doi:10.1016/j.ijhydene.2008.10.043
Kyazze G, Martinez-Perez N, Dinsdale R, Premier GC, Hawkes FR, Guwy AJ, Hawkes DL (2006) Influence of substrate concentration on the stability and yield of continuous biohydrogen production. Biotechnol Bioeng 93(5):971–979. doi:10.1002/bit.20802
Lay CH, Sung IY, Kumar G, Chu CC, Lin CY (2012) Optimizing biohydrogen production from mushroom cultivation waste using anaerobic mixed cultures. Int J Hydrogen Energy 37:16473–16478. doi:10.1016/j.ijhydene.2012.02.135
Lee YW, Chung J (2010) Bioproduction of hydrogen from food waste by pilot-scale combined hydrogen/methane fermentation. Int J Hydrogen Energy 35:11746–11755. doi:10.1016/j.ijhydene.2010.08.093
Levin D (2004) Biohydrogen production: prospects and limitations to practical application. Int J Hydrogen Energy 29(2):173–185. doi:10.1016/S0360-3199(03)00094-6
Li D, Yuan Z, Sun Y, Ma L, Li L (2010) Sequential anaerobic fermentative production of hydrogen and methane from organic fraction of municipal solid waste. Chin J Appl Environ Biol 2009:250–257. doi:10.3724/SP.J.1145.2009.00250
Liang TM, Cheng SS, Wu KL (2002) Behavioral study on hydrogen fermentation reactor installed with silicone rubber membrane. Int J Hydrogen Energy 27:1157–1165. doi:10.1016/S0360-3199(02)00099-X
Lin CY, Lee CY, Cheng Tseng I, Shiao IZ (2006) Biohydrogen production from sucrose using base-enriched anaerobic mixed microflora. Process Biochem 41(4):915–919. doi:10.1016/j.procbio.2005.10.010
Lin CY, Wu SY, Lin PJ, Chang JS, Hung CH, Lee KS, Lay CH, Chu CY, Cheng CH, Chang AC, Wu JH, Chang FY, Yang LH, Lee CW, Lin YC (2011) A pilot-scale high-rate biohydrogen production system with mixed microflora. Int J Hydrogen Energy 36:8758–8764. doi:10.1016/j.ijhydene.2010.07.115
Lin CY, Lay CH, Sen B, Chu CY, Kumar G, Chen CC, Chang JS (2012) Fermentative hydrogen production from wastewaters: a review and prognosis. Int J Hydrogen Energy 37(20):15632–15642. doi:10.1016/j.ijhydene.2012.02.072
Liu D, Liu D, Zeng RJ, Angelidaki I (2006) Hydrogen and methane production from household solid waste in the two-stage fermentation process. Water Res 40:2230–2236. doi:10.1016/j.watres.2006.03.029
Liu L, Pang C, Wu S, Dong R (2014) Optimization and evaluation of an air-recirculated stripping for ammonia removal from the anaerobic digestate of pig manure. Process Saf Environ Prot. doi:10.1016/j.psep.2014.08.006
Lukyanov BN, Andreev DV, Parmon VN (2009) Catalytic reactors with hydrogen membrane separation. Chem Eng J 154:258–266. doi:10.1016/j.cej.2009.04.023
Luo G, Xie L, Zhou Q, Angelidaki I (2011) Enhancement of bioenergy production from organic wastes by two-stage anaerobic hydrogen and methane production process. Bioresour Technol 102:8700–8706. doi:10.1016/j.biortech.2011.02.012
Malamis S, Katsou E, Di Fabio S, Bolzonella D, Fatone F (2014) Biological nutrients removal from the supernatant originating from the anaerobic digestion of the organic fraction of municipal solid waste. Crit Rev Biotechnol 34:244–257. doi:10.3109/07388551.2013.791246
Mangayil R, Karp M, Santala V (2012) Bioconversion of crude glycerol from biodiesel production to hydrogen. Int J Hydrogen Energy 37(17):12198–12204. doi:10.1016/j.ijhydene.2012.06.010
Mathews J, Wang G (2009) Metabolic pathway engineering for enhanced biohydrogen production. Int J Hydrogen Energy 34(17):7404–7416. doi:10.1016/j.ijhydene.2009.05.078
Meier L, Perez R, Azocar L, Rivas M, Jeison D (2015) Photosynthetic CO2 uptake by microalgae: an attractive tool for biogas upgrading. Biomass Bioenergy 73:102–109. doi:10.1016/j.biombioe.2014.10.032
Mendes D, Mendes A, Madeira LM, Iulianelli A, Sousa JM, Basile A (2010) The water-gas shift reaction: from conventional catalytic systems to Pd-based membrane reactors-a review. Asia Pac J Chem Eng 5:111–137. doi:10.1002/apj.364
Monlau F, Sambusiti C, Barakat A, Guo X-M, Latrille E, Trably E, Steyer J-P, Carrere H (2012) Predictive models of biohydrogen and biomethane production based on the compositional and structural features of lignocellulosic materials. Environ Sci Technol 46:12217–12225. doi:10.1021/es303132t
Monlau F, Kaparaju P, Trably E, Steyer JP, Carrere H (2015) Alkaline pretreatment to enhance one-stage CH4 and two-stage H2/CH4 production from sunflower stalks: mass, energy and economical balances. Chem Eng J 260:377–385. doi:10.1016/j.cej.2014.08.108
Montalvo S, Guerrero L, Borja R et al (2012) Application of natural zeolites in anaerobic digestion processes: a review. Appl Clay Sci 58:125–133. doi:10.1016/j.clay.2012.01.013
Moon C, Jang S, Yun YM, Lee MK, Kim DH, Kang WS, Kwak SS, Kim MS (2015) Effect of the accuracy of pH control on hydrogen fermentation. Bioresour Technol 179:595–601. doi:10.1016/j.biortech.2014.10.128
Motte JC, Trably E, Escudie R, Hamelin J, Steyer JP, Bernet N, Delgenes JP, Dumas C (2013) Total solids content: a key parameter of metabolic pathways in dry anaerobic digestion. Biotechnol Biofuels 6(1):164. doi:10.1186/1754-6834-6-164
Motte JC, Trably E, Hamelin J, Escudie R, Bonnafous A, Steyer JP, Dumas C (2014) Total solid content drives hydrogen production through microbial selection during thermophilic fermentation. Bioresour Technol 166:610–615. doi:10.1016/j.biortech.2014.05.078
Nasr N, Elbeshbishy E, Hafez H, Nakhla G, Hesham El Naggar M (2012) Comparative assessment of single-stage and two-stage anaerobic digestion for the treatment of thin stillage. Bioresour Technol 111:122–126. doi:10.1016/j.biortech.2012.02.019
Ngo TA, Kim MS, Sim SJ (2011) High-yield biohydrogen production from biodiesel manufacturing waste by Thermotoga neapolitana. Int J Hydrogen Energy 36(10):5836–5842. doi:10.1016/j.ijhydene.2010.11.057
Nielsen HB, Angelidaki I (2008) Strategies for optimizing recovery of the biogas process following ammonia inhibition. Bioresour Technol 99:7995–8001. doi:10.1016/j.biortech.2008.03.049
Nikooyeh K, Jeje AA, Hill JM (2007) 3D modeling of anode-supported planar SOFC with internal reforming of methane. J Power Sources 171:601–609. doi:10.1016/j.jpowsour.2007.07.003
Niu K, Zhang X, Tan WS, Zhu ML (2010) Characteristics of fermentative hydrogen production with KlebsiellapneumoniaeECU-15 isolated from anaerobic sewage sludge. Int J Hydrogen Energy 35:71–80. doi:10.1016/j.ijhydene.2009.10.071
Ntaikou I, Antonopoulou G, Lyberatos G (2010) Biohydrogen production from biomass and wastes vía dark fermentation: a review. Waste Biomass Valor 1:21–39. doi:10.1007/s12649-009-9001-2
Orecchini F (2006) The era of energy vectors. Int J Hydrogen Energy 31:1951–1954. doi:10.1016/j.ijhydene.2006.01.015
O-Thong S, Prasertsan P, Intrasungkha N, Dhamwichukorn S, Birkeland NK (2007) Improvement of biohydrogen production and treatment efficiency on palm oil mill effluent with nutrient supplementation at thermophilic condition using an anaerobic sequencing batch reactor. Enzyme Microbial Technology 41(5):583–590. doi:10.1016/j.enzmictec.2007.05.002
Park MJ, Jo JH, Park D, Lee DS, Park JM (2010) Comprehensive study on a two-stage anaerobic digestion process for the sequential production of hydrogen and methane from cost-effective molasses. Int J Hydrogen Energy 35:6194–6202. doi:10.1016/j.ijhydene.2010.03.135
Parthasarathy P, Narayanan KS (2014) Hydrogen production from steam gasification of biomass—influence of process parameters on hydrogen yield: a review. Renew Energy 66:570–579. doi:10.1016/j.renene.2013.12.025
Patterson T, Esteves S, Dinsdale R, Guwy A, Maddy J (2013) Life cycle assessment of biohydrogen and biomethane production and utilisation as a vehicle fuel. Bioresour Technol 131:235–245. doi:10.1016/j.biortech.2012.12.109
Pottering H-G, Necas P (2009) Promotion of the use of energy from renewable sources and amending and subsequently repleaing Directives 2001/77/EC and 2003/30/EC. Off J Eur Union 140:16–62
Qiao Z, Wang Z, Yuan S, Wang J, Wang S (2015) Preparation and characterization of small molecular amine modified PVAm membranes for CO2/H2 separation. J Membr Sci 475:290–302. doi:10.1016/j.memsci.2014.10.034
Quéméneur M, Hamelin J, Latrille E, Steyer JP, Trably E (2011) Functional versus phylogenetic fingerprint analyses for monitoring hydrogen-producing bacterial populations in dark fermentation cultures. Int J Hydrogen Energy 36(6):3870–3879. doi:10.1016/j.ijhydene.2010.12.100
Rabiee H, Soltanieh M, Mousavi SA (2014) Ghadimi A (2014) Improvement in CO2/H2 separation by fabrication of poly(ether-b-amide6)/glycerol triacetate gel membranes. J Membr Sci 469:43–58. doi:10.1016/j.memsci.2014.06.026
Rafrafi Y, Trably E, Hamelin J, Latrille E, Meynial-Salles I, Benomar S, Giudici-Orticoni M, Steyer JP (2013) Sub-dominant bacteria as keystone species in microbial communities producing bio-hydrogen. Int J Hydrogen Energy 38(12):4975–4985. doi:10.1016/j.ijhydene.2013.02.008
Rajagopal R, Massé DI, Singh G (2013) A critical review on inhibition of anaerobic digestion process by excess ammonia. Bioresour Technol 143:632–641. doi:10.1016/j.biortech.2013.06.030
Ramírez-Morales JE, Tapia-Venegas E, Nemestóthy N, Bakonyi P, Bélafi-Bakó K, Ruiz-Filippi G (2013) Evaluation of two gas membrane modules for fermentative hydrogen separation. Int J Hydrogen Energy 38:14042–14052. doi:10.1016/j.ijhydene.2013.08.092
Ramírez-Morales J, Tapia-Venegas E, Toledo-Alarcón J, Ruiz-Filippi G (2015) Simultaneous production and separation of biohydrogen in mixed culture systems by continuous dark fermentation. Water Sci Technol 71:1271–1285
Ren N, Li J, Li B, Wang Y, Liu S (2006) Biohydrogen production from molasses by anaerobic fermentation with a pilot-scale bioreactor system. Int J Hydrogen Energy 31:2147–2157. doi:10.1016/j.ijhydene.2006.02.011
Ren NQ, Tang J, Liu BF, Guo WQ (2010) Biological hydrogen production in continuous stirred tank reactor systems with suspended and attached microbial growth. Int J Hydrogen Energy 35:2807–2813. doi:10.1016/j.ijhydene.2009.05.010
Robeson LM (2008) The upper bound revisited. J Membr Sci 320:390–400. doi:10.1016/j.memsci.2008.04.030
Robledo-Narváez PN, Muñoz-Páez KM, Poggi-Varaldo HM, Ríos-Leal E, Calva-Calva G, Ortega-Clemente LA, Rinderknecht-Seijas N, Estrada-Vázquez C, Ponce-Noyola MT, Salazar-Montoya JA (2013) The influence of total solids content and initial pH on batch biohydrogen production by solid substrate fermentation of agroindustrial wastes. J Environ Manag 128:126–137. doi:10.1016/j.jenvman.2013.04.042
Saady NMC (2013) Homoacetogenesis during hydrogen production by mixed cultures dark fermentation: unresolved challenge. Int J Hydrogen Energy 38:13172–13191. doi:10.1016/j.ijhydene.2013.07.122
Salemme L, Simeone M, Chirone R, Salatino P (2014) Analysis of the energy efficiency of solar aided biomass gasification for pure hydrogen production. Int J Hydrogen Energy 39:14622–14632. doi:10.1016/j.ijhydene.2014.07.041
Salerno MB, Park W, Zuo Y, Logan BE (2006) Inhibition of biohydrogen production by ammonia. Water Res 40:1167–1172. doi:10.1016/j.watres.2006.01.024
Schievano A, Tenca A, Scaglia B, Merlino G, Rizzi A, Daffonchio D, Oberti R, Adani F (2012) Two stage versus single-stage thermophilic anaerobic digestion: comparison of energy production and biodegradation efficiencies. Environ Sci Technol 46:8502–8510. doi:10.1021/es301376n
Schievano A, Tenca A, Lonati S, Manzini E, Adani F (2014) Can two-stage instead of one-stage anaerobic digestion really increase energy recovery from biomass? Appl Energy 124:335–342. doi:10.1016/j.apenergy.2014.03.024
Scholes CA, Stevens GW, Kentish SE (2010) The effect of hydrogen sulfide, carbon monoxide and water on the performance of a PDMS membrane in carbon dioxide/nitrogen separation. J Membr Sci 350:189–199. doi:10.1016/j.memsci.2009.12.027
Serna-Maza A, Heaven S, Banks CJ (2014) Ammonia removal in food waste anaerobic digestion using a side-stream stripping process. Bioresour Technol 152:307–315. doi:10.1016/j.biortech.2013.10.093
Shalini SS, Joseph K (2013) Start-up of the SHARON and ANAMMOX process in landfill bioreactors using aerobic and anaerobic ammonium oxidising biomass. Bioresour Technol 149:474–485. doi:10.1016/j.biortech.2013.09.104
Shi XY, Jin DW, Sun QY, Li WW (2010) Optimization of conditions for hydrogen production from brewery wastewater by anaerobic sludge using desirability function approach. Renew Energy 35(7):1493–1498. doi:10.1016/j.renene.2010.01.003
Shin HS, Youn JH, Kim SH (2004) Hydrogen production from food waste in anaerobic mesophilic and thermophilicacidogenesis. Int J Hydrogen Energy 29:1355–1363. doi:10.1016/j.ijhydene.2003.09.011
Show KY, Lee DJ, Chang JS (2011) Bioreactor and process design for biohydrogen production. Bioresour Technol 102(18):8524–8533. doi:10.1016/j.biortech.2011.04.055
Show KY, Lee DJ, Tay JH, Lin CY, Chang JS (2012) Biohydrogen production: current perspectives and the way forward. Int J Hydrogen Energy 37:15616–15631. doi:10.1016/j.ijhydene.2012.04.109
Singh L, Wahid ZA (2015) Methods for enhancing bio-hydrogen production from biological process: a review. J Ind Eng Chem 21:70–80. doi:10.1016/j.jiec.2014.05.035
Siriwongrungson V, Zeng RJ, Angelidaki I (2007) Homoacetogenesis as the alternative pathway for H2 sink during thermophilic anaerobic degradation of butyrate under suppressed methanogenesis. Water Res 41:4204–4210. doi:10.1016/j.watres.2007.05.037
Sivaramakrishna D, Sreekanth D, Himabindu V, Anjaneyulu Y (2009) Biological hydrogen production from probiotic wastewater as substrate by selectively enriched anaerobic mixed microflora. Renew Energy 34(3):937–940. doi:10.1016/j.renene.2008.04.016
Tang GL, Huang J, Sun ZJ, Tang QQ, Yan CH, Liu GQ (2008) Biohydrogen production from cattle wastewater by enriched anaerobic mixed consortia: influence of fermentation temperature and pH. J Biosci Bioeng 106(1):80–87. doi:10.1263/jbb.106.80
Tenca A, Schievano A, Lonati S, Malagutti L, Oberti R, Adani F (2011) Looking for practical tools to achieve next-future applicability of dark fermentation to produce bio-hydrogen from organic materials in continuously stirred tank reactors. Bioresour Technol 102(17):7910–7916. doi:10.1016/j.biortech.2011.05.088
Traversi D, Villa S, Lorenzi E, Degan R, Gilli G (2012) Application of a real-time qPCR method to measure the methanogen concentration during anaerobic digestion as an indicator of biogas production capacity. J Environ Manag 111:173–177. doi:10.1016/j.jenvman.2012.07.021
Ueno Y, Fukui H, Goto M (2007) Operation of a two-stage fermentation process producing hydrogen and methane from organic waste. Environ Sci Technol 41:1413–1419. doi:10.1021/es062127f
Valdez-Vazquez I, Poggi-Varaldo HM (2009) Alkalinity and high total solids affecting H2 production from organic solid waste by anaerobic consortia. Int J Hydrogen Energy 34:3639–3646. doi:10.1016/j.ijhydene.2009.02.039
Vanginkel S, Oh S, Logan B (2005) Biohydrogen gas production from food processing and domestic wastewaters. Int J Hydrogen Energy 30(15):1535–1542. doi:10.1016/j.ijhydene.2004.09.017
Varde KS, Frame GM (1984) A study of combustion and engine performance using electronic hydrogen fuel injection. Int J Hydrogen Energy 9:327–332. doi:10.1016/0360-3199(84)90085-5
Vatsala T, Raj S, Manimaran A (2008) A pilot-scale study of biohydrogen production from distillery effluent using defined bacterial co-culture. Int J Hydrogen Energy 33(20):5404–5415. doi:10.1016/j.ijhydene.2008.07.015
Vaughn JT, Koros WJ, Johnson JR, Karvan O (2012) Effect of thermal annealing on a novel polyamide–imide polymer membrane for aggressive acid gas separations. J Membr Sci 401–402:163–174. doi:10.1016/j.memsci.2012.01.047
Vaxelaire J (2001) Moisture sorption characteristics of waste activated sludge. J Chem Technol Biotechnol 76:377–382. doi:10.1002/jctb.392
Velsen AFM (1979) Adaptation of methanogenic sludge to high ammonia-nitrogen concentrations. Water Res 13:995–999. doi:10.1016/0043-1354(79)90194-z
Venkata Mohan S, Lalit Babu V, Sarma PN (2007a) Anaerobic biohydrogen production from dairy wastewater treatment in sequencing batch reactor (AnSBR): effect of organic loading rate. Enzyme Microbial Technol 41(4):506–515. doi:10.1016/j.enzmictec.2007.04.007
Venkata Mohan S, Vijaya Bhaskar Y, Murali Krishna P, Chandrasekhara Rao N, Lalit Babu V, Sarma P (2007b) Biohydrogen production from chemical wastewater as substrate by selectively enriched anaerobic mixed consortia: influence of fermentation pH and substrate composition. Int J Hydrogen Energy 32(13):2286–2295. doi:10.1016/j.ijhydene.2007.03.015
Villante C, Genovese A (2012) Hydromethane: a bridge towards the hydrogen economy or an unsustainable promise? Int J Hydrogen Energy 37:11541–11548. doi:10.1016/j.ijhydene.2012.03.066
Walker M, Iyer K, Heaven S, Banks CJ (2011) Ammonia removal in anaerobic digestion by biogas stripping: an evaluation of process alternatives using a first order rate model based on experimental findings. Chem Eng J 178:138–145. doi:10.1016/j.cej.2011.10.027
Wang J, Wan W (2009) Factors influencing fermentative hydrogen production: a review. Int J Hydrogen Energy 34(2):799–811. doi:10.1016/j.ijhydene.2008.11.015
Wang X, Zhao Y (2009) A bench scale study of fermentative hydrogen and methane production from food waste in integrated two-stage process. Int J Hydrogen Energy 34:245–254. doi:10.1016/j.ijhydene.2008.09.100
Wang H, Paul DR, Chung TS (2013) Surface modification of polyimide membranes by diethylenetriamine (DETA) vapor for H2 purification and moisture effect on gas permeation. J Membr Sci 430:223–233. doi:10.1016/j.memsci.2012.12.008
Willquist K, Zeidan AA, Van Niel E (2010) Physiological characteristics of the extreme thermophile Caldicellulosiruptorsaccharolyticus: an efficient hydrogen cell factory. Microb Cell Fact 9:89. doi:10.1186/1475-2859-9-89
Willquist K, Nkemka VN, Svensson H, Pawar S, Ljunggren M, Karlsson H, Murto M, Hulteberg C, Niel EWJ, Liden G (2012) Design of a novel biohythane process with high H2 and CH4 production rates. Int J Hydrogen Energy 37:17749–17762. doi:10.1016/j.ijhydene.2012.08.092
Wong YM, Wu TY, Juan JC (2014) A review of sustainable hydrogen production using seed sludge via dark fermentation. Renew Sustain Energy Rev 34:471–482. doi:10.1016/j.rser.2014.03.008
Wu SY, Hung CH, Lin CN, Chen HW, Lee AS, Chang JS (2006) Fermentative hydrogen production and bacterial community structure in high-rate anaerobic bioreactors containing silicone-immobilized and self-flocculated sludge. Biotechnol Bioeng 93:934–946. doi:10.1002/bit.20800
Wu X, Zhu J, Dong C, Miller C, Li Y, Wang L, Yao W (2009) Continuous biohydrogen production from liquid swine manure supplemented with glucose using an anaerobic sequencing batch reactor. Int J Hydrogen Energy 34:6636–6645. doi:10.1016/j.ijhydene.2009.06.058
Xia A, Jacob A, Herrmann C, Tabassum MR, Murphy JD (2015) Production of hydrogen, ethanol and volatile fatty acids from the seaweed carbohydrate mannitol. Bioresour Technol 193:488–497. doi:10.1016/j.biortech.2015.06.130
Xiao J, Hay W, Wu TY, Juan JC (2013) Biohydrogen production through fermentation using waste as a future prospects of hydrogen usage. Biofuels Bioprod Biorefin. doi:10.1002/bbb
Yang H, Shao P, Lu T, Shen J, Wang D, Xu Z, Yuan X (2006) Continuous bio-hydrogen production from citric acid wastewater via facultative anaerobic bacteria. Int J Hydrogen Energy 31(10):1306–1313. doi:10.1016/j.ijhydene.2005.11.018
Yu H, Zhu Z, Hu W, Zhang H (2002) Hydrogen production from rice winery wastewater in an upflow anaerobic reactor by using mixed anaerobic cultures. Int J Hydrogen Energy 27:1359–1365. doi:10.1016/S0360-3199(02)00073-3
Zeidan A, Van Niel E (2010) A quantitative analysis of hydrogen production efficiency of the extreme thermophile Caldicellulosiruptor owen sensis OLT. Int J Hydrogen Energy 35(3):1128–1137. doi:10.1016/j.ijhydene.2009.11.082
Zhang J, Wang Q (2013) Buffering and nutrient effects of white mud from ammonia–soda process on thermophilic hydrogen fermentation from food waste. Int J Hydrogen Energy 38(31):13564–13571. doi:10.1016/j.ijhydene.2013.08.047
Zhang H, Bruns MA, Logan BE (2006) Biological hydrogen production by Clostridium acetobutylicum in an unsaturated flow reactor. Water Res 40:728–734. doi:10.1016/j.watres.2005.11.041
Zhao BH, Yue ZB, Zhao QB, Mu Y, Han-Qing Yua HQ, Haradab H, Li YY (2008) Optimization of hydrogen production in a granule-based UASB reactor. Int J Hydrogen Energy 33:2454–2461. doi:10.1016/j.ijhydene.2008.03.008
Zheng H, O’Sullivan C, Mereddy R, Zeng RJ, Duke M, Clarke WP (2010) Experimental and theoretical investigation of diffusion processes in a membrane anaerobic reactor for bio-hydrogen production. Int J Hydrogen Energy 35:5301–5311. doi:10.1016/j.ijhydene.2010.03.002
Acknowledgments
F.Paillet’s Ph.D. work was supported by the TRIFYL center, administrative department syndicate for treatment and valorization of municipal solid waste, the French Environment and Energy Management Agency (ADEME) and the French Institute for Agricultural and Food Research (INRA). A.Marone ‘s postdoctoral program was funded by the Marie Curie IntraEuropean Fellowship WASTE2BIOHY (FP7-MC-IEF-326974) under the 7th Framework Programme of the European Community. The work also beneficiated of the funds from the French-Chilean exchange program ECOS-CONICYT under the Project Nos. C12E06 (ECOMODH2), FONDECYT 1120659 and GRAIL 613667 (FP7-KBBE-2013).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Tapia-Venegas, E., Ramirez-Morales, J.E., Silva-Illanes, F. et al. Biohydrogen production by dark fermentation: scaling-up and technologies integration for a sustainable system. Rev Environ Sci Biotechnol 14, 761–785 (2015). https://doi.org/10.1007/s11157-015-9383-5
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11157-015-9383-5