Abstract
Thyroid gland has been implicated in the regulation of many functions using endocrine, paracrine and autocrine signals. Functional thyroid follicular cells derived from stem cells attracted a great interest from researchers as a strategy for thyroid’s regenerative therapy. Thyroid has a very low rate of turnover; however, studies showed that the regenerative ability is enhanced following diseases or thyroidectomy, which promotes the role of stem cell. The objective of this review is to summarize the morphological characterization and the expression of stem cell genes/markers in the thyroid. Also, to highlight the mechanisms of tumor formation in thyroid via its stem cells. The most important thyroid stem cell’s markers are: stem cell antigen 1 (SCA-1), octamer-binding transcription 4 (OCT-4), p63, CD34+ CD45-, paired box gene 8 (PAX-8), thyroid transcription factor 1 (TTF-1), thyroid transcription factor 2 (TTF-2), hematopoietically expressed homeobox protein HHEX, the transcription factor GATA-4, hepatocyte nuclear factor 4-α (HNF-4-α) and homeobox transcription factor Nanog (hNanog). This review highlights the functional characterization describing the mechanisms of stem cell’s differentiation into functional thyroid follicle and proposing mechanisms involving in cancer formation through one of these cell types: fetal cell, thyroblasts, prothyrocytes, certain genetic mutation in the mature thyroid cells or presence of a special type of cells (cancer stem cell) which are responsible for different types of cancer formation. Understanding the mechanisms of thyroid’s stem cell in cancer formation and the expression of the biomarkers in normal and abnormal thyroid status are promising physiological tools in promoting thyroid regeneration and in provision management for thyroid cancer.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Thyroid gland plays a key role in controlling several main functions in the body, among which is the control of rate of cellular metabolism. It is unique in its structure and functions, because its hormones are stored in special follicles in an inactive form called thyroglobulin. Thyroid hormones are released into the circulation to regulate the energy production in the cells and to exert other important physiological functions, and also, function locally as paracrine signals with C cells that are imbedded in the thyroid. The main factor that controls its production and release is the Thyroid Stimulating Hormone (TSH) produced and released by the anterior pituitary gland.
2 Origin and histology of thyroid gland
Thyroid gland is located in the neck and surrounded by a thin fibrous capsule that sends fine septa to the interior of the gland. Thyroid is intimately related to the parathyroid glands, which are embedded in its posterior aspect. The parenchyma of the thyroid gland consists principally of two cell types: follicular cells (FC), and parafollicular cells (also known as clear cells or C cells). The follicular cells are usually cuboidal in shape and line a spherical follicle by forming a single layer. The height of these FC differs according to the activity of the thyroid gland; the more active, the taller are the cells. The follicles are filled with a homogenous material known as the colloid, which is composed of thyroglobulin, the un-iodinated form of the thyroid hormones (prohormones). The parafollicular cells are found in the spaces between the follicles or between the FC and the basal lamina of the follicles. These parafollicular cells are the source of Calcitonin hormone, which decreases calcium ions level in the blood. The two types of cells (FC and C cells) have different embryological origins, but during development, they join and intermix in the gland substance. However, the parafollicular cells are more concentrated in the central parts of the lateral lobes [1]. The FC originate from a mass of endoderm that grows from the base of the tongue during the fourth week of intrauterine development and descends through the neck till it reaches its desired location in front of the lower part of larynx and upper part of the trachea. On the other hand, C cells arise from the ultimobranchial body, a group of neural crest-derived epithelial cells that detach from the ventral part of the fourth pharyngeal pouch during the fifth week of intrauterine development and migrate to the newly developed thyroid gland and implant in its dorsal wall and differentiate to C cells [2]. For long time, it has been believed that neural crest cells are the origin of C cells, but recent investigations, based on genetic lineage studies in mice, showed that C cells arise from the definitive embryonic endoderm [3, 4].
3 Thyroid stem cells/ progenitor cells
Thyroid progenitor cells are generated/ derived from embryonic stem cells during development.
Stem cells are a special type of cells that possess an unrestricted ability to self-renew and to develop into various types of mature cells [5]. Four types of stem cells were described: 1) Totipotent stem cells, represented by the zygote, which produces the embryo and the extraembryonic membranes, 2) Pluripotent stem cells, like the embryonic stem cells that form all tissues and organs of the embryo, 3) Multipotent stem cells, these are able to form more than one specialized cell type, like the adult stem cells of various organs (skin, gut, bone marrow, liver, …), and 4) Unipotent stem cells, which produce only one type of specialized cells, like spermatogonia and oogonia in the testis and ovary, respectively [6]. Thomas et al. [7] divided stem cells into three types: embryonic, fetal and adult types, and considered cancer stem cells as a special category. Stem cells reside in a special environment (niche) that provides the necessary signals to promote the persistence of these stem cells [7]. Investigating these stem cells holds a great promise for understanding the mechanisms of many diseases and subsequently finding a cure for them.
In recent years, considerable interest has been directed to the existence of stem cells in the thyroid gland and the possible mechanisms of thyroid regeneration following injury. Thyroid gland cells have a very low rate of turnover, estimated to be five times during the life time [8]. Long time ago, researchers observed that subtotal thyroidectomy led to hyperplasia of thyroid cells, suggesting cell proliferation [9]. It has been postulated that adult thyroid gland possesses stem cell population estimated to be ~ 0.1% of all thyroid cells [8]. The first attempt to characterize these cells was performed by Hoshi et al. [10], who demonstrated the presence of side population (SP) cells in thyroid gland of adult mice. These SP cells resemble the stem cells in expressing number of genes like stem cell antigen 1 (SCA-1) and Octamer-binding transcription 4 (OCT-4). Another study provided a proof for the existence of stem cells in the mature thyroid of mice [11], the study examined the regenerative capacity of thyroid after inducing Hashimoto thyroiditis in the mice. They found remarkable regenerative capacity 100 days following the induction of the disease. They also described a role of the cell adhesion molecule, cluster of differentiation 24 (CD24), in thyroid regeneration. Restricted thyroidectomy has been used as a model for regeneration of thyroid in the mouse [1]. It has been found that following partial thyroidectomy, the central zone of the thyroid showed proliferation, in terms of increase in the amount cells reactive for bromodeoxyuridine and cells that have clear cytoplasm. Bromodeoxyuridine, a chemical analogue to thymidine, is usually used in the recognition of dividing cells in the tissues [12]. Ozaki et al. [1] reported the presence of immature cells in the thyroid gland following partial thyroidectomy, that can participate in the regeneration of the gland. The researchers proposed that the immature cells are either derived from stem/progenitor cells or from de-differentiated C cells and follicular cells [1]. Okamoto et al. [13] presented a model to investigate the mechanism of thyroid regeneration following partial thyroidectomy. They described the appearance of SCA1+ cells within the mesenchymal areas between the follicles 7 days post thyroidectomy. Kimura [14] proposed that these SCA1+ cells, in the aforementioned model, are resident adult thyroid stem/progenitor cells that have been stimulated by partial thyroidectomy, or bone marrow-derived cells that participated in thyroid regeneration following injury. The study also described another model of thyroid regeneration, and designated it as Model II, in which the amount of thyroidectomy is massive compared to that presented by Okamoto et al. [13], which Kimura designated as Model I. The proposed mechanism of regeneration in Model II involves the conversion of a previously differentiated thyroid cells into immature cells that contribute to regeneration of the thyroid gland [14]. The aforementioned text presents a confirmation for the role of thyroid stem cells in thyroid tissue repair and regeneration.
Other group of studies were conducted on human subjects and presented for the first time a strong evidence for the existence of stem cell population in the thyroid of humans [15, 16] and showed the expression of p63, a stem cell marker, in some thyroid cells [15]. While Thomas et al. [16] demonstrated the expression of some markers of stem cells in goitrous thyroid of adult human subjects. Side population cells have also been reported in adult human thyroid glands by Lan et al. [17], who isolated these stem-like cells from cultures of goitrous thyroid glands. They characterized these cells as having high nuclear/cytoplasmic ratio and expressing OCT-4, one of the stem cell marker genes. They estimated the side population cells as forming 0.1% of all cells examined in the study. In 2008, Fierabracci et al. [18] confirmed those observations in human thyroid gland. They found that CD34+ CD45− cells were able to differentiate into functional thyroid follicle cells that produce thyroid hormone. CD stands as cluster of differentiation protocol, which identifies cell surface antigens. CD34 is a transmembrane phosphoglycoprotein, and one of the markers of the undifferentiated stem cells [19]. CD45 is a transmembrane protein that is present on all differentiated hematopoietic cells, except erythrocytes and plasma cells.
Human embryonic progenitor cells and human induced pluripotent stem cells could differentiate into any cell population in the human body. The significant step in generating organ’s progenitor cells is the differentiation into definitive endoderm for many organs including the thyroid [20]. Recently, scientists started to understand the signals produced by the surrounding environment in order to influence specific differentiation of stem cells and production of specific cell lineage. Most of these studies were conducted on mice, and the first in vitro study that succeeded in producing thyroid-like cells from embryonic stem (ES) cells is by Lin et al. [21]. Subsequent studies also demonstrated this differentiation of ES cells into thyroid-like cells in mice [22,23,24,25,26] and humans [27]. These studies investigated the effect of number of hormones/growth factors on the differentiation process from ES cells to thyrocytes. In 2012, a group of investigators succeeded in generating functional thyroid follicles from mouse embryonic stem cells, which overexpress two transcription factors required essentially for thyroid development; NKX2–1 and PAX 8 [28]. These induced follicles were successfully used to treat experimentally–induced hypothyroidism in vivo. Mice and human thyroid stem cells were identified in mature thyroids in vivo and in vitro with successful differentiation into thyroid-like cells [29]. The complex process of differentiation of ES cells to thyroid cells is expressed by Onyshchenko et al. [30] who reported that stimulation of human ES cells by thyrotropin (thyroid stimulating hormone) only is not enough to form thyroid cells. In the developing thyroid of mice, epithelial release of angiogenic factor and vascular endothelial growth factor-A are required for endothelial cell recruitment and growth. Hereafter, endothelial cells regulate follicular epithelium re-organization, folliculogenesis and differentiation, as well as thyrocytes and C cell differentiation. The lack of blood supply makes embryonic endothelial progenitor cells recover folliculogenesis by expanding its lumen and activate expression of calcitonin in C cells [31].
Results of Murata et al. [32] illustrate that the side population cell-derived thyroid cell line (SPTL cells) are capable of differentiation into thyroid to some extent. After restricted thyroidectomy, a little SPTL cells were present in part of the follicles, most of which expressed NKX2–1, a transcription factor important for thyroid differentiation and functions, also, greatly expressed genes required in epithelial-mesenchymal transition, as confirmed by analysis of RNA seq and showed a gene-expression model comparable to anaplastic thyroid cancer. SPTL cells develop to follicle-like structures in cultures.
The abovementioned account on the state of research on the stem cells of thyroid gland shows that researchers are trying to understand the mechanism of thyroid regeneration following injury or disease. This understanding carries a promise for improving therapeutical approaches to treat thyroid diseases.
4 Roles of thyroid stem cells in thyroid cancer
According to the American Cancer Society, thyroid cancer is the commonest type of cancer of endocrine glands. Recent studies in Saudi Arabia have shown that there is a rise in the rate of occurrence of thyroid cancer among Saudi females. Breast cancer and thyroid cancer are the most common types of cancer among the Saudi female population [33, 34]. In the United States, thyroid cancer accounts for 96% of all malignancies of endocrine glands and is the second most common malignancy among adolescents [35]. There are different types of thyroid cancer, and these are classified according to the level of differentiation of cancer cells into either differentiated or undifferentiated types. The differentiated types include papillary, follicular and medullary thyroid cancer. The undifferentiated type is represented by the anaplastic thyroid cancer, which has a very bad prognosis. The medullary thyroid cancer arises from C cells of the thyroid gland, whereas the others arise from the follicular cells. The differentiated types of thyroid cancer have the best prognosis among the different types of thyroid cancer [36].
A special category of stem cells is that found in cancerous tissue, Cancer Stem Cells (CSCs) [35], also known as tumor-initiating cells (TICs) as designated and recommended by Gao et al. [36]. It is not clear whether these CSCs are originally derived from the normal cells that have de-differentiated or actually stem cells from the start [7]. However, the widely accepted notion is that CSCs are derived from the original stem cells of thyroid gland that have undergone certain mutational changes [36, 37]. Zhang et al. [38] presented a very interesting hypothesis that postulated a different origin of each type of thyroid cancer cells. They stated that the undifferentiated type, the anaplastic cancer, arises directly from stem cells; whereas the medullary type arises from the progenitor C cells. Burstein et al. [39] supposed a role of p63-positive stem cells in the pathogenesis of papillary carcinoma. Interestingly, Takano and Amino [40] hypothesized that the origin of the anaplastic thyroid cancer is the remnants from fetal thyroid cells. Zane et al. [35] presented three models for cancer formation in the thyroid gland. These models are: 1) multistep carcinogenesis model, 2) fetal cell carcinogenesis model, and 3) cancer stem cell model. The first model implies that the initial step is occurrence of genetic mutation in the thyrocytes, which leads to one of the differentiated forms of thyroid cancer. In the following step, another mutation is going to transform the differentiated form into the undifferentiated form (anaplastic thyroid cancer). The second model, fetal cell carcinogenesis; proposed that the different types of thyroid cancer originate from thyroid cells at different stages of development; for example, the undifferentiated form develops from the thyroid stem cells, whereas, the differentiated papillary form develops from the thyroblasts. On the other hand, the follicular type of the differentiated cancer arises from the prothyrocytes [41]. The third model of carcinogenesis, the model of cancer stem cell, proposes that a few cells have the characteristics of stem cells and are capable of producing the tumor cells. These cancer stem cells could arise following genetic alterations in the normal stem cells. Figure 1 summarizes in a schematic representation these three models of mechanism of cancer formation in thyroid gland.
Schematic diagram summarizes the 3 theories of cancer formation in thyroid gland. a: shows the steps of normal development of thyroid cells. b: represents the fetal cell theory of cancer formation that proposes that the undifferentiated form of thyroid cancer arises from thyroid stem cells, whereas the differentiated forms arise from the thyroblasts or prothyrocytes. c: is for the multistep theory that postulates occurrence of certain genetic mutation in the mature thyroid cells that leads to dedifferentiation and cancer formation over number of steps. d: is for cancer stem cell theory that hypothesizes presence of a special type of cells (cancer stem cells) which are responsible for cancer formation
Accumulative evidence that supports the concept of the presence of subpopulation of thyroid cancer cells that demonstrate features specific for stem cells is now well accepted. These putative cancer-initiating cells lead to tumorigenesis because of their properties to 1) undergo self-renewal, 2) differentiate into different kinds of cancer cells, 3) give rise to metastases and play a definitive role in malignant progression, and 4) resist the chemo- and radiotherapy. TICs were isolated and recognized using particular biomarkers (CD133), sphere formation assay, the side population and aldehyde dehydrogenase activity tests [36]. Although TICs are mostly unknown, they attracted the interest of researchers in order to reveal details on their cellular pathway and clarify the reasons of their therapeutic resistance and finding out anti-cancer drugs [36,37,38,39,40,41,42]. Despite the putative role of the TICs in metastasis of cancer cells, the papillary type of thyroid cancer is known for its lack of metastasis [43, 44]. In dissimilarity, with anaplastic thyroid carcinoma, papillary thyroid carcinoma is classified as a non-aggressive type with lower rate of mortality. The documented cases of distant metastasis of papillary cancer, or transformation to anaplastic type were in the gland itself or neighbouring lymph nodes, and presented as case reports [43, 44]. Therefore, it is not yet understood how these TICs in papillary thyroid cancer do not lead to metastasis.
The first study that identified side-population cells in thyroid cancer is performed in 2007 by Mitsutake et al. [45] who identified the proportion of SP cells among different types of thyroid cancer in cultured human cell lines. They found that cancer stem cells are not only represented by SP cells because injection of SP and non-SP cells derived from thyroid cancer into nude mice led to formation of tumors. Nagayama et al. [46] described two methods to prove the presence of thyroid CSCs. The first one is in vivo injection of these cells into immune-deficient mice and document the occurrence of tumor. While the other method is in vitro formation of spheres in cell cultures (thyrospheres).
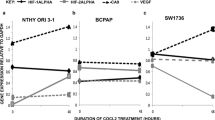
In an attempt to understand the underlying mechanism(s) that underlie(s) the growth and maintenance of thyroid cancer, Mahkamova et al. [47] investigated the role of hypoxia in this type of cancer in human cell cultures. They found that the Hypoxia-inducible factors (HIF) signals have a significant role in the maintenance of stem cells in side population of thyroid cancer. They reported that two factors: HIF-1alpha and HIF-2 alpha were expressed in thyroid cancers. Stanniocalcin (STC) is a glycoprotein implicated in calcium and phosphate homeostasis, and present in various organs as type 1 (STC1) or type 2 (STC2) [48]. The degree of expression of STC has been found to be related to the prognosis of different types of cancers; the higher the expression, the poorer is the prognosis [49]. STC1 has been found to be expressed in both mice thyroid SP cells and thyroid cancer cells [50].
For characterizing, investigating the stem cells of the thyroid and their role in pathogenesis of cancer, Saito et al. [51] succeeded in establishing what they called “thyroid organoids” in culture of mice thyroid cells, they transferred genetically-manipulated organoids into mouse recipients, which developed poorly differentiated thyroid cancer (PDTC). The genetic alteration that they performed is the high expression of oncogenic NRAS (NRASQ61R) in the organoids of thyroid obtained from mice with p53 knockout. It was concluded that mutations in p53 and NRAS genes of thyroid stem cells contribute to the development of the PDTC and anaplastic types of thyroid cancer, this new model (culture system of thyroid organoid) is a possible research tool for investigating thyroid functions in health and diseases.
Research work in the field of thyroid CSCs carries very promising solutions for cancer in terms of finding out specific therapeutic protocols that target the CSCs.
5 Markers used for characterizing thyroid stem cells
Number of studies described specific cellular markers for thyroid cells at different stages of development. Some of these studies were performed on mice and example of these is the one carried out by Antonica et al. [28], who worked on generating functional thyroid follicles from mouse embryonic stem cells. The progenitor/stem cells were found to express specific markers like paired box gene 8 (PAX-8), thyroid transcription factor 1 (TTF-1), and thyroid transcription factor 2 (TTF-2). However, in another murine study, the precursor cells were found to express thyrotropin receptor (TSH-R), and the fully differentiated thyroid follicles express thyroid peroxidase (TPO), thyroglobulin (Tg), and sodium/iodide symporter (NIS) [21]. Moreover, it was postulated that co-expression of PAX-8 and TTF-1 in embryonic stem cells is essential for the development of follicular structures in thyroid gland [26, 28]. Zane et al. [35] also reported the expression of TTF-1, TTF-2, and PAX-8 during development. These markers are likely indicative of thyroid stem cells [7]. Van Vliet [52] reported that the markers of early differentiation of thyroid cells (viz. TTF-1, TTF-2 and PAX-8) persist throughout the different phases of development of these cells (Van Vlient paper shows a representation of this continuous expression of the early thyroid genes). The SP cells that were described by Hoshi et al. [10] were found to be of two types SP1 and SP2 according to the expression of SCA-1 marker; SP1 are positive for this marker and SP2 are negative; both SP cells express Oct-4 marker. STC1 has been found to be expressed in mice thyroid SP cells [50]. Klonisch et al. [53] reported the expression of hematopoietically expressed homeobox protein HHEX in the early undifferentiated thyroid cells. The mature thyroid follicles start to express the markers of thyroid differentiation: Tg, TPO, and NIS [54], while Klonisch et al. [53] described the thyroid stem cells as residing within clusters of cells defined as solid cell nests, and express tumour protein 63 (p63).
Regarding human studies, Thomas et al. [16] demonstrated expression of octamer-binding protein 4 (Oct-4), the transcription factor GATA-4, hepatocyte nuclear factor 4-α (HNF-4-α) and PAX-8 in stem cells isolated from goitrous thyroid. Fierabracci et al. [18] found that CD34+ /CD45− cells represent the stem cells in human thyroid gland. These cells also express Oct-4 marker and homeobox transcription factor Nanog (hNanog). Lan et al. [17] characterized isolated stem-like cells from cultures of goitrous thyroid glands as expressing Oct-4, and found that these stem cells differentiated into thyroid cells expressing Pax-8, Tg, NIS and TPO. Oct-4 was also identified in thyroid cancer stem cells by Zheng et al. [55]. STC1 has been found to be expressed in thyroid cancer cells [50]. It has been reported that markers that are expressed by thyroid cancer cells differ according to the type of the cancer [35]. For example, the anaplastic thyroid cancer cells express the onco-fetal fibronectin and lack the markers of differentiation. On the other hand, papillary thyroid cancer cells express the onco-fetal fibronectin and the thyroglobulin (Tg) markers. In accordance with the notion of expression of specific markers by different types of thyroid cancer, Friedman et al. [56] described the expression of CD133 in anaplastic type of thyroid cancer but not in the other types. Mirshahidi et al. [57] described two subpopulation of papillary thyroid cancer stem cells according to the expression of aldehyde dehydrogenase ALDH; positive and negative. They suggested that the cooperation between these two subpopulations is crucial for the initiation and propagation of the tumor. Table 1 presents the different biomarkers that have been so far identified in the different types of thyroid cells.
6 Regulation of functions of stem cells
The modulation of function of the stem cells is the result of integration between internal genetic programs within the stem cells and external factors governed by the surrounding environment. It has been found that different signal pathways are involved in the control of the differentiation of stem cells. One of these pathways involves the deiodenases group of enzymes that determine the bioavailability of thyroid hormones [5]. The deiodenases play a role in the modification of thyroid hormone signals in adult stem cells, and this will determine the fate of the stem cell: self-renewal, proliferation or differentiation. Lack of expression of the deiodenases has been implicated in several pathological processes including cancer [59]. The role of the deiodenases in controlling the function of the stem cells in muscles has been investigated by Ambrosio et al. [60]. They found that deiodenases are crucial in monitoring the homeostasis and regenerative ability of the muscle cells by influencing the stem cells.
In a recent review, Cicatiello et al. [37] presented an evidence for the role of thyroid hormone (TH) in the pathogenesis of different types of cancer. This influence is due to the effect of TH on the CSCs that are believed to be the origin of the tumor. They stated that deiodenases play an important role in the regulation of action of TH; therefore, these enzymes would have an influence on the cancer initiation and progression. Three types of deiodenases are described: (D1 and D2) that catalyze production of the TH, and D3 that promotes degradation of the hormone [61]. Up- or downregulation of these enzymes have been described in different types of cancer [37, 62]. In their review, they postulated a beneficial therapeutic effect of TH in preventing and monitoring the progression of cancer in different types of tissues, i.e., TH can be used as an anti-cancer drug. Another study reported a role for the thyrotropin on the growth of the CSCs, which were found to be CD133+ in immune-deficient mice [56]. Therefore, it was postulated that developing a drug that targets this activated thyrotropin signalling could be an efficient tool in treating thyroid cancer.
Another functional marker that has been shown to have a role in the function of the CSCs in thyroid is intracellular reactive oxygen species (ROS) [63]. The level of ROS status is implicated in the metabolic programming of the cells; it was demonstrated that thyroid CSCs have a low level of ROS. They attributed this low level to the decreased mitochondrial oxidative phosphorylation and increased glutathione synthesis. Shimamura et al. [63] suggested that drugs that target ROS could help in the treatment of thyroid cancer.
Zhao et al. [64] postulated a role for the cell cycle checkpoint kinase 2 (CHK2) in the pathogenesis of thyroid cancer. They investigated this putative role in 100 patients with papillary thyroid cancer and found an elevated expression of this enzyme, which is linked to the progression of the cancer. So, they suggested a link between CHK2 and the survival of the circulating cancer cells.
7 Conclusion
Thyroid gland controls the energy and multiple functions in the body in coordination with other hormonal and neural factors. Thyroid stem cells were postulated in the 90s of the last century, but interest in investigating these cells started only during the last decade. The population of stem cells perform important roles in maintaining its own pool, as well as in regeneration and maintenance of thyroid functions. Defect in stem cell differentiation, DNA mutation and genetic alteration may lead to multiple diseases including cancer. This review summarized the current knowledge of thyroid stem cells in terms of methods of identification and mechanisms of their functions in health and disease.
Morphological characterization of thyroid stem cells focused on the expression of stem cell genes/markers. Examples of these markers are: stem cell antigen 1 (SCA-1), Octamer-binding transcription 4 (OCT-4), p63, CD34+ CD45-, paired box gene 8 (PAX-8), thyroid transcription factor 1 (TTF-1), thyroid transcription factor 2 (TTF-2), hematopoietically expressed homeobox protein HHEX, the transcription factor GATA-4, hepatocyte nuclear factor 4-α (HNF-4-α) and homeobox transcription factor Nanog (hNanog). In order to investigate the regenerative ability of thyroid cells and examine the putative roles of stem cells, researchers used different experimental animal models, like induction of inflammatory disease or performing partial thyroidectomy. Some of the postulated mechanisms of regeneration is the de-differentiation of mature thyroid cells, activation of the stem cells in the gland or migration of bone marrow-derived cells into thyroid gland. Stem cell populations and progenitors were also studied in human thyroid. Three putative mechanisms for cancer formation are proposed in thyroid: 1) multistep carcinogenesis model, 2) fetal cell carcinogenesis model, and 3) cancer stem cell model. Various mechanisms have been proposed that explain the maintenance of thyroid cancer, such as expression of Hypoxia-inducible factors (HIF) or expression of Stanniocalcin (STC). Understanding the underling mechanisms and the expression of the biomarkers in normal and abnormal status are promising physiological tools in promoting thyroid regeneration and in provision of direct management for thyroid cancer.
References
Ozaki T, Matsubara T, Seo D, Okamoto M, Nagashima K, Sasaki Y, et al. Thyroid regeneration: characterization of clear cells after partial thyroidectomy. Endocrinology. 2012;153(5):2514–25.
Sadler, T. W. and Langman, J. M. E. (2010) Langman's medical embryology. 11th ed., international ed. / T.W. Sadler ; original illustrations by Jill Leland ; computer illustrations by Susan L. Sadler-Redmond ; scanning electron micrographs by Kathy Tosney ; ultrasound images by Nancy Cheschier and Hytham Imseis. edn. Philadelphia: Wouters Kluwer/Lippincott Williams & Wilkins.
Johansson E, Andersson L, Örnros J, Carlsson T, Ingeson-Carlsson C, Liang S, et al. Revising the embryonic origin of thyroid C cells in mice and humans. Development. 2015;142(20):3519–28.
Nilsson M, Williams D. On the origin of cells and derivation of thyroid Cancer: C cell story revisited. Eur Thyroid J. 2016;5(2):79–93.
Salvatore D. Deiodinases and stem cells: an intimate relationship. J Endocrinol Investig. 2018;41(1):59–66.
Khanlarkhani N, Baazm M, Mohammadzadeh F, Najafi A, Mehdinejadiani S, Sobhani A. Multipotent stem cell and reproduction. J Stem Cells. 2016;11(4):219–29.
Thomas D, Friedman S, Lin RY. Thyroid stem cells: lessons from normal development and thyroid cancer. Endocr Relat Cancer. 2008;15(1):51–8.
Dumont JE, Lamy F, Roger P, Maenhaut C. Physiological and pathological regulation of thyroid cell proliferation and differentiation by thyrotropin and other factors. Physiol Rev. 1992;72(3):667–97.
Johansen R, Gardner RE, Galante M, Marchi FF, Ledwich TW, SOLEY MH, et al. An experimental study of thyroid regeneration following subtotal thyroidectomy. Surg Gynecol Obstet. 1951;93(3):303–9.
Hoshi N, Kusakabe T, Taylor BJ, Kimura S. Side population cells in the mouse thyroid exhibit stem/progenitor cell-like characteristics. Endocrinology. 2007;148(9):4251–8.
Chen CY, Kimura H, Landek-Salgado MA, Hagedorn J, Kimura M, Suzuki K, et al. Regenerative potentials of the murine thyroid in experimental autoimmune thyroiditis: role of CD24. Endocrinology. 2009;150(1):492–9.
Lehner B, Sandner B, Marschallinger J, Lehner C, Furtner T, Couillard-Despres S, et al. The dark side of BrdU in neural stem cell biology: detrimental effects on cell cycle, differentiation and survival. Cell Tissue Res. 2011;345(3):313–28.
Okamoto M, Hayase S, Miyakoshi M, Murata T, Kimura S. 'Stem cell antigen 1-positive mesenchymal cells are the origin of follicular cells during thyroid regeneration. PLoS One. 2013;8(11):e80801.
Kimura S. Thyroid regeneration: how stem cells play a role? Front Endocrinol (Lausanne). 2014;5:55.
Reis-Filho JS, Preto A, Soares P, Ricardo S, Cameselle-Teijeiro J, Sobrinho-Simões M. p63 expression in solid cell nests of the thyroid: further evidence for a stem cell origin. Mod Pathol. 2003;16(1):43–8.
Thomas T, Nowka K, Lan L, Derwahl M. Expression of endoderm stem cell markers: evidence for the presence of adult stem cells in human thyroid glands. Thyroid. 2006;16(6):537–44.
Lan L, Cui D, Nowka K, Derwahl M. Stem cells derived from goiters in adults form spheres in response to intense growth stimulation and require thyrotropin for differentiation into thyrocytes. J Clin Endocrinol Metab. 2007;92(9):3681–8.
Fierabracci A, Puglisi MA, Giuliani L, Mattarocci S, Gallinella-Muzi M. Identification of an adult stem/progenitor cell-like population in the human thyroid. J Endocrinol. 2008;198(3):471–87.
Satterthwaite AB, Burn TC, Le Beau MM, Tenen DG. Structure of the gene encoding CD34, a human hematopoietic stem cell antigen. Genomics. 1992;12(4):788–94.
Korostylev A, Mahaddalkar PU, Keminer O, Hadian K, Schorpp K, Gribbon P, et al. A high-content small molecule screen identifies novel inducers of definitive endoderm. Mol Metab. 2017;6(7):640–50.
Lin RY, Kubo A, Keller GM, Davies TF. Committing embryonic stem cells to differentiate into thyrocyte-like cells in vitro. Endocrinology. 2003;144(6):2644–9.
Arufe MC, Lu M, Kubo A, Keller G, Davies TF, Lin RY. Directed differentiation of mouse embryonic stem cells into thyroid follicular cells. Endocrinology. 2006;147(6):3007–15.
Arufe MC, Lu M, Lin RY. Differentiation of murine embryonic stem cells to thyrocytes requires insulin and insulin-like growth factor-1. Biochem Biophys Res Commun. 2009;381(2):264–70.
Ma R, Latif R, Davies TF. Thyrotropin-independent induction of thyroid endoderm from embryonic stem cells by activin a. Endocrinology. 2009;150(4):1970–5.
Jiang N, Hu Y, Liu X, Wu Y, Zhang H, Chen G, et al. Differentiation of E14 mouse embryonic stem cells into thyrocytes in vitro. Thyroid. 2010;20(1):77–84.
Ma R, Latif R, Davies TF. Thyroid follicle formation and thyroglobulin expression in multipotent endodermal stem cells. Thyroid. 2013;23(4):385–91.
Arauchi A, Matsuura K, Shimizu T, Okano T. Functional thyroid follicular cells differentiation from human-induced pluripotent stem cells in suspension culture. Front Endocrinol (Lausanne). 2017;8:103.
Antonica F, Kasprzyk DF, Opitz R, Iacovino M, Liao XH, Dumitrescu AM, et al. Generation of functional thyroid from embryonic stem cells. Nature. 2012;491(7422):66–71.
Davies TF, Latif R, Minsky NC, Ma R. Clinical review: the emerging cell biology of thyroid stem cells. J Clin Endocrinol Metab. 2011;96(9):2692–702.
Onyshchenko MI, Panyutin IG, Panyutin IV, Neumann RD. Stimulation of cultured h9 human embryonic stem cells with thyroid stimulating hormone does not lead to formation of thyroid-like cells. Stem Cells Int. 2012;2012:634914.
Hick AC, Delmarcelle AS, Bouquet M, Klotz S, Copetti T, Forez C, et al. Reciprocal epithelial:endothelial paracrine interactions during thyroid development govern follicular organization and C-cells differentiation. Dev Biol. 2013;381(1):227–40.
Murata T, Iwadate M, Takizawa Y, Miyakoshi M, Hayase S, Yang W, et al. An adult mouse thyroid side population cell line that exhibits enriched epithelial-mesenchymal transition. Thyroid. 2017;27(3):460–74.
Hussain F, Iqbal S, Mehmood A, Bazarbashi S, ElHassan T, Chaudhri N. Incidence of thyroid cancer in the Kingdom of Saudi Arabia, 2000-2010. Hematol Oncol Stem Cell Ther. 2013;6(2):58–64.
Alghamdi IG, Hussain II, Alghamdi MS, Dohal AA, Almalki SS, El-Sheemy MA. The incidence rate of thyroid cancer among women in Saudi Arabia: an observational descriptive epidemiological analysis of data from Saudi Cancer registry 2001-2008. J Immigr Minor Health. 2015;17(3):638–43.
Zane M, Scavo E, Catalano V, Bonanno M, Todaro M, De Maria R, et al. Normal vs cancer thyroid stem cells: the road to transformation. Oncogene. 2016;35(7):805–15.
Gao YJ, Li B, Wu XY, Cui J, Han JK. Thyroid tumor-initiating cells: increasing evidence and opportunities for anticancer therapy (review). Oncol Rep. 2014;31(3):1035–42.
Cicatiello AG, Ambrosio R, Dentice M. Thyroid hormone promotes differentiation of colon cancer stem cells. Mol Cell Endocrinol. 2017;459:84–9.
Zhang P, Zuo H, Ozaki T, Nakagomi N, Kakudo K. Cancer stem cell hypothesis in thyroid cancer. Pathol Int. 2006;56(9):485–9.
Burstein DE, Nagi C, Wang BY, Unger P. 'Immunohistochemical detection of p53 homolog p63 in solid cell nests, papillary thyroid carcinoma, and hashimoto's thyroiditis: a stem cell hypothesis of papillary carcinoma oncogenesis. Hum Pathol. 2004;35(4):465–73.
Takano T, Amino N. Fetal cell carcinogenesis: a new hypothesis for better understanding of thyroid carcinoma. Thyroid. 2005;15(5):432–8.
Takano T. Fetal cell carcinogenesis of the thyroid: theory and practice. Semin Cancer Biol. 2007;17(3):233–40.
Lin RY. Thyroid cancer stem cells. Nat Rev Endocrinol. 2011;7(10):609–16.
Benedict M, Costa J. Metastatic papillary thyroid carcinoma with multifocal synchronous transformation to anaplastic thyroid carcinoma. Case Rep Pathol. 2016;2016:4863405.
Papp S, Asa SL. When thyroid carcinoma goes bad: a morphological and molecular analysis. Head Neck Pathol. 2015;9(1):16–23.
Mitsutake N, Iwao A, Nagai K, Namba H, Ohtsuru A, Saenko V, et al. Characterization of side population in thyroid cancer cell lines: cancer stem-like cells are enriched partly but not exclusively. Endocrinology. 2007;148(4):1797–803.
Nagayama Y, Shimamura M, Mitsutake N. Cancer stem cells in the thyroid. Front Endocrinol (Lausanne). 2016;7:20.
Mahkamova K, Latar N, Aspinall S, Meeson A. Hypoxia increases thyroid Cancer stem cell-enriched side population. World J Surg. 2018;42(2):350–7.
Yeung BH, Law AY, Wong CK. Evolution and roles of stanniocalcin. Mol Cell Endocrinol. 2012;349(2):272–80.
Lin S, Guo Q, Wen J, Li C, Lin J, Cui X, et al. Survival analyses correlate stanniocalcin 2 overexpression to poor prognosis of nasopharyngeal carcinomas. J Exp Clin Cancer Res. 2014;33:26.
Hayase S, Sasaki Y, Matsubara T, Seo D, Miyakoshi M, Murata T, et al. Expression of stanniocalcin 1 in thyroid side population cells and thyroid cancer cells. Thyroid. 2015;25(4):425–36.
Saito Y, Onishi N, Takami H, Seishima R, Inoue H, Hirata Y, et al. Development of a functional thyroid model based on an organoid culture system. Biochem Biophys Res Commun. 2018;497(2):783–9.
Van Vliet G. Development of the thyroid gland: lessons from congenitally hypothyroid mice and men. Clin Genet. 2003;63(6):445–55.
Klonisch T, Hoang-Vu C, Hombach-Klonisch S. Thyroid stem cells and cancer. Thyroid. 2009;19(12):1303–15.
Davies TF, Ando T, Lin RY, Tomer Y, Latif R. Thyrotropin receptor-associated diseases: from adenomata to graves disease. J Clin Invest. 2005;115(8):1972–83.
Zheng X, Cui D, Xu S, Brabant G, Derwahl M. Doxorubicin fails to eradicate cancer stem cells derived from anaplastic thyroid carcinoma cells: characterization of resistant cells. Int J Oncol. 2010;37(2):307–15.
Friedman S, Lu M, Schultz A, Thomas D, Lin RY. 'CD133+ anaplastic thyroid cancer cells initiate tumors in immunodeficient mice and are regulated by thyrotropin. PLoS One. 2009;4(4):e5395.
Mirshahidi S, Simental A, Lee SC, De Andrade Filho PA, Peterson NR, Cao W, et al. Subpopulations of cancer stem cells found in papillary thyroid carcinoma. Exp Cell Res. 2018;362(2):515–24.
Grosse-Gehling P, Fargeas CA, Dittfeld C, Garbe Y, Alison MR, Corbeil D, et al. CD133 as a biomarker for putative cancer stem cells in solid tumours: limitations, problems and challenges. J Pathol. 2013;229(3):355–78.
Canettieri G, Franchi A, Sibilla R, Guzmán E, Centanni M. Functional characterisation of the CRE/TATA box unit of type 2 deiodinase gene promoter in a human choriocarcinoma cell line. J Mol Endocrinol. 2004;33(1):51–8.
Ambrosio R, De Stefano MA, Di Girolamo D, Salvatore D. Thyroid hormone signaling and deiodinase actions in muscle stem/progenitor cells. Mol Cell Endocrinol. 2017;459:79–83.
Visser TJ. Thyroid hormone transport across the placenta. Ann Endocrinol (Paris). 2016;77(6):680–3.
Casula S, Bianco AC. Thyroid hormone deiodinases and cancer. Front Endocrinol (Lausanne). 2012;3:74.
Shimamura M, Yamamoto K, Kurashige T, Nagayama Y. Intracellular redox status controls spherogenicity, an in vitro cancer stem cell marker, in thyroid cancer cell lines. Exp Cell Res. 2018;370(2):699–707.
Zhao W, Chen S, Hou X, Chen G, Zhao Y. CHK2 promotes Anoikis and is associated with the progression of papillary thyroid Cancer. Cell Physiol Biochem. 2018;45(4):1590–602.
Author information
Authors and Affiliations
Contributions
EA designed the idea, EA and KA drafted and revised the manuscript, KA designed the figure while EA drafted the table. Both authors approved the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that there is no conflict of interest.
Author disclosure statement
No competing financial interests exist.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Al-Suhaimi, E.A., Al-Khater, K. Functions of stem cells of thyroid glands in health and disease. Rev Endocr Metab Disord 20, 187–195 (2019). https://doi.org/10.1007/s11154-019-09496-x
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11154-019-09496-x