Abstract
The production of biogas in landfills, its composition and the problems resulting from its generation are all reviewed. Biofiltration is a promising option for the control of emissions to atmosphere of the methane contained in biogas issued from the smaller and/or older landfills. A detailed review of the methane biofiltration literature is presented. The microorganisms, mainly the methanotrophs, involved in the methane biodegradation process, and their needs in terms of oxygen and carbon dioxide utilization, are described. Moreover, the influence of nutrients such as copper, nitrogen and phosphorus, and the process operating conditions such as temperature, pH and moisture content of the biofilter bed, are also presented. Finally, the performance of various filter beds, in terms of their elimination capacities, is presented for laboratory scale biofilters and landfill covers.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
Biogas results from the anaerobic degradation of organic wastes. Every year, thousands of tons of the greenhouse gas (GHG), methane (CH4), are produced in landfills, some of which escapes directly to the atmosphere. Even if GHG emissions associated with landfills represent only a small percentage (3.4% for Canada) of the national total of GHG emissions from all sectors, it is important to note that landfills generally constitute the most important sources of anthropogenic CH4. For example, in Canada and the United States, around 25% and 34% respectively, of the total methane emissions are directly related to landfill installations (Environnement Canada 2006; EPA 2006). About 10,000 landfills presently exist in Canada and the average waste production per inhabitant in year 2000 was 1,020 kg, of which some 73.2% was discarded to landfills. The wastes have generated in year 2001 GHG emissions, mainly in the form of CH4, at a level around 25 × 106 metric tons, when expressed as the carbon dioxide (CO2) equivalent (Environnement Canada 2006). The recent ratification by Canada of the Kyoto protocol forces this country, along with several others, to find new alternatives for the control of CH4 emissions. Indeed, Canada has committed itself to reduce its GHG emissions by 6%, compared to the 1990 level, during the period from 2008 to 2012, by targeting some particular gaseous compounds, such as CH4, for major attention (Kyoto protocol 1998).
Methane, as a GHG, is some 21–25 times more detrimental to the environment than CO2 and its lifespan in the atmosphere is ∼12 years (Hütsch et al. 1994; Goossens 1996; Hettiaratchi and Stein 2001; Kumar et al. 2004). Various technologies such as combustion can be used to control the CH4 emissions issued from landfills but, for the older and/or smaller landfills, traditional technologies are not very applicable and thus the biofiltration approach could be a promising solution. This process is one of the oldest of biotechnologies used in the treatment of polluted air. In the beginning, the process was employed only for the elimination of odors (March 1994). Thereafter, biofiltration, applied to contaminated air, proved to be also reliable for the elimination of volatile organic compounds (VOCs) and volatile inorganic compounds (VICs) (Jorio et al. 2003; Delhoménie and Heitz 2005).
The idea of using biofiltration for CH4 elimination derives from the fact that some bacterial species are able to degrade CH4 while generating oxidation by-products such as water (H2O), CO2, salts and biomass, all products much less harmful for the environment than the initial substrate. On an annual basis, at least 10–25% of the total CH4 emitted from landfills is oxidized by microorganisms (Nozhevnikova et al. 1993; Mancinelli 1995; Chanton and Liptay 2000; Christophersen et al. 2000; EPA 2005; Stralis-Pavese et al. 2006). Moreover, biofiltration creates environmental problems (such as CO2 production) to a lesser extent, in comparison with regular chemical oxidation processes. Also, biofiltration often offers the advantage of being performed at normal atmospheric pressure and temperature, thus resulting in lower ranges operational costs than traditional technologies (Ottengraf 1986).
2 Sanitary landfills
A sanitary landfill is an installation arranged to receive wastes and to retain the products of their decomposition so that they cease to constitute a threat for human or animal health (Popov 2005; Zamorano et al. 2006). Several types of landfills presently exist, some, known as closed-landfills, prevent the migration of liquid phase species from these sites towards the exterior environment. They are often used for the long-term storage of dangerous wastes. However, the majority of landfills are only partially closed, thereby allowing the collection and treatment of the leachate, or kept open, leading to the gradual migration and dispersal of the leachate within the immediate ecosystem (Warmer Bulletin 2000; Nikiema et al. 2004a; Zamorano et al. 2006). Sanitary landfills can receive and process, over the period of their active life, more than a million metric tons of wastes (Desideri et al. 2003; Zamorano et al. 2006; Spokas et al. 2006). For small cities and towns of less than 35,000 inhabitants, a municipal landfill of 20–30 m in depth is able to receive up to 200,000 m3 of waste during its lifetime and is classified as a small landfill (Börjesson et al. 2001; Park et al. 2004). The choice of a suitable site must be the subject of quite detailed attention. Factors commonly taken into account are; the long term availability of the site, which will be devoted to this exclusive use over a period of at least 30 years; its geological stability and characteristics. The site must also be of suitable size, and be located as far as possible from both residential and commercial areas, though remaining of easy access and servicing (Gielecki 1997).
Wastes, after their arrival on the site, are dehumidified if necessary, and moderately compacted, generally using bulldozers, to reduce their density to values bordering on 0.7–0.9 m3 per metric ton before storage (Warmer Bulletin 2000; Zamorano et al. 2006). At the end of each day’s operations, the densified wastes are covered with an inert layer: e.g. compacted mineral material, such as clay soil, of about 0.15 m height, in order to control the harmful effects of waste’s decomposition (such as odors) and losses, and to reduce the risk of fires. When an operational section of the site is completely filled, a final cover, composed of 0.6–1.0 m of clay and 0.2–0.6 m of soil, is applied to isolate it. The goal of this operation is thus to limit and even prevent the infiltration of H2O into the thus deposited wastes (Zamorano et al. 2006).
3 Biogas
3.1 Biogas composition
Once stored in landfills, wastes degrade biologically, thereby generating biogas (Popov 2005). This biogas contains mainly CH4, a colorless and odorless GHG, explosive when its concentration lies in the range 5–15% v/v in air (Perry et al. 1997; Tagaris et al. 2003), and CO2, able to cause respiratory problems when its concentration is greater than 0.5% for a prolonged exposure (Toutant 1994; Reginster 1999; Nikiema et al. 2004a). The CH4 concentrations in biogas, as mentioned in the literature, generally vary from 30 to 70% v/v while the CO2 concentration varies between 20 and 50% v/v (Humer and Lechner 1999b; Kallistova et al. 2005; Murphy and McCarthy 2005; Tsai 2006; Zamorano et al. 2006).
In the biogas, some sulfur compounds are present in small proportions (typically less than 0.2% v/v), such as hydrogen sulfide (H2S), mercaptans and thiols. These are responsible for the unpleasant odors that often emerge from poorly maintained landfills and can cause to humans and animals nausea, illness and in extreme cases death (Ma et al. 1996; Reginster 1999; Warmer Bulletin 2000). The biogas also generally contains some chlorinated compounds (less than 40 ppmv), among which are vinyl chloride, dichloromethane and tetrachloroethylene, all carcinogenic for humans and animals (Brosseau and Heitz 1994; Reginster 1999; Warmer Bulletin 2000; Scheutz et al. 2000; Zamorano et al. 2006).
Biogas can also contain trace amounts of various VOCs (less than 70 ppmv), such as benzene, a carcinogenic compound, toluene and the xylenes. Hydrogen (H2), a by-product of the waste decomposition, can also be found in biogas at small concentrations, < 0.2% v/v, along with nitrogen (< 5% v/v) and sometimes oxygen (O2) (< 1% v/v) (Reginster 1999; Warmer Bulletin 2000; ZWA 2006). Moreover, biogas is generally water saturated (Warmer Bulletin 2000; Spokas et al. 2006). Even when all of these compounds are found in biogas of various origins, their concentrations can be very variable and depend on the type of the stored waste and the age of the landfill. Table 1 presents typical concentrations for several compounds generally found in biogas.
3.2 Biogas production
One metric ton of municipal waste can generate between 135 and 375 m3 of biogas (Humer and Lechner 1999b; Warmer Bulletin 2000; Aye and Widjaya 2006; Murphy and McCarthy 2005; Zamorano et al. 2006). Many parameters influence the quantity and the rate of biogas production over time (Goossens 1996; Ozkaya et al. 2006). First, the age of the site is a determining factor in the production of biogas, due to commencement of waste decomposition, which can begin approximately 3 months after the waste storage installation and is subsequently spread over some 20–50 years (Bajic and Zeiss 2001; Zamorano et al. 2006). During the early years of a sanitary landfill’s life (when it is being established and filled), the rate of generated biogas released increases rapidly, from 0 to 11 m3 metric ton−1 year−1 (Reginster 1999; Kumar et al. 2004) and thereafter, a slow and continuous decline in the gas emission follows. After some 30–50 years, rates of biogas production become very low and almost cease (Reginster 1999).
The rate of biogas production also depends on the waste bed internal temperature and, to a lesser extent, on the external climatic conditions, such as the ambient temperature (Kumar et al. 2004). The optimal temperature for the production of biogas is 35–37°C (Kettunen and Rintala 1997). The lowering of the temperature to 24°C in a controlled environment, such as within a digester, causes a reduction in the rate of biogas production of ∼50% (Crill 1991; Nguyen et al. 2006). On the other hand, according to Chanton and Liptay (2000), variations in the production of biogas from an older landfill, as caused by seasonal temperature changes, are weak because the composting reactions of the organic wastes, located inside the deeper installed beds, ensures a near constant year round temperature of ∼50°C (Straka et al. 1999; Hudgins and Green 2000).
Another important parameter is the waste’s moisture content that should ideally remain between 50 and 60% wt/wt. This factor can be controlled during the wastes’ initial compaction, i.e. just before their placement in the long-term storage. The wetter the wastes, the greater their rate of degradation. However, a waste bed that is excessively wet (i.e. more than 65% wt/wt moisture content) may cause settlement in the site material and produces substantial amounts of leachate needing to be handled. On the other hand, when wastes are not wet enough (less than 30% wt/wt moisture content), they degrade more slowly because the microbial activity is inhibited. Therefore, it results in an increase of the lifespan of the wastes. However, the mechanical stability of the landfill is good, reducing the risk of safety hazards generation (Reinhart and Al-Yousfi 1996; Warmer Bulletin 2000; Hudgins and Green 2000).
The type of waste stored in the landfill can also influence both the composition and the quantities of the generated biogas produced. Organic wastes produce a biogas principally containing CH4 and CO2, in contrast to synthetic wastes that can be practically inert, like glass, or introduce into the biogas specific substances such as H2S, in the case of certain plastics degradation (Brosseau and Heitz 1994). Finally, the physical characteristics of the landfill, e.g. the bed depth, and its chemical characteristics, such as the pH, also play important roles in determining the production rate of the biogas. For maximum biogas production, the bed must be of sufficient depth to ensure that its interior regions provide for an anaerobic environment in which the relevant microorganisms can thrive, and the pH must also generally be close to neutral, i.e. between 6.8 and 7.2 (Yongzhi and Hu 2002; Kettunen and Rintala 1997).
3.3 Methane in the biogas
Methane, the atomically simplest and most stable hydrocarbon, is one of the important components in biogas. Its synthesis in organic waste beds is performed in three steps. Initially, polymers of the organic matter are hydrolyzed by the heterotrophic bacteria to form monomers. These molecules are then subject to fermentation which leads to the production of the organic and soluble products, composed mainly of acetates, formates and alcohols. By-products arising during this process step are CO2 and H2 (Le Mer and Roger 2001). These by-products are then converted to acetate in the presence of acetogenic bacteria, with simultaneous acidification, according to the following reaction:
All of these steps are strictly anaerobic. Acetate and other organic acids are then decomposed to CH4 and CO2 by the methanogenic microorganisms, all belonging to the domain Archaea (Hudgins and Green 2000; Le Mer and Roger 2001; Ozkaya et al. 2006). These microorganisms are strictly anaerobic (i.e. the tolerated dissolved oxygen concentrations do not excede the low micromolar range) and they are widely found in various environments such as anaerobic digestors, anoxic sediments, flooded soils and landfills. The acidification and methane generation steps are synchronized and mutualistic associations of microorganisms belonging to different genera are often observed at this late stage of methanogenesis, creating reciprocally favorable conditions, each moving the reaction equilibrium of the other in the most favorable direction (Whitman et al. 1999; Le Mer and Roger 2001).
3.4 Biogas valorization
Some landfills have active biogas collection systems (made as gas wells) but even in these cases, the quantities of recovered gases are usually only between 40% and 60% of the actually produced gas quantities (Humer and Lechner 1999a, b; Bajic and Zeiss 2001; Christophersen and Kjeldsen 2001; Popov 2005; Zamorano et al. 2006; Spokas et al. 2006). Newer more efficient techniques, including the use of synthetic cover materials, now allow for up to 90% gas collection effectiveness to be reached (Spokas et al. 2006). The biogas thus collected can subsequently be used in a variety of processes.
Combustion: This option is applicable only if the generated CH4 concentration in the biogas and the overall biogas quantities are important, i.e. more than 30% (which occurs during the first 25 years of the landfill) and 50 m3 h−1, respectively (Reginster 1999; Bajic and Zeiss 2001; Streese et al. 2001; Haubrichs and Widmann 2006). The calorific value of biogas is typically around 20,000 kJ m−3, i.e. about half that of the calorific value of natural gas and thus, the hot gases generated from biogas combustion can be best used as an energy source for the production of electricity and/or to generate hot water or steam (Goossens 1996; Desideri et al. 2003; Tsai 2006; Zamorano et al. 2006; Spokas et al. 2006). This valorization process allows at least, the partial meeting of the energy demand for the wastes processing site and for other clients located in its neighborhood. The investment cost required to install and operate such technology, considering a global collection and energy recovery efficiency of 50%, in a landfill, already equipped with biogas collection systems, is 3.1$ US/ton CO2 equivalent of CH4 eliminated (Ayalon et al. 2001). Estimates made by the Environmental Protection Agency (EPA) in 1996 indicated that the recovered energy from biogas, issued from the landfills across the whole USA, could be used to meet the needs of some 2.3 million homes (Goossens 1996). However, this solution is not universally economic at present because of the low cost of natural gas. Moreover, the addition of biogas to the natural gas network may deteriorate the quality and lifetime of the latter (Brosseau and Heitz 1994; Ewall 1999).
Other alternatives: A catalytic flow reversal reactor technology concept was developed by Natural Resources Canada (USDE 2005). The main goal of this process is the elimination of CH4 when its concentration in air lies between the values of 0.1–1% v/v. The methane is oxidized in a packed bed reactor, the exit product gases having a temperature ranging from 600 to 800°C. Heat can then be recovered from it, either to produce electricity or to satisfy various local heating needs. Another alternative for the CH4 content in biogas valorization consists of transforming this compound into methanol. This latter product can then be sold to chemical processors (Ewall 1999; Popov 2005).
3.5 Biogas elimination
Flaring: Sometimes, collected biogas is simply burned in flares. This CH4 elimination method is done with minimal facilities and without energy recuperation, the objective being to avoid the risk of explosion caused by the presence of CH4 in the air. However, this disposal method can be environmentally harmful, when dangerous compounds, such as dioxins, are generated during the combustion and are released to the atmosphere (Gielecki 1997; Jaffrin et al. 2003). Flaring of landfill biogas requires about 1.2 $ US/ton eq CO2 of CH4 eliminated (Ayalon et al. 2001). This treatment process can be used only when the amounts of biogas to be treated exceed 10–15 m3 h−1, while the biogas CH4 concentration remains greater than 20% v/v (Haubrichs and Widmann 2006).
Biological oxidation: Many landfill installations are, even today, still deprived of collection systems for the biogas produced. And even where such systems are in place, it is still difficult, and usually uneconomic, to utilize traditional valorization techniques for the older or smaller landfills (Bajic and Zeiss 2001). In these cases, other processes may need to be used to eliminate the dangers created by the CH4 presence in the atmosphere-released biogas. A possible solution is the use of biofiltration, a biological oxidation process. This idea comes from the fact that some bacteria are able to degrade air pollution compounds, such as CH4. This process already provides for the elimination of some 10–100% of the CH4 escaping from the upper layers of landfills, depending on local climatic conditions (Nozhevnikova et al. 1993; Kightley et al. 1995; Czepiel et al. 1996; Chanton et al. 1999; Christophersen et al. 2000; Bajic and Zeiss 2001; EPA 2005; Stralis-Pavese et al. 2006).
4 Methane biofiltration
4.1 Configuration
A biofilter is a three-phase bioreactor: the filter bed constitutes the solid phase, the biofilm, the liquid phase and the gaseous pollutants, the gas phase. Contact between the microorganisms and the polluting CH4 takes place in the biofilm, immobilized on the filter bed. The majority of biofilters, as used in lab-scale experiments, are closed systems. The air supply is ensured by a forced ventilation system. Gases circulation in the biofilter can be effected from either top to bottom or conversely. Closed biofilters are compact systems that can be assembled from several stages. Different performance parameters like Inlet load (IL), Elimination capacity (EC) and conversion (X) used in biofiltration are defined in Table 2. In a closed biofilter, maintaining the operational parameters unchanged is also a relatively easy practice, resulting in good performance, with CH4 X values as high as 90% (Dammann et al. 1999; Streese et al. 2001; Gebert et al. 2001; Du Plessis et al. 2003; Nikiema et al. 2005). The biofilter can also be an open system generally organized within the landfill covers. Usually, in this case, the flow of the polluted gas in the bed proceeds upwards, while the O2 diffuses from the ambient air into the bed (passive ventilation). The main disadvantage of this process lies in the difficulty of controlling the operational parameters, such as the temperature and moisture levels. Moreover, transfer of O2 to the bed’s lowest layers is a very important limiting factor for the overall performance (Kjeldsen et al. 1997; Gebert et al. 2001). For example, removal efficiencies of up to 60% can be obtained, when the empty bed residence times (EBRT) is at least an hour, with an open biofilter, installed on a landfill site (Du Plessis et al. 2003; Gebert and Groengroeft 2006a, b).
Laboratory-scale experiments, using a forced ventilation at the top of the biofilter in order to simulate the natural behavior of landfill covers, have been reported by several authors (Hilger et al. 2000a, b; Hettiaratchi and Stein 2001; Stein and Hettiaratchi 2001). The best EC obtained with this operational mode was achieved in the range of 325 and 400 g m−2 d−1 (Hettiaratchi and Stein 2001). The IL of CH4 is another important parameter. Various ILs have been tested at the laboratory scale and are reported in the literature, as presented in Table 3, ranging from 200 to 1700 g m−2 d−1. For an IL close to 300 g m−2 d−1, a conversion of 50% was obtained, as against 100% when the IL was only of 186 g m−2 d−1 (Hettiaratchi et al. 2000). An experiment reported by Humer and Lechner (1999b) on a sandy soil bed, showed the same tendency. However, according to Humer and Lechner (1999b), a flow rate of too low value could lead to poor performance if the filter bed porosity is not high enough.
In the case of methane biooxidation, EBRTs are typically between a few minutes to several hours, due to methane’s low-biodegradability (Dammann et al. 1999; Hettiaratchi and Stein 2001; Du Plessis et al. 2003; Nikiema et al. 2004b, 2005). In contrast, for VOCs and VICs biofiltration, EBRTs are in general, between 30 and 120 s (Jorio and Heitz 1999). The required operating volumes can reach as much as 100 times those used for treating the same amount of odors (Streese and Stegmann 2003). Indeed, the size of the biofilter should be at a scale of at least 1 m3 of filter bed for achieving flow rates of CH4 in the range of 0.01–2.5 m3 h−1 (Straka et al. 1999; Stresse and Stegmann 2003; Haubrichs and Widmann 2006). The height of the open biofilters with passive ventilation, used for CH4 elimination, must also be lower than 1 m (Kjeldsen et al. 1997; Boeckx and Van Cleemput 2000; Stein and Hettiaratchi 2001; Stein et al. 2001; Park et al. 2002; Tagaris et al. 2003). Open systems are usually less expensive, at least 15%, than closed systems. In 2001, for the non-easily degradable, volatile organic pollutants, the costs for the installation of open biofilters were between 0.25 and 0.4$ for each m3 d−1 of polluted gas to be treated (we assume this cost will probably be similar to that for CH4). In addition, the industry consensus on capital and operating costs must be considered, and recently, these costs were 0.5–1.8$ and 0.07–0.1$ per m3 d−1 of polluted gas, respectively (Janni et al. 2001).
4.2 Microorganisms
4.2.1 Methanotrophs
The specific bacteria responsible for the decomposition of CH4 are known as methanotrophs and constitute a sub-group of the methylotrophs, i.e. bacteria specialized in the degradation of those compounds having only one carbon atom. Earlier, methanotrophs were identified only according to their morphology, their intracytoplasmic membranes structure and some of their physiological characteristics. Since then, DNA analysis has aided the identification of the genera of methanotrophs (Hanson and Hanson 1996; Lidstrom 2001).
There are three basic steps in the decomposition of CH4. The first reaction step consists of the oxidation of CH4 to methanol, utilizing the enzyme MMO (Hanson and Hanson 1996; Auman and Lidstrom 2002). The methanol thus obtained is transformed into formaldehyde. The latter compound can be subsequently used in a dissimilatory pathway (i.e. being oxidized to CO2, with formate as an intermediate) or via several types of assimilatory pathways, leading to the synthesis of cell components, necessary for the growth of methanotrophs (Hanson and Hanson 1996).
The genera of methanotrophs are grouped into three main types. The genera Methylomonas, Methylomicrobium, Methylobacter, Methylocaldum, Methylophaga, Methylosarcina, Methylothermus, Methylohalobius and Methylosphaera belong to type I. They assimilate formaldehyde by the ribulose monophosphate pathway and their cellular membranes are mainly made up of fatty acids with 16, or sometimes 14 atoms of carbon (Hanson and Hanson 1996; Tsubota et al. 2005, Kalyuzhnaya et al. 2005; Heyer et al. 2005; Stralis-Pavese et al. 2006). Methylocystis, Methylocella, Methylocapsa and Methylosinus constitute the type II and they use the serine pathway for their formaldehyde assimilation. Their cellular membranes contain fatty acids of 18 carbons, arranged around the cell periphery (Hanson and Hanson 1996; Börjesson et al. 1998; Dedysh et al. 2000; Dedysh et al. 2002; Nikiema et al. 2005). Methylococcus, known as type X, combines the properties of types I and II i.e. fatty acids with 16 carbons and the assimilation of formaldehyde through both the ribulose monophosphate cycle and the serine pathway. The recently completed genomic sequence of Methylococcus capsulatus confirmed the presence of genes directing both pathways (Hanson and Hanson 1996; Wise et al. 1999; Kelly et al. 2005). Aerobic methanotrophic bacteria are essentially unable to grow on substrates containing C–C bonds as the only carbon source and thus can be considered as obligate C1 metabolizers. The genus Methylocella seems however to be an exception to this rule, being able to use compounds such as acetate, pyruvate, succinate, malate, and ethanol (Dedysh et al. 2005; Horz et al. 2005).
Methylococcus (type X), Methylothermus and Methylocaldum (type I) are moderately thermophilic and their optimal growth temperatures vary from 42°C, for the majority, to 62°C. Methylomonas, Methylobacter and Methylosphaera, all of type I, are psychrophilic, developing over a range of temperatures, from 5 to 15°C (Trotsenko and Khmelenina 2002). Methylobacter, type I bacteria, have an optimum growth temperature of around 6°C, while Methylosphaera develop better, between 10 and 13°C, in sea water (Berestovskaya et al. 2002). Mention is made that several methanotrophic communities have the capability of adapting to various temperatures, as long as these lie between 0 and 55°C. However, at temperatures lower than 0°C, the multiplication of the bacteria stops (Humer and Lechner 1999b). Methylocystis and Methylosinus, bacteria composing type II, are acidophilic. They exhibit a maximum growth rate in acidic media, in the pH range from 5 to 5.5. Methylomicrobium (type I bacteria) are distributed between the group of halophilic, being at ease in saline media having sodium chloride concentrations ranging from 0.5 to 5.6% wt/wt, and that of the alcaliphilic, for which the optimal pH ranges between 7.5 and 10 (Trotsenko and Khmelenina 2002).
4.2.1.1 Methane monooxygenase enzyme
A specific enzyme known as methane monooxygenase or MMO characterizes the methanotrophs. The MMO is the key enzyme allowing methanotrophs to perform the decomposition of CH4 (Hanson and Hanson 1996). This enzyme exists in two forms: particulate MMO (pMMO) and soluble MMO (sMMO). The pMMO enzyme can be both found in and synthesized by all methanotrophs, except Methylocella, but the sMMO is almost always present in bacteria of type II and X. However, some Methylomonas strains (type I), possessing the sMMO enzyme, have already been found (Auman and Lidstrom 2002).
It is known that methanotrophs containing pMMO (mainly type I) grow more rapidly and are more specific to CH4 than those having the sMMO (type II and X) (Henckel et al. 2000; Reay and Nedwell 2004). These differences are noticed when the CH4 concentration is lower than 1,000 ppmv of CH4 (Segers 1998). Thus, type I bacteria with pMMO develop quickly when the experimental conditions permit and become dominant in environments when such rapid growth is allowed (Henckel et al. 2000). However, they are sensitive to variations in nutrients availability, mainly the nitrogen and copper, and in the CH4 concentrations. On the other hand, populations of type II and X bacteria, having the sMMO, are quasi-steady and very stable in various environments, such as the landfill covers (Henckel et al. 2000; Crossman et al. 2004). In addition, sMMO also has affinities for a variety of compounds, such as methanol, several chlorinated compounds and hydrocarbons, among which are the alkanes, olefinic hydrocarbons and aromatic compounds (Hanson and Hanson 1996; Dunfield et al. 1999; Vorholt 2002; Hilger and Humer 2003; Erwin et al. 2005; Hesselsoe et al. 2005; Lindner et al. 2005).
4.2.1.2 Oxygen and carbon dioxide needs of methanotrophs
All of the methanotrophs species can be found in small quantities in any environments exposed simultaneously to significant amounts of CH4 and O2 (Börjesson et al. 1998; Dammann et al. 1999). For example, Methylomonas and Methylobacter (type I), Methylocystis and Methylosinus (type II) as well as Methylococcus (type X) have already been isolated from the cover soils of several landfills (Börjesson et al. 1998). However, the distribution of methanotrophs within a filtering material is not a random process since each type of bacteria develops preferentially in that portion offering the most advantageous conditions for its growth (Henckel et al. 2000; Gebert et al. 2003). An O2 concentration of 21% v/v, associated with a CH4 concentration less than 1,000 ppmv better supports the growth of type I bacteria. On the other hand, when the CH4 concentration is superior to 1% v/v and the concentration of O2 is low (about 1% v/v), type II bacteria develop better (Hanson and Hanson 1996; Henckel et al. 2000; Crossman et al. 2004). However, there are exceptions to this scheme and some type I bacteria have their growth stimulated only in the presence of an appreciable concentration of CH4 (> 1% v/v), and correspondingly, a low amount of O2 (< 1% v/v) (Henckel et al. 2000; Erwin et al. 2005). Bender and Conrad (1994), Czepiel et al. (1996) and Stein and Hettiaratchi (2001) have shown that, by increasing the O2 concentration from 3 to 20% v/v in the gas mixture, the CH4 conversion varies only slightly (less than 10%). However, a decrease of O2 concentrations from 3 to 1% causes the fall off of CH4 oxidation of more than 50%. However, during the experiments of Stein and Hettiaratchi (2001), the maximal CH4 elimination was obtained at O2 concentration between 0.75 and 1.6%.
The presence of CO2 in a biofilter at the same time as the CH4 modifies the behavior of the microorganisms present. According to Acha et al. (2002), the activity of the methanotrophs, using the serine pathway for the assimilation of formaldehyde obtained during the decomposition process of CH4, requires some CO2 input (partial pressure of CO2 around 11.6 kPa) (Acha et al. 2002).
4.2.2 Non-methanotrophic bacteria
Nitrifying bacteria, responsible for the decomposition of ammonia (NH3), can also degrade CH4, but their performance rate is less than 5% that of the pure methanotrophic populations (Hanson and Hanson 1996; Bodelier and Frenzel 1999). Also, some bacteria involved in the decomposition of methanol are capable of degrading CH4, but only if the CH4 concentrations remain below 10% v/v. The optimal growth temperature for these bacteria is around 35°C (Hughes et al. 2002). There are also certain anaerobic bacteria that are able to degrade CH4. Such bacteria are active when immersed in aqueous media. These bacteria work in tandem with those involved in reducing sulfates, the reaction requiring additional sources of carbon such as acetate or lactate (Hanson and Hanson 1996; Kotelnikova 2002; Valentine 2002). The minimal sulfate concentration in the system must be approximately 1 mmol l−1 (Segers 1998). The hypothesis of coupling between sulfate reduction and anaerobic methane oxidation is also supported by studies on a landfill-leachate plume (Grossman et al. 2002) and in ground water (Van Stempvoort et al. 2005). However, experiments to isolate these anaerobic bacteria remain unsuccessful to date (Conrad 1996; Segers 1998; Kotelnikova 2002). Recently, a microbial consortium has been isolated, found to be performing methane oxidation, coupled to nitrate reduction, in the absence of oxygen. The consortium includes two microorganisms: a bacterium and an archaeon, belonging to as yet unknown species (Raghoebarsing et al. 2006).
4.3 Inoculation and incubation
When contact is created between methanotrophs and CH4 in a biofilter, an induction step, during which X is weak (0–10% of the steady state conversion), always precedes the optimal system functioning. This lag phase is due to the activation and growth of the methanotrophic bacteria (Bender and Conrad 1995; Henckel et al. 2000) and its duration is determined by the operating conditions (CH4 concentration, temperature and moisture of the filter bed). During the experiments carried out by Henckel et al. (2000) in microcosms maintained under a CH4 continuous flow environment, some 6 and 19 days were required to reach steady X, respectively for high (10,000 ppmv) and low (1,000 ppmv) CH4 concentrations. In order to aid the establishment of the specific and competitive methanotrophic population in the filter bed, inoculation of the bed by selected methanotrophic bacteria is usually performed, even if the success of this practice is not guaranteed.
At the laboratory scale, another common practice involves incubation, consisting of a prolonged exposure (several days or weeks) of the filter bed to significant CH4 concentrations, ranging between 1,000 and 200,000 ppmv. The higher the CH4 concentration, the more the growth of the methanotrophs is promoted. The consequence then is a rapid increase in the oxidation rate (Bender and Conrad 1995; Hanson and Hanson 1996; Henckel et al. 2000; Le Mer and Roger 2001; Crossman et al. 2004; Mor et al. 2006). For example, the oxidation rate for a CH4 at initial concentration of 100,000 ppmv is around 0.8 g CH4 kg soil−1 d−1 which is 10 times higher than the value observed for a CH4 initial concentration of 10,000 ppmv (Bender and Conrad 1995). Since all bacteria do not develop within the same range of CH4 concentrations, the choice of the incubation parameters must be made judiciously. At the end of the induction phase, a peak value in the conversion up to 3 times that obtained for a steady operation (e.g. X = 64%) can be noted (Hettiaratchi and Stein 2001; Abichou et al. 2006a).
4.4 Parameters
4.4.1 Filter bed
The filter bed is the solid phase on which the biofilm containing the microorganisms is to be formed. It must present sufficient space for the development of microorganisms and it should also have a texture providing a great moisture-holding capacity, in addition to appropriate bacteriological and mechanical properties. It must also be inexpensive (Humer and Lechner 1999a, b; Bajic and Zeiss 2001; Nikiema et al. 2004b). Various experiments, conducted at the laboratory scale, have been performed to test various filter bed structures, using natural materials such as soils and composts or synthetic materials. The results obtained are presented in Table 3 and will be expressed in terms of the IL, EC and X. Composts of various origins (solid wastes, vegetable wastes, clarification sludges...) were tested during the CH4 biofiltration. Compost, made from mature yard wastes yielded the best results with EC up to 590 g m−2 d−1 and at values for X of between 90 and 100%, during more than 100 days of continuous filter operation (Haubrichs and Widmann 2006). Compost, made from dead leaves, also yielded good results (Hettiaratchi and Stein 2001; Wilshusen et al. 2004). In addition, the time required to reach 100%, conversion is less for the mature compost than that for freshly generated compost, being some 15 and 55 days respectively. This result makes the mature compost a preferred framework for the biofiltration of CH4 (Humer and Lechner 1999b).
The soils most often employed are those of landfills covers (Hettiaratchi et al. 2000; Hilger et al. 2000a), but agricultural soils, soils derived from mountains, forests and rice plantations, peat bogs and swamps, have also been tested in CH4 biofiltration (Dobbie and Smith 1996; Hütsch 1998b; Del Grosso et al. 2000; Hettiaratchi et al. 2000; Cai and Mosier 2000; Nozhevnikova et al. 2001; Stein and Hettiaratchi 2001; Novikov and Stepanov 2002; Kravchenko 2002). All of these soils contain different proportions of sand, clay, silica and organic matter. The most effective soils for CH4 elimination are those taken directly from the upper layers of landfill covers. An EC of 435 g m−2 d−1, corresponding to an X value of greater than 80%, has been reported in the literature (Park et al. 2002). The addition to a soil of organic residues, such as vegetable residues (beet leaves, wheat straw), clarification sludge or composts, can improve its CH4 elimination. The EC values, reported from these modifications (100–200 g m−2 d−1), correspond to some 40–100% of CH4 conversion, and remain below the EC obtained during similar experiments with compost-based beds (Börjesson et al. 1998; De Visscher et al. 1999; Humer and Lechner 1999b; Park et al. 2002). The mean size of the soil particles must preferably lie between 0.5 and 2 mm (Bender and Conrad 1995; Kightley et al. 1995; Börjesson et al. 1998; Hettiaratchi et al. 2000; Min et al. 2002). Indeed, when particle sizes are less than 0.02 mm, the bed tends to become packed, preventing the effective diffusion of pollutants in the gas phase and then negatively affecting the conversion (Bender and Conrad 1995; Le Mer and Roger 2001; Min et al. 2002).
With either synthetic or inert filter materials, a few interesting results were obtained during the CH4 biofiltration. An experiment, involving biofiltration by percolation with glass particles, has been reported (Sly et al. 1993). For a residence time of 20 min and an IL of around 200 g m−2 d−1, more than 95% of CH4 conversion was achieved. But the best EC reported in the literature is 700 g m−2 d−1, obtained by Nikiema et al. (2004b) during their experiments with an inorganic-packed bed biofilter of 0.018 m3, the gas flow rate being 6 m3 d−1 and the CH4 concentration maintained at between 7,000 and 7,500 ppmv.
4.4.2 Nutrients
Nutrients such as copper, nitrogen and phosphorus are strong determining factors for the success of CH4 biofiltration, since they are necessary for the growth of the microorganisms (Trotsenko and Khmelenina 2002). These nutrients, unless already present in the filter bed in a bioavailable form, must be added to the solution used to humidify the filter bed (Nikiema et al. 2005).
4.4.2.1 Copper
It has been shown that, while copper inhibits the sMMO enzyme at concentrations superior to 1μ mol l−1, it supports the synthesis of the pMMO for concentrations between 1 and 5μ mol l−1 (Hanson and Hanson 1996). Thus, by adjusting the bed copper concentration, it is possible, in various cases, to develop a medium rich in bacteria of types I or II (Wise et al. 1999; Erwin et al. 2005). It has also been noted that, in adding around 0.02 g of copper, in the form of CuCl2, per kg of paddy soil, CH4 oxidation is slightly stimulated (an increase of around 5%) (Mohanty et al. 2000).
4.4.2.2 Nitrogen compounds
Nitrogen element is an important nutrient for the methanotrophic bacteria. This element is usually provided to microorganisms in an inorganic form: e.g. nitrate (NO −3 ), ammonium (NH +4 ) or nitrite (NO −2 ) ions. Various tests have been performed and described in the literature to determine the influence of each of these compounds. Usually, they were undertaken with soils from various environmental sites, such as landfills, rice paddies, containing indigenous populations of microorganisms. The influence of NH +4 and NO −3 seems to be variable (Hütsch 1998a, b; Bodelier and Laanbroek 2004; Reay and Nedwell 2004). The sources of NH +4 most frequently tested are ammonium chloride, ammonium sulfate and urea. For NO −3 , sodium nitrate and potassium nitrate are the most studied. On some occasions, ammonium nitrate was used as a nitrogen source (Kightley et al. 1995; Hettiaratchi et al. 2000). Hettiaratchi et al. (2000) reported an example of improvement of CH4 elimination by some 100%, following the addition of nitrogen (25 mg N per kg soil) in the form of NH +4 or NO −3 . But, according to Chiemchaisri et al. (2001a), 30 mg N per kg soil or more, added in the form of NH +4 or NO −3 inhibit the CH4 elimination. In the case of NH +4 , many authors also report the risk of competition between CH4 and NH +4 when the latter was provided as a nitrogen source (Mancinelli 1995; Boeckx and Van Cleemput 1996; Humer and Lechner 1999b; Sitaula et al. 2000; Novikov and Stepanov 2002). Indeed, methanotrophs can convert NH +4 to NO −2 . During the experiments conducted by Novikov and Stepanov (2002), 12–28% of the methanotrophic population was dedicated to a nitrification step instead of the CH4 oxidation. In soils however, the decrease of CH4 elimination rate was observed only after the nitrogen concentration reached 10–200 mg N–NH +4 kg soil−1 (Bronson and Mosier 1994; Cai and Mosier 2000; Hettiaratchi et al. 2000; Novikov and Stepanov 2002; Park et al. 2004). But, the importance of this inhibition depends on the type of soil (Novikov and Stepanov 2002; Wang and Ineson 2003; Reay and Nedwell 2004) and can be further accentuated if other operating conditions, such as moisture content, are not satisfactory (Cai and Mosier 2000). Generally, an increase of the N–NH +4 concentration results in a higher percentage of inhibition at constant CH4 concentration. Conversely, an increase of CH4 concentration results in a lower percentage of inhibition at constant N–NH +4 content (De Visscher et al. 1999; Cai and Mosier 2000; Kravchenko 2002). Therefore, the inhibitory effect of NH +4 could be minimized if higher CH4 concentrations were continuously provided to the filter media.
For the case of equal nitrogen supply, NH +4 will be less inhibiting than NO −3 (Kravchenko 2002; Wang and Ineson 2003). But, according to Mancinelli (1995), NO −3 instead of NH +4 is the preferred source of fixed nitrogen for the methanotrophs. Le Mer and Roger (2001) stated that the presence of NO −3 can improve CH4 elimination. Potassium nitrate has been used for the culture of methanotrophs since 1970 as a component of the “nitrogen minimal salt” (NMS) nutrient solution, which includes 0.14 g of N–NO −3 per liter (Whittenbury et al. 1970). During experiments with an inorganic filter material, conducted by Nikiema et al. (2005), the authors noted that increasing nitrogen content supplied as sodium nitrate, from 0.14 to 0.75 g N l−1 in the nutrient solution, led to 5 times increase in the EC, from 130 to 700 g m−2 d−1. However, a further increase of nitrogen content (> 0.75 g N l−1) resulted in a decrease of the CH4 oxidation conversion. During other experiments in soils, variations in the nitrogen supply between 25 and 100 mg N–NO −3 kg soil−1 did not show any noticeable influence on the biological elimination of CH4 (Boeckx and Van Cleemput 1996; Park et al. 2002). A NO −3 inhibition in soils was reported for high concentrations of around 2,500 mg N kg soil−1 (Kumaraswamy et al. 2001).
Nitrite is well known as an inhibiting compound for methane elimination by methanotrophs (King and Schnell 1994; Mancinelli 1995; Boeckx and Van Cleemput 1996; Hanson and Hanson 1996). This compound can be generated when incomplete nitrification processes occurs in the filter media (Dunfield and Knowles 1995; Kravchenko 2002).
Sometimes, the inhibitory effect associated with the nitrogen content is otherwise caused by the salt effect. Indeed, the addition of salts containing inorganic nitrogen can change the overall ionic content of the soil (Hanson and Hanson 1996; King and Schnell 1998; Kravchenko 2002). Also, the influence of the nitrogen content is noticeable, especially in the case of low CH4 concentrations, less than 100 ppmv (King and Schnell 1994).
Finally, it is important to mention that some methanotrophs are capable of N2 fixation and express nitrogenase (Murrell and Dalton 1983; Kim and Graham 2001; Dedysh et al. 2002; Bodelier and Laanbroek 2004). Until recently, only Type II methanotrophs and Type X Methylococcus were thought to be capable of nitrogen fixation (Oakley and Murrell 1988; Dedysh et al. 2000). More recent work has revealed nitrogenase activity by the acetylene reduction route, and the presence of nifH genes generated by polymerase chain reaction amplification in a variety of methanotrophic species, belonging to both Types I and II (Auman and Lidstrom 2001; Boulygina et al. 2002). These data suggest that methanotrophs can play a significant role in nitrogen fixation in several natural environments, such as freshwater lakes (Zani et al. 2000). However the importance of N2 fixation during biofiltration of methane remains to be assessed.
4.4.2.3 Phosphorus
Generally speaking, phosphorus is of universal importance in promoting the growth of bacteria. However, despite its evident importance, it appears (from a close examination of the relevant literature) that only Kightley et al. (1995) have tried to obtain basic understanding of this element’s contribution to the CH4 biofiltration process. In their published studies, they have shown that the addition of a quantity of clarification sludge nutrient to an ordinary soil-based filter bed (final nutrient concentrations present in the soil: 0.1 g P per kg and 0.1 g N per kg) increased the rate of conversion of CH4 by ∼26%. On the other hand however, the addition of some 0.1 g of P–K2HPO4 nutrient per kg of the same soil did not result in any noticeable effect on promoting the CH4 elimination property of the soil (Kightley et al. 1995; Hettiaratchi et al. 2000; Le Mer and Roger 2001). Thus, the role and activity of phosphorus, in the above described circumstances, remains unclear and further investigations will therefore be required to elucidate the mechanisms leading eventually to either the promotion of the bacterial growth or to its inhibition.
4.4.2.4 Other elements
Potassium sulfate or manganese oxide increases the oxidation of CH4 (Kumaraswamy et al. 2001). Addition of lime provides a soil-based bed with a neutral pH and thus appears to be interesting for CH4 biofiltration (Hilger et al. 2000b). Excessive concentrations of sodium chloride and potassium chloride are both CH4 elimination inhibitors (Cai and Yan 1999; Kravchenko 2002; Gebert et al. 2003), probably due to their osmotic effects.
4.4.3 Operating conditions
4.4.3.1 Temperature
Methane oxidation is exothermic and, theoretically releases about 880 kJ per mole CH4. In case of bio-oxidation, the larger portion of this energy is used for the anabolic reactions during CH4 biodegradation. The other portion is transferred to both the filtering material and to the mixture of gases that traverses it. The reaction heat released creates a temperature gradient in the biofilter, between its lower and upper surfaces (Humer and Lechner 1999b; Nikiema et al. 2004b; Nikiema et al. 2005). The significance of this thermal gradient depends on the input gas flow rate, the conversion, the type of filtering material and various other influential parameters. For example, a temperature change of around 4°C is noted for an inlet gases flow rate of 3.6 m3 d−1, when the volumetric EC in a compost-based biofilter is 840 g CH4 m−3 d−1 (Streese et al. 2001). With an inorganic material, Nikiema et al. (2004b, 2005) did not observe any temperature gradient in the biofilter.
Tests on the influence of temperature during CH4 biofiltration were conducted with common filter materials, such as soils and composts. In general, the optimal bed temperature is usually found to lie between 29 and 30°C for composts (Dammann et al. 1999; Streese et al. 2001; Mor et al. 2006) and between 25 and 36°C for soils (Whalen et al. 1990; Bender and Conrad 1995; Hanson and Hanson 1996; Boeckx and Van Cleemput 1996; Visvanathan et al. 1999; Cai and Yan 1999; Christophersen et al. 2000; Min et al. 2002; Mingxing and Jing 2002; Park et al. 2004). Apart from these intervals, the decrease in the conversion was important. For example, it fell by around 50% when the temperature was reduced from 30 to 20°C or from 29 to 24°C (Dammann et al. 1999; Streese et al. 2001). Between −5 and 10°C as the ambient temperature, the biological elimination of CH4 in an opened biofilter system (landfill cover soil) is considerably decreased, i.e. more than 80% compared to the value at 15°C (Christophersen et al. 2000; Le Mer and Roger 2001). Therefore, the influence of temperature on the biological process constitutes the major limit for open biofilters, mainly during the winter season, when temperature falls to values lower than the limit that can be tolerated by the microorganisms consuming the CH4 (Humer and Lechner 1999b).
On the other hand, if higher temperatures (>35°C) stimulate the activity of some methanotrophs, it should be noted that in such cases, the biofilter beds dry more quickly; this in turn leading to a decrease in the conversion rate (Visvanathan et al. 1999).
4.4.3.2 pH of the filter bed
From a practical viewpoint, the pH of the filter bed is a parameter of lesser importance because the biodegradation of CH4 does not generate intermediate or final products capable of influencing significantly the pH. The optimal pH values for the oxidation of CH4 are in fact the same as those promoting the growth in the majority of methanotrophs bacteria. These are, in general, neutrophiles but they can, according to Hanson and Hanson (1996), tolerate pH values between 5.5 and 8.5. However, abrupt variations in the pH are adverse to methane elimination. A permanent inhibition was noted when the pH of the soil was changed by around 2 units, from 6.8 to 4.7 or from 6.8 to 9.0. This inhibition was partial for a unit variation, from 6.8 to 5.9 or 6.8 to 7.7, over the same operating conditions. This observation brought these present authors to propose a more restricted range of operating pH values, being that from 5.9 to 7.7 (Arif et al. 1996). In soil-based filter beds, the optimum pH ranges between the values of 6.7 and 8.1 (Bender and Conrad 1995) while for peat, the range lies between 5 and 6.5 (Le Mer and Roger 2001).
4.4.3.3 Filter bed moisture
The filter bed moisture content is another key factor that determines the performance of the biofilter (Börjesson et al. 1998). When the moisture is too high, it acts as a rate-limiting factor by preventing the flow and transfer of CH4 and O2 (Humer and Lechner 1999b; Cai and Yan 1999; McLain 2000; Mingxing and Jing 2002; McLain et al. 2002; Park et al. 2002). The optimal filter bed water content depends on both the gas flow rate and the type of filter bed (soil, compost or other material employed) (Christophersen et al. 2000). Table 4 presents some typical water contents suggested in the literature. Optimal moisture content of soil materials (from the upper layers of landfills) ideally lies between 13 and 15.5% wt/wt, on a dry basis (Whalen and Reeburgh 1996; Boeckx and Van Cleemput 1996; Chiemchaisri et al. 2001b; Stein and Hettiaratchi 2001; Jäckel et al. 2001; Park et al. 2002, 2004). However, Giani et al. (2002) reported a case for which the optimal moisture content of the landfill cover soil, used for biofiltration, was 25–30% wt/wt on a dry basis (at moisture values lower than 15%, the EC of CH4 was reduced by 50% or more, compared to the maximal value). For composts or biological residues, optimal bed moisture lies between 25% and 50% wt/wt (Humer and Lechner 1999b). Methane conversion levels in soils that are less wet than the optimum level are lower than those attained in greater moisture content soils (Boeckx and Van Cleemput 1996; Cai and Yan 1999; Stein and Hettiaratchi 2001). Indeed, for a moisture content of around 745 g kg paddy soil−1, i.e. approximately 265% of the optimal moisture (280 g kg paddy soil−1), the conversion was only 24% of the maximum conversion. When the moisture content of the same material was changed to around 150 g kg soil−1, the conversion fell to only 1% of that of the soil at its optimal moisture conversion (Cai and Yan 1999).
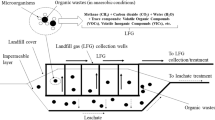
5 Landfill covers
Open biofilters are an attractive alternative for the older or smaller landfills, when gas collection systems cannot be installed for biogas valorization or elimination (Du Plessis et al. 2003; Berger et al. 2005). To our knowledge, there are no industrial applications related to the CH4 biofiltration process in North America at the present time. However, at least the subjects of 3 patents registered worldwide, are more or less related to landfill biogas treatment, using in situ filters (Bergmann et al. 1998; Lee et al. 2002; Contec and Landkeis 2004). Landfill covers that permit a natural biological elimination of CH4 could be considered as natural open biofilters. These covers are usually made of soils, sand or clay and represent the daily and final cover of the wastes in the landfill. Methane elimination in such covers is caused by the presence of methanotrophic populations. The behavior of a landfill cover is similar to that of an open biofilter equipped with passive aeration, except that the IL is usually low. Indeed, the mean IL of CH4 in landfills covers generally lie between 50 and 340 g CH4 m−2 d−1 (Jones and Nedwell 1993; Bogner et al. 1997; Humer and Lechner 2001; Perera et al. 2002; De Visscher and Van Cleemput 2003; Park et al. 2004; Gebert and Groengroeft 2006a; Abichou et al. 2006a, b). Börjesson and Svensson (1997) have noted that diurnal CH4 emissions are up to 100% higher than the daily values, depending on the ambient temperature and air pressure. Also, CH4 fluxes are themselves very variable and are usually not evenly distributed (Börjesson et al. 1998; Segers 1998; Gebert et al. 2001; Gebert and Groengroeft 2006a). Important rates of irrigation of the biofilter bed by rain may cause a decrease in the EC, up to 40%, by preventing the flow of biogas (Berger et al. 2005; Horz et al. 2005). On the other hand, even with a well constructed collection system, leaks always exist in such landfill covers, leading to the development of very important levels of emission in certain zones, up to 9,000 g CH4 m−2 d−1 (Maurice et al. 1999; Chanton and Liptay 2000; Bajic and Zeiss 2001; Spokas et al. 2006). The covering of the whole landfill with a 0.1–0.6 m layer of mulch or compost helps to avoid uncontrolled CH4 emissions from older landfills, when IL < 90 g m−2 d−1 (Chanton and Liptay 2000; Mor et al. 2006).
The performance of the landfill cover in the treatment of CH4 is influenced by two main parameters: the temperature and the available oxygen concentration. During winter, the CH4 conversion within landfill covers is reduced to around 3–10% (Chanton et al. 1999; Chanton and Liptay 2000; Giani et al. 2002; Spokas et al. 2006). However, at an ambient temperature of 2°C, Christophersen et al. (2000) have noted that it was still possible to biodegrade all of the CH4 produced in older landfills if IL is inferior to 70 g m−2 d−1. Indeed, the microbial activity, combined with the isolation effect of the bed, contributes to keeping the inner bed layer at temperatures 5–8°C higher than the ambient temperature (Berger et al. 2005). In summer, the CH4 conversion can reach 50% or more (Börjesson et al. 1998; Chanton et al. 1999; Chanton and Liptay 2000; Perera et al. 2002; Spokas et al. 2006). On the other hand, the diffusion of atmospheric O2 is limited and generally, an oxygenated zone of only 0.6–0.8 m is observed (Nozhevnikova et al. 1993; Börjesson and Svenson 1997; Klusman and Dick 2000; Christophersen and Kjeldsen 2001; Chiemchaisri et al. 2001b; Perera et al. 2002; Tagaris et al. 2003; Crossman et al. 2004; Kallistova et al. 2005).
The landfill cover height must be at least 0.7 m for achieving best results (Giani et al. 2002). In order to reduce the influence of temperature, and the problems related to O2 diffusion, on the landfill covers and also for open biofilters, many authors have favored the use of multi-layer beds (Bajic and Zeiss 2001; Streese and Stegmann 2003; Berger et al. 2005). For example, at the lowest bed level (0.25–0.9 m above entry point), a material, with the mean porosity such as soils or sand, is provided. This layer is employed for the retention of the filter bed humidity, in order to avoid quick bed drying events. The most important part of the overall CH4 elimination process (typically 60%) will take place in the second layer, made of compost, for example (Bajic and Zeiss 2001; Berger et al. 2005). On a landfill site, the use of composts of ∼0.3–0.6 m deep, instead of soils as an oxidation layer, can double the overall CH4 elimination because of the availability of nutrients for the bacteria, while the higher porosity level leads in turn to a more satisfactory diffusion of the O2 uptake (Hilger and Humer 2003). A third layer may be used at the top of the biofilter as a heat retention blanket, which will provide a particularly important practical feature to the biofilter when the atmospheric temperature falls during the winter season (Straka et al. 1999; Kallistova et al. 2005).
6 Conclusion
An important source of GHG emissions is that related to methane contained in biogas and released from sanitary landfills. In the present paper, a brief review of the composition, the production and valorization of the biogas is described. When this valorization is not possible, an alternative treatment lies in the biofiltration remediation of CH4 emissions, particularly from older and smaller landfills. The main part of this paper focuses on this biotechnology.
The biofilter can be either an open or a closed system, equipped with either an active or a passive oxygen feed system. The use of open systems, whilst being more financially interesting, can also permit methane conversions of 60% and even more in specific cases, even if control of the process operational laboratory scale parameters is barely feasible. But, to our knowledge, there is no application in North America for this landfill technology. However, landfill covers play the role of a natural biofilter and eliminate up to 320 g CH4 m−2 m d−1 On the other hand, closed systems are often more compact and provide for the better management and control of the operational parameters. The bed volume required for CH4 control in a biofilter is at least 1 m3 bed for a CH4 gas flow in the range of 0.01–2.5 m3 h−1.
The majority of authors appear to agree on the point that matured compost constitutes a satisfactory filter material for supporting the biofiltration of CH4. Indeed, both the presence of nutrients in the compost, in addition to its physical properties supports the growth of methanotrophs. The filter bed optimal temperature appears to lie between 29 and 30°C, and its optimal moisture level is found to lie between 25% and 50% wt/wt, on a wet basis. However, interesting results could also be obtained when using an inorganic-based bed biofilter.
Methane biofiltration is, at the same time, both a simple and a complex process. Indeed, even if the overall phenomenon of the reaction seems to be well known, many aspects are still misunderstood and contradictory theories are proposed, especially in relation to reaction optimization like nutrients, for long-term operations.
References
Abichou T, Chanton J, Powelson D, Fleiger J, Escoriaza S, Yuan L, Stern J (2006a) Methane flux and oxidation at two types of intermediate landfill covers. Waste Manag 26(11):1305–1312
Abichou T, Powelson D, Chanton J, Escoriaza S, Stern J (2006b) Characterization of methane flux and oxidation at a solid waste landfill. J Environ Eng 132(2):220–228
Acha V, Alba J, Thalasso F (2002) The absolute requirement for carbon dioxide for aerobic methane oxidation by a methanotrophic-heterotrophic soil community of bacteria. Biotechnol Lett 24(9):675–679
Arif MAS, Houwen F, Verstraete W (1996) Agricultural factors affecting methane oxidation in arable soil. Biol Fertil Soil 21(1–2):95–102
Auman AJ, Lidstrom ME (2002) Analysis of sMMO-containing type I methanotrophs in Lake Washington sediment. Environ Microbiol 4(9):517–524
Auman AJ, Speake CC, Lidstrom ME (2001) nifH sequences and nitrogen fixation in Type I and Type II methanotrophs. Appl Environ Microbiol 67(9):4009–4016
Ayalon O, Avnimelech Y, Shechter M (2001) Solid waste treatment as a high-priority and low-cost alternative for greenhouse gas mitigation. Environ Manag 27(5):697–704
Aye L, Widjaya ER (2006) Environmental and economic analyses of waste disposal options for traditional markets in Indonesia. Waste Manag 26(10):1180–1191
Bajic Z, Zeiss C (2001) Methane oxidation in alternative landfill cover soils. In: Proceedings from the 24th Annual Landfill Gas Symposium, March 19–22, Dallas, Texas, USA, SWANA-Solid Waste Association of North America, Silver Spring, MD, USA, pp 145–151
Bender M, Conrad R (1994) Methane oxidation activity in various soils and freshwater sediments—Occurrence, characteristics, vertical profiles, and distribution on grain size fractions. J Geophys Res Atmos 99(D8):16531–16540
Bender M, Conrad R (1995) Effect of CH4 concentrations and soil conditions on the induction of CH4 oxidation activity. Soil Biol Biochem 27(12):1517–1527
Berestovskaya Y, Vasil’eva LV, Chestnykh OV, Zavarzin GA (2002) Methanotrophs of the psychrophilic microbial community of the russian arctic tundra. Microbiology 71(4):460–466
Berger J, Fornes LV, Ott C, Jager J, Wawra B, Zanke U (2005) Methane oxidation in a landfill cover with capillary barrier. Waste Manag 25(4):369–373
Bergmann J-U, Eichmann H-L, Klein T (1998) Device for removing gases from a landfill. Rehau AG & CO, Patent number: EP 0884117 (Priority number: DE19724430), Rehau, 6 p
Bodelier PLE, Frenzel P (1999) Contribution of methanotrophic and nitrifying bacteria to CH4 and NH4 oxidation in the rhizosphere of rice plants as determined by new methods of discrimination. Appl Environ Microbiol 65(5):1826–1833
Bodelier PLE, Laanbroek HJ (2004) Nitrogen as a regulatory factor of methane oxidation on soils and sediments. FEMS Microbiol Ecol 47:265–277
Boeckx P, Van Cleemput O (1996) Methane oxidation in a neutral landfill cover soil—influence of moisture content, temperature, and nitrogen-turnover. J Environ Qual 25(1):178–183
Boeckx P, Van Cleemput O (2000) Methane oxidation in landfill cover soils. In: Singh SN (ed) Trace gas emissions and plants. Kluwer Academic Publishers, pp 197–213.
Bogner JE, Spokas KA, Burton EA (1997) Kinetics of methane oxidation in a landfill cover soil—Temporal variations, a whole landfill oxidation experiment, and modeling of net CH4 emissions. Environ Sci Technol 31(9):2504–2514
Börjesson G, Svensson BH (1997) Seasonal and diurnal methane emissions from a landfill and their regulation by methane oxidation. Waste Manag Res 15(1):33–54
Börjesson G, Chanton J, Svensson BH (2001) Methane oxidation in two Swedish landfill covers measured with carbon-13 to carbon-12 isotope ratios. J Environ Qual 30:369–376
Börjesson G, Sundh I, Tunlid A, Frostegard A, Svensson BH (1998) Microbial oxidation of CH4 at high partial pressures in an organic landfill cover soil under different moisture regimes. FEMS Microbiol Ecol 26(3):207–217
Boulygina ES, Kuznetsov BB, Marusina AI, Tourova TP, Kravchenko IK, Bykova SA, Kolganova TV, Galchenko VF (2002) A study of nucleotide sequences of nifH genes of some methanotrophic bacteria. Microbiology 71(4):425–432
Bronson KF, Mosier AR (1994) Suppression of methane oxidation in aerobic soil by nitrogen fertilizers, nitrification inhibitors, and urease inhibitors. Biol Fertil Soil 17:263–268
Brosseau J, Heitz M (1994) Trace gas compound emissions from municipal landfill sanitary sites. Atmos Environ 28(2):285–293
Cai ZC, Mosier AR (2000) Effect of NH4Cl addition on methane oxidation by paddy soils. Soil Biol Biochem 32(11/12):1537–1545
Cai ZC, Yan XY (1999) Kinetic model for methane oxidation by paddy soil as affected by temperature, moisture and N addition. Soil Biol Biochem 31(5):715–725
Chanton J, Liptay K (2000) Seasonal variation in methane oxidation in a landfill cover soil as determined by an in situ stable isotope technique. Global Biogeochem Cycles 14(1):51–60
Chanton JP, Rutkowski CM, Mosher B (1999) Quantifying methane oxidation from landfills using stable isotope analysis of downwind plumes. Environ Sci Technol 33(21):3755–3760
Chiemchaisri W, Visvanathan C, Wu, JS (2001a) Biological activities of methane oxidation in tropical landfill cover soils. J Solid Waste Technol Manag 27(3–4):129–136
Chiemchaisri W, Wu JS, Visvanathan C (2001b) Methanotrophic production of extracellular polysaccharide in landfill cover soils. Water Sci Technol 43(6):151–159
Christophersen M, Kjeldsen P (2001) Lateral gas transport in soil adjacent to an old landfill: factors governing gas migration. Waste Manag Res 19(6):579–594
Christophersen M, Linderod L, Jensen PE, Kjeldsen P (2000) Methane oxidation at low temperatures in soil exposed to landfill gas. J Environ Qual 29(6):1989–1997
Conrad R (1996) Soil microorganisms as controllers of atmospheric trace gases (H2, CO, CH4, OCS, N2O, and NO). Microbiol Rev 60(4):609–640
Contec (Contec Ingenieurgesellschaft fuer Energie und Umwelttechnik) and Landkeis (Landkeis Freudenstadt Abfallwirtschaftsbetrieb) (2004) Methane oxidation filter for the treatment of landfill lean and/or waste gases from municipal waste landfills. Ger. Gebrauchsmusterschrift, Patent number: DE 202004013278, 6 p
Crill PM (1991) Seasonal patterns of methane uptake and carbon dioxide release by a temperate woodland soil. Global Geochem Cycles 5(4):319–334
Crossman ZM, Abraham F, Evershed RP (2004) stable isotope pulse-chasing and compound specific stable carbon isotope analysis of phospholipid fatty acids to assess methane oxidizing bacterial populations in landfill cover soils. Environ Sci Technol 38(5):1359–1367
Czepiel PM, Mosher B, Crill PM, Harriss, RC (1996) Quantifying the effect of oxidation on landfill methane emissions. J Geophys Res Atmos 101(D11):16721–16729
Dammann B, Streese J, Stegmann R (1999) Microbial oxidation of methane from landfills in biofilters. In: Proceedings of Sardinia 99, 7th International Waste Management and Landfill Symposium, S. Margherita di Pula, Cagliari, Italy, 4–9 October 1999, Published by SWANA-Solid Waste Association of North America, Silver Spring, MD, USA, pp 517–524
De Visscher A, Van Cleemput O (2003) Simulation model for gas diffusion and methane oxidation in landfill cover soils. Waste Manage 23:581–591
De Visscher A, Thomas D, Boeckx P, Van Cleemput O (1999) Methane oxidation in simulated landfill cover soil environments. Environ Sci Technol 33(11):1854–1859
Dedysh SN, Khmelenina VN, Suzina NE, Trotsenko YA, Semrau JD, Liesack W, Tiedje JM (2002) Methylocapsa acidiphila gen nov, sp nov, a novel methane-oxidizing and dinitrogen-fixing acidophilic bacterium from Sphagnum bog. Int J Syst Evol Microbiol 52(1):251–261
Dedysh SN, Knief C, Dunfield PF (2005) Methylocella species are facultatively methanotrophic. J Bacteriol 187(13):4665–4670
Dedysh SN, Liesack W, Khmelenina VN, Suzina NE, Trotsenko YA, Semrau JD, Bares AM, Panikov NS, Tiedje JM (2000) Methylocella palustris gen nov, sp nov, a new methane-oxidizing acidophilic bacterium from peat bogs, representing a novel subtype of serine-pathway methanotrophs. Int J Syst Evol Microbiol 50(3):955–969
Del Grosso SJ, Parton WJ, Mosier AR, Ojima DS, Potter CS, Borken W, Brumme R, Butterbach-Bahl K, Crill PM, Dobbie K, Smith KA (2000) General CH4 oxidation model and comparisons of CH4 oxidation in natural and managed systems. Global Biogeochem Cycles 14(4):999–1019
Delhoménie M-C, Heitz M (2005) Biofiltration of air: a review. Press Crit Rev Biotechnol 25(1–2):53–72
Desideri U, Di Maria F, Leonardi D, Proietti S (2003) Sanitary landfill energetic potential analysis: a real case study. Energy Conver Manag 44:1969–1981
Dobbie KE, Smith KA (1996) Comparison of CH4 oxidation rates in woodland, arable and sand aside soils. Soil Biol Biochem 28(10–11):1357–1365
Du Plessis CA, Strauss JM, Sebapalo EMT, Riedel K-HJ (2003) Empirical model for methane oxidation using a composted pine bark biofilter. Fuel 82:1359–1365
Dunfield P, Knowles R (1995) Kinetics of methane oxidation by nitrate, nitrite ans ammonium in a humisol. Appl Environ Microbiol 61(8):3129–3135
Dunfield PF, Liesack W, Henckel T, Knowles R, Conrad, R (1999) High-affinity methane oxidation by a soil enrichment culture containing a type II methanotroph. Appl Environ Microbiol 65(3):1009–1014
Environnement Canada (2006) Landfill Gas.www.ec.gc.ca/nopp/lfg/en/index.cfm (Visited in August 2006)
EPA (2005) Inventory of US greenhouse Gas emissions and sink: 1990–2003. US Environmental Protection Agency, Washington, USA. www.yosemite.epa.gov/oar/globalwarming.nsf/UniqueKeyLookup/RAMR69V4ZS/$File/05_complete_report.pdf (Visited in August 2006)
EPA (2006) Methane: sources and emissions, Human-related sources. www.epa.gov/methane/sources.html#landfills (Visited in August 2006)
Erwin DP, Erickson IK, Delwiche ME, Colwell FS, Strap JL and Crawford RL (2005) Diversity of oxygenase genes from methane- and ammonia-oxidizing bacteria in the Eastern Snake River Plain aquifer. Appl Environ Microbiol 71(4):2016–2025
Ewall M (1999) Primer on Landfill Gas as "Green" Energy. Report for Energy Justice network, www.penweb.org/issues/energy/green4.html (Visited in August 2006)
Gebert J, Groengroeft A (2006a) Passive landfill gas emission—Influence of atmospheric pressure and implications for the operation of methane-oxidizing biofilters. Waste Manag 26:245–251
Gebert J, Groengroeft A (2006b) Performance of a passively vented field-scale biofilter for the microbial oxidation of landfill methane. Waste Manag 26:399–407
Gebert J, Groengroeft A, Miehlich G (2001) Microbial reduction of methane and trace gas emissions in a biofilter. In: Proceedings from the 8th International Waste Management and Landfill Symposium S Margherita di Pula, Cagliari, Italy, 1–5 October 2001, Published by SWANA-Solid Waste Association of North America, Silver Spring, MD, USA, pp 585–593
Gebert J, Groengroeft A, Miehlich G (2003) Kinetics of microbial landfill methane oxidation in biofilter. Waste Manag 23:609–619
Giani L, Bredenkamp J, Eden I (2002) Temporal and spatial variability of CH4 dynamics of landfill cover soils. J Plant Nutr Soil Sci 165:205–210
Gielecki M (1997) Renewable Energy Annual 1996. Report prepared by the Energy Information Administration, US Department of Energy, Washington, DOE/ELA-0603(96), Distribution category UC-950, 180 p
Goossens MA (1996) Landfill gas power plants. Renew Energy 9(1–4):1015–1018
Grossman EL, Cifuentes LA, Cozzarelli IM (2002) Anaerobic methane oxidation in a landfill-leachate plume. Environ Sci Technol 36:2436–2442
Hanson RS, Hanson TE (1996) Methanotrophic bacteria. Microbiol Rev 60(2):439–471
Haubrichs R, Widmann R (2006) Evaluation of aerated biofilter systems for microbial methane oxidation of poor landfill gas. Waste Manag 26:408–416
Henckel T, Roslev P, Conrad R (2000) Effects of O2 and CH4 on presence and activity of the indigenous methanotrophic community in rice field soil. Environ Microbiol 2(6):666–679
Hesselsoe M, Boysen S, Iversen N, Jorgensen L, Murrell J, McDonald I, Radajewski S, Thestrup H, Roslev P (2005) Degradation of organic pollutants by methane grown microbial consortia. Biodegradation 16(5):435–448
Hettiaratchi JPA, Stein VB (2001) Methanobiofilters (MBFs) and landfill cover systems for CH4 emission mitigation. In: Proceedings of the 17th International Conference on Solid Waste Technology and Management, Philadelphia, PA, Oct. 20–24, Published by the J Solid Waste Technol Manag, Silver Spring, MD, USA, pp 465–476
Hettiaratchi JPA, Stein VB, Achari G (2000) Biofiltration: A cost-effective technique for controlling methane emissions from sub-surface sources. In: Singhal, Mehrotra (eds) 6th Environmental Issues and Management of Waste in Energy and Mineral Production, Balkema Rotterdam, Netherlands, pp 291–299
Heyer J, Berger U, Hardt M, Dunfield PF (2005) Methylohalobius crimeensis gen nov, sp nov, a moderately halophilic, methanotrophic bacterium isolated from hypersaline lakes of Crimea. Int J Syst Evol Microbiol 55(5):1817–1826
Hilger H, Humer M (2003) Biotic landfill cover treatments for mitigating methane emissions. Environ Monitor Assess 84(1–2):71–84
Hilger HA, Cranford DF, Barlaz MA (2000a) Methane oxidation and microbial exopolymer production in landfill cover soil. Soil Biol Biochem 32(4):457–467
Hilger HA, Wollum AG, Barlaz MA (2000b) Landfill methane oxidation response to vegetation, fertilization, and liming. J Environ Qual 29(1):324–334
Horz H-P, Rich V, Avrahami S, Bohannan BJ M (2005) Methane-oxidizing bacteria in a California upland grassland soil: diversity and response to simulated global change. Appl Environ Microbiol 71(5):2642–2652
Hudgins M, Green L (2000) Innovative landfill gas and odor control using an aerobic landfill system. In: Odors and VOC Emissions 2000, Conference Proceedings, Cincinnati, OH, United States, Apr. 16–19, Published by Water Environment Federation, pp 619–641
Hughes KL, Daneel RA, Senior E (2002) Physiological characterization of a methanol-oxidizing microbial association isolated from landfill final covering soil. South Afr J Sci 98:434–437
Humer M, Lechner P (1999a) Methane oxidation in compost cover layers on landfills. In: Proceedings of Sardinia 99, 7th International Waste Management and Landfill Symposium, S Margherita di Pula, Cagliari, Italy, 4–8 October 1999, Published by SWANA-Solid Waste Association of North America, Silver Spring, MD, USA, pp 403–410
Humer M, Lechner P (1999b) Alternative approach to the elimination of greenhouse gases from old landfills. Waste Manag Res 17(6):443–452
Humer M, Lechner P (2001) Microbial methane oxidation for the reduction of landfill gas emissions. J Solid Waste Technol Manag 27(3–4):146–151
Hupe K, Heyer KU, Stegmann R (1998) Hazardous sites and landfills utilize compost. Biocycle 39(6):79
Hütsch BW (1998a) Methane oxidation in arable soil as inhibited by ammonium, nitrite, and organic manure with respect to soil pH. Biol Fertil Soil 28:27–35
Hütsch BW (1998b) Tillage and land use effects on methane oxidation rates and their vertical profiles in soil. Biol Fertil Soil 27:284–292
Hütsch BW, Webster CP, Powlson DS (1994) Methane oxidation in soil as affected by land use, soil pH, N fertilization. Soil Biol Biochem 26(12):1613–1622
Jäckel U, Schnell S, Conrad R (2001) Effect of moisture, texture and aggregate size of paddy soil on production and consumption of CH4. Soil Biol Biochem 33(7–8):965–971
Jaffrin A, Bentounes N, Joan AM, Makhlouf S (2003) Landfill biogas for heating greenhouses and providing carbon dioxide supplement for plant growth. Biosyst Eng 86(1):113–123
Janni KA, Maier WJ, Kuehn TH, Yang CH, Bridges BB, Vesley D, Nellis MA (2001) Evaluation of Biofiltration of Air—an innovative air pollution control strategy. ASHRAE Trans 107(1):198–214
Jones HA, Nedwell DB (1993) Methane emission and methane oxidation in landfill cover soil. FEMS Microbiol Lett 102(3–4):185–195
Jorio H, Heitz M (1999) Traitement de l’air par biofiltration. Can J Civil Eng 26:402–424
Jorio H, Payre G, Heitz M (2003) Mathematical modeling of gas-phase biofilter performance. J Chem Technol Biotechnol 78:834–846
Kallistova AY, Kevbrina MV, Nekrasova VK, Glagolev MV, Serebryanaya MI, Nozhevnikova AN (2005) Methane oxidation in landfill cover soil. Microbiology 74(5):608–614
Kalyuzhnaya MG, Stolyar SM, Auman AJ, Lara JC, Lidstrom ME, Chistoserdova L (2005) Methylosarcina lacus sp nov, a methanotroph from Lake Washington, Seattle, USA, and emended description of the genus Methylosarcina. Int J Syst Evol Microbiol 55(6):2345–2350
Kelly DP, Anthony C, Murrell JC (2005) Insights into the obligate methanotroph Methylococcus capsulatus. Trends Microbiol 13(5):195–198
Kettunen RH, Rintala JA (1997) The effect of low temperature (5–29°C) and adaptation on the methanogenic activity of biomass. Appl Microbiol Biotechnol 48:570–576
Kightley D, Nedwell DB, Cooper M (1995) Capacity for methane oxidation in landfill cover soils measured in laboratory-scale soil microcosms. Appl Environ Microbiol 61(2):592–601
Kim HJ, Graham DW (2001) Effect of oxygen level on simultaneous nitrogenase and sMMO expression and activity in Methylosinus trichosporium OB3b and its sMMOC mutant, pp319: aerotolerant N2 fixation in PP319. FEMS Microbiol Lett 201(2):133–138
King GM, Schnell S (1994) Effect of increasing atmospheric methane concentration on ammonium inhibition of soil methane consumption. Nature 370:282–284
King GM, Schnell S (1998) Effects of ammonium and non-ammonium salt additions on methane oxidation by Methylosinus trichosporium OB3b and Maine forest soils. Appl Environ Microbiol 64(1):253–257
Kjeldsen P, Dalager A, Broholm K (1997) Attenuation of methane and nonmethane organic compounds in landfill gas affected soils. J Air Waste Manag Assoc 47(12):1268–1275
Klusman RW, Dick CJ (2000) Seasonal variability in CH4 emissions from a landfill in a cool, semiarid climate. J Air Waste Manag Assoc 50(9):1632–1636
Kotelnikova S (2002) Microbial production and oxidation of methane in deep subsurface. Earth Sci Rev 58(3–4):367–395
Kravchenko IK (2002) Methane oxidation in boreal peat soils treated with various nitrogen compounds. Plant Soil 242(1):157–162
Kumar S, Mondal AN, Gaikwad SA, Devotta S, Singh RN (2004) Qualitative assessment of methane emission inventory from municipal solid waste disposal sites: a case study. Atmos Environ 38:4921–4929
Kumaraswamy S, Ramakrishnan B, Sethunathan N (2001) Methane production and oxidation in an anoxic rice soil as influenced by inorganic redox species. J Environ Qual 30(6):2195–2201
Kyoto protocol (1998) Kyoto protocol to the United Nations framework convention on climate change. www.unfccc.int/resource/docs/convkp/kpeng.pdf (Visited in August 2006)
Le Mer J, Roger P (2001) Production, oxidation, emission and consumption of methane by soils: A review. Euro J Soil Biol 37(1):25–50
Lee GY, Cho YL, Yang CR, Lee DH, Won YM, Lee YJ, Park YJ (2002) Landfill structure using concept of multi-layered reactors and method for operating the same. ENV21 Co., Ltd., PCT International Application, Patent number: WO0243883, S Korea, 65 p
Lidstrom ME (2001) Aerobic methylotrophic prokaryotes. In: Dworkin (ed) The Prokaryotes: An Evolving Electronic Resource for the Microbiological Community, 3rd edn, release 3.7. Springer-Verlag, New York, Available on-line at http://link.springer-ny.com/link/service/books/10125/ (Visited in August 2006)
Lindner AS, Semrau JD, Adriaens P (2005) Substituent effect on the oxidation of substituted biphenyl cogeners by type II methanotrop strain CSC1. Arch Microbiol 183:266–276
Ma TH, Xu C, Liao S, H McConnel, Jeong BS, Won CD (1996) In situ monitoring with the Tradescantia bioassays on the genotoxicity of gaseous emissions from a closed landfill site and an incinerator. Mut Res 359:39–52
Mancinelli RL (1995) The regulation of methane oxidation in soil. Annu Rev Microbiol 49:581–605
March R (1994) Biofiltration for emissions abatement—Réduction des émissions par biofiltration. Euro Coatings J 7–8:528
Maurice C, Ettala M, Lagerkvist A (1999) Effects of leachate irrigation on landfill vegetation and subsequent methane emissions. Water Air Soil Pollut 113(1):203–216
McLain J (2000) Rising CO2 threatens forest methane sink. 100th General Meeting of the American Society for Microbiology, Los Angeles, California, USA, May 21–25, 2000, Published by the Journal of American Society for Microbiology, Paper N-101.
McLain TEJ, Kepler BT, Ahmann MD (2002) Belowground factors mediating changes in methane consumption in a forest soil under elevated CO2. Global Biogeochem Cycles 16(3):(23) 1–14
Min H, Chen ZY, Wu WX, Chen MC (2002) Microbial aerobic oxidation of methane in paddy soil. Nutr Cycling Agroecosyst 64(1–2):79–85
Mingxing W, Jing L (2002) CH4 emission and oxidation in Chinese rice paddies. Nutrient Cycling Agroecosystems 64:43–55
Mohanty RS, Bharati K, Deepa N, Adhya KT (2000) Influence of heavy metals on methane oxidation in tropical rice soils. Ecotoxicol Environ Saf 47:277–284
Mor S, De Visscher A, Ravindra K, Dahiya RP, Chandra A, Van Cleemput O (2006) Induction of enhanced methane oxidation in compost: temperature and moisture responses. Waste Manag 26(4):381–388
Murphy JD, McCarthy K (2005) The optimal production of biogas for use as a transport fuel in Ireland. Renew Energy 30:2111–2127
Murrell JC, Dalton H (1983) Nitrogen fixation in obligate methanotrophs. J Gen Microbiol 129(11):3481–3486
Nguyen PHL, Kuruparan P, Visvanathan C (2006) Anaerobic digestion of municipal solid waste as a treatment prior to landfill. Bioresource Technol 98(2):380–387
Nikiema J, Bibeau L, Lavoie J, Brzezinski R, Comeau J F, Vigneux J, Heitz M (2004a) Atténuation de l’effet de serre par biofiltration du méthane émis par les lieux d’enfouissement sanitaire. 7\(2^{i\grave{e}me}\) Congrès de l’Association Canadienne Française pour l’Avancement des Sciences (ACFAS), Université du Québec à Montréal, Canada, May 10–14
Nikiema J, Bibeau L, Lavoie J, Brzezinski R, Vigneux J, Heitz M (2004b) Biogas, a real problem: Biofiltration, a promising solution. In: Proceedings of the USC-CSC-TRG Conference on Biofiltration, October 20–22, Los Angeles, California, Published by The Reynolds Group, Tustin, California, USA, pp 73–80
Nikiema J, Bibeau L, Lavoie J, Brzezinski R, Vigneux J, Heitz M (2005) Biofiltration of methane: an experimental study. Chem Eng J 113(2–3):111–117
Novikov VV, Stepanov AL (2002) Coupling of microbial processes of methane and ammonium oxidation in soils. Microbiology 71(2):234–237
Nozhevnikova AN, Lifshitz AB, Lebedev VS, Zavarzin GA (1993) Emission of methane into the atmosphere from landfills in the former USSR. Chemosphere 26(1–4):401–417
Nozhevnikova AN, Nekrasova VK, Kevbrina MV, Kotsyurbenko OR (2001) Production and oxidation of methane at low temperature by the microbial population of municipal sludge checks situated in north-east Europe. Water Sci Technol 44(4):89–95
Oakley CJ, Murrell JC (1988) nifH genes in the obligate methane oxidizing bacteria. FEMS Microbiol Lett 49:53–57
Ottengraf SPP (1986) Exhaust gas purification. In: Rehm HJ, Reed G (eds) Biotechnology, a comprehensive treatise, vol 8. VCH Verlagsgesellschaft, Weinheim, Germany, pp 426–452
Ozkaya B, Demir A, Bilgili MS (2006) Neural network prediction model for the methane fraction in biogas from field-scale landfill bioreactors. Environ Modelling Softw (in press)
Park S, Brown KW, Thomas JC (2002) The effect of various environmental and design parameters on methane oxidation in a model biofilter. Waste Manag Res 20(5):434–444
Park SY, Brown KW, Thomas JC (2004) The use of biofilters to reduce atmospheric methane emissions from landfills: part I biofilter design. Water Air Soil Pollut 155(1–4):63–85
Perera LAK, Achari G, Hettiaratchi JPA (2002) Determination of source strength of landfill gas: a numerical modeling approach. J Environ Eng ASCE 128(5):461–471
Perry RH, Green DW, Maloney JO (1997) Perry’s chemical engineers’ handbook, 7th edn. McGraw-Hill, New York
Popov V (2005) A new landfill system for cheaper landfill gas purification. Renew Energy 30(7):1021–1029
Raghoebarsing AR, Pol A, van de Pas-Schoonen KT, Smolders AJP, Ettwig KF, Rijpstra WIC, Schouten S, Sinninghe Damsté JS, Op den Camp HJM, Jetten MSM, Strous M (2006) A microbial consortium couples anaerobic methane oxidation to denitrification. Nature 440:918–921
Reay DS, Nedwell DB (2004) Methane oxidation in temperate soils: effects of inorganic N. Soil Biol Biochem 36:2059–2065
Reginster J (1999) Problématique de la décharge de Mont-Saint-Guibert: État de la situation et risques pour la population. Report of the “Société Publique d’Aide à la Qualité de l’Environnement de la Région wallonne”, Published by Association des Habitants de Louvain-la-Neuve, Louvain-la-Neuve, Belgique, 160 p
Reinhart DR, Al-Yousfi AB (1996) The impact of leachate recirculation on municipal solid waste landfill operating characteristics. Waste Manag Res 14:337–346
Scheutz C, Winther K, Kjeldsen P (2000) Removal of halogenated organic compounds in landfill gas by top covers containing zero-valent iron. Environ Sci Technol 34(12):2557–2563
Segers R (1998) Methane production and methane consumption-a review of processes underlying wetland methane fluxes. Biogeochemistry 41(1):23–51
Sitaula BK, Hansen S, Sitaula JIB, Bakken LR (2000) Methane oxidation potentials and fluxes in agricultural soil: effects of fertilization and soil compaction. Biogeochemistry 48(3):323–339
Sly LI, Bryant LJ, Cox JM, Anderson JM (1993) Development of a biofilter for the removal of methane from coal mine ventilation atmospheres. Appl Microbiol Biotechnol 39(3):400–404
Spokas K, Bogner J, Chanton JP, Morcet M, Aran C, Graff C, Golvan YM-L, Hebe I (2006) Methane mass balance at three landfill sites: what is the efficiency of capture by gas collection systems?. Waste Manag 26(5):516–525
Stein VB, Hettiaratchi JPA (2001) Methane oxidation in three Alberta soils: influence of soil parameters and methane flux rates. Environ Technol 22(1):101–111
Stein VB, Hettiaratchi JPA, Achari G (2001) A numerical model for biological oxidation and migration of methane in soils. ASCE Prac Period Hazard Toxic Radioactive Waste Manag 5(4):225–234
Straka F, Crha J, Musilova M, Kuncarova M (1999) LFG-Biofilters on old landfills. In: Proceedings of Sardinia 99, 7th International Waste Management and Landfill Symposium, S Margherita di Pula, Cagliari, Italy, 4–9 October 1999, Published by SWANA-Solid Waste Association of North America, Silver Spring, MD, USA, pp 507–516
Stralis-Pavese N, Bodrossy L, Reichenauer TG, Weilharter A, Sessitsch A (2006) 16S rRNA based T-RFLP analysis of methane oxidizing bacteria—Assessment, critical evaluation of methodology performance and application for landfill site cover soils. Appl Soil Ecol 31:251–266
Streese J, Stegmann (2003) Microbial oxidation of methane from old landfills in biofilters. Waste Manag 23:573–580
Streese J, Dammann B, Stegmann R (2001) Reduction of methane and trace gas emissions from former landfills in biofilters. In: Proceedings of the 8th International Waste Management and Landfill Symposium S Margherita di Pula, Cagliari, Italy, 1–5 October 2001, Published by SWANA-Solid Waste Association of North America, Silver Spring, MD, USA, pp 575–584
Tagaris E, Sotiropoulou R-EP, Pilinis C, Halvadakis CP (2003) A methodology to estimate odors around landfill sites: the use of methane as an odor index and its utility in landfill sitting. J Air Waste Manag Assoc 53(5):629–634
Toutant C (1994) L’atmosphère terrestre, ses ennemis et leur contrôle. In: Éditions Odile Germain, Québec, Canada, p 250
Trotsenko YA, Khmelenina VN (2002) Biology of extremophilic and extremotolerant methanotrophs. Arch Microbiol 177(2):123–131
Tsai WT (2006) Bioenergy from landfill gas (LFG) in Taiwan. Renew Sustainable Energy Rev 11(2):331–344
Tsubota J, Eshinimaev BTs, Khmelenina VN, Trotsenko YA (2005) Methylothermus thermalis gen nov, sp nov, a novel moderately thermophilic obligate methanotroph from a hot spring in Japan. Int J Syst Evol Microbiol 55:1877–1884
USDE (United States Department of Energy) (2005) US climate change technology program—technology options for the near and long term. Report, published by USDE, August 2005, 210 p
Valentine DL (2002) Biogeochemistry and microbial ecology of methane oxidation in anoxic environments: a review. Antonie van Leeuwenhoek 81:271–282
Van Stempvoort D, Maathuis H, Jaworski E, Mayer B, Rich K (2005) Oxidation of fugitive methane in ground water linked to bacterial sulphate reduction. Ground Water 43(2):187–199
Visvanathan C, Pokhrel D, Cheimchaisri W, Hettiaratchi JPA, Wu JS (1999) Methanotrophic activities in tropical landfill cover soils: effects of temperature, moisture content and methane concentration. Waste Manag Res 17(4):313–323
Vorholt JA (2002) Cofactor-dependent pathways of formaldehyde oxidation in methylotrophic bacteria. Arch Microbiol 178(4):239–249
Wang Z-P, Ineson P (2003) Methane oxidation in a temperate coniferous forest soil: effects of inorganic N. Soil Biol Biochem 35:427–433
Warmer Bulletin (2000) Landfill. Information Sheet, Published by Residua, Skipton, North Yorkshire, United Kingdon, 4 p
Whalen SC, Reeburgh WS (1996) Moisture and temperature sensitivity of CH4 oxidation in boreal soils. Soil Biol Biochem 28(10):1271–1281
Whalen SC, Reeburgh WS, Sandbeck KA (1990) Rapid methane oxidation in a landfill cover soil. Appl Environ Microbiol 56:3405–3411
Whitman WB, Bowen TL, Boone DR (1999) The methanogenic bacteria. In: Dworkin M (ed) The prokaryotes: an evolving electronic resource for the microbiological community, 3rd edn, release 3.0. Springer-Verlag, New York, Available on-line at http://link.springer-ny.com/link/service/books/10125/ (Visited in August 2006)
Whittenbury R, Phillips KC, Wilkinson JF (1970) Enrichment, isolation and some properties of methane-utilizing bacteria. J Gen Microbiol 61(2):205–218
Wilshusen JH, Hettiaratchi JPA, Stein VB (2004) Long-term behavior of passively aerated compost, methanotrophic biofilter columns. Waste Manage 24(7):643–653
Wise MG, McArthur JV, Shimkets LJ (1999) Methanotroph diversity in landfill soil: isolation of novel type I, type II methanotrophs whose presence was suggested by culture-independent 16S ribosomal DNA analysis. Appl Environ Microbiol 65(11):4887–4897
Yongzhi W, Hu W (2002) Research and application of biogas decontamination system. Report prepared by the Mianzhu Rural Energy Bureau and the Mianzhu Environmental Protection Bureau, Sichuan, China, Copyright for the Ecological Sanitation Research, Stockholm
Zamorano M, Pérez Pérez JI, Pavés IA, Ridao AR (2006) Study of the energy potential of the biogas produced by an urban waste landfill in Southern Spain. Renew Sustainable Energy Rev (In press)
Zani S, Mellon MT, Collier JL, Zehr JP (2000) Expression of nifH genes in natural microbial assemblages in Lake George, New York, detected by reverse transcriptase PCR. Appl Environ Microbiol 66:3119–3124
ZWA-Zero Waste America (2006) Landfills: Hazardous to the environment. www.zerowasteamerica.org/Landfills.htm (Visited in August 2006)
Acknowledgements
The authors gratefully acknowledge the Natural Sciences and Engineering Research Council of Canada (NSERC) for their financial contribution to the project and express their gratitude to Dr. P. Lanigan for text review. One of the authors (J. Nikiema) would like to thank the NSERC for providing a scholarship for her doctoral studies (Canada Graduate Scholarships Program).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Nikiema, J., Brzezinski, R. & Heitz, M. Elimination of methane generated from landfills by biofiltration: a review. Rev Environ Sci Biotechnol 6, 261–284 (2007). https://doi.org/10.1007/s11157-006-9114-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11157-006-9114-z