Abstract
Myo-inositol (Myo-Ins) plays an important role in thyroid function and autoimmunity. Myo-Ins is the precursor for the synthesis of phosphoinositides, which takes part in the phosphatidylinositol (PtdIns) signal transduction pathway, and plays a decisive role in several cellular processes. In the thyroid cells, PtdIns is involved in the intracellular thyroid-stimulating hormone (TSH) signaling, via Phosphatidylinositol (3,4,5)-trisphosphate (PtdIns(3,4,5)P3) (PIP-3). Moreover, the phosphatidyl inositol 3 kinases (PI3K) family of lipid kinases regulates diverse aspects of T, B, and Tregs lymphocyte behaviour. Different mouse models deficient for the molecules involved in the PIP3 pathway suggest that impairment of PIP3 signaling leads to dysregulation of immune responses and, sometimes, autoimmunity. Studies have shown that cytokines modulate Myo-Ins in thyroid cells. Moreover, clinical studies have shown that after treatment with Myo-inositol plus seleniomethionine (Myo-Ins + Se), TSH levels significantly declined in patients with subclinical hypothyroidism due to autoimmune thyroiditis. The treatment was accompanied by a decline of antithyroid autoantibodies. After treatment serum CXCL10 levels declined, confirming the immune-modulatory effect of Myo-Ins. Additional research is necessary in larger population to evaluate the effect on the quality of life, and to study the mechanism of the effect on chemokines.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
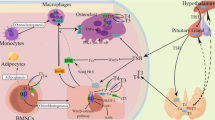
Inositol(s) (INS) are hexahydroxycyclohexanes (C6H12O6) deriving from cyclohexane, and nine different stereoisomeric forms exist, constituted by a ring with six carbon atoms each bound to a hydroxyl group. Isomers derive from the epimerization of the six hydroxyl groups [1]. INS is the main element of phytates [2]. Myo-inositol (Myo-Ins) is more than 99% of the intracellular INS of most tissues. These molecules derive from the diet (beans, citrus fruits, except from lemon, nuts, and cereals with elevated bran content) [3] and endogenous biosynthesis (Myo-Ins is produced from glucose [4], mainly from liver and kidney). Noteworthy, Myo-Ins plays a key biological role and looks to be essential for our health. Myo-Ins is a precursor in the synthesis of phosphatidylinositol (PtdIns) polyphosphates, that have important physiological functions [5]. Cellular membranes have the precursor of inositol trisphosphate (InsP3), phosphatidyl-Myo-Ins, a second messenger that participates to the regulation of numerous signals [6]. In cells, INS derivatives constitute the structural lipids, and take part in crucial biochemical pathways, as glucose metabolism, regulation of cell proliferation, morphogenesis, fertility and cytoskeleton rearrangement [7, 8]. INS are involved in hormones signaling, which is the case of thyroid stimulating hormone (TSH), insulin and follicle stimulating hormone (FSH). In the thyroid, modifications and imbalances in the inositol metabolism impair steps which require a fine regulation in hormone biosynthesis, storage, and secretion. In thyroid cells, TSH stimulates inositol phosphate formation in a concentration-dependent manner [9]. Thus, it was shown that the action of TSH in thyroid tissue was mediated also by another second-messenger system, in addition to cyclic AMP (cAMP).
Both the phosphatidylinositol 4,5-bisphosphate (PIP2) and the cAMP cascade control thyroid hormones (THs) synthesis. These metabolic pathways are connected to the activation and inhibition of the hydrogen peroxide synthesis; indeed, the thyroid gland is a unique endocrine organ requiring H2O2 for the hormone assembly. The physiologic hydrogen peroxide production is a restrictive factor in the organification process (as for example the reaction that permits inorganic iodine to bind tyrosine residues by thyroid peroxidase) [10]. If this process is impaired, several illnesses can arise; indeed, the overproduction and lack of H2O2 degradation may explain, at least partially, serious pathologies such as thyroiditis and tumors of this gland. On the other hand, the failure in the hydrogen peroxide production or the impairment of its positive control system may cause diseases, such as congenital hypothyroidism [11]. Experimental researches have shown that partial organification defects can be caused by TSH-receptor (TSH-R) mutations, with also a complete loss of inositol phosphates (IP) signaling [12].
Phosphatidyl inositol 3 kinases (PI3K) signaling is a complex network of interactions, that guarantee the right cell responses and to maintain immune homeostasis [13]. In different mouse models deficient for the molecules involved in PIP3 cascade it has been shown that impairment of PIP3 signaling can dysregulate immune responses and, sometimes, autoimmunity [14]. PI3K signaling plays a central role in determining B cell fate [15]. Furthermore, the inositol poly-phosphatases are determinant for the effector and regulatory functions of the T cell compartment. Moreover, PI3K controls chemokine responsiveness and antigen-driven changes in lymphocyte trafficking. Consequently, human health can be damaged by the breakdown of immune system in this way giving rise to autoimmune diseases.
We aim to review the role of Myo-Ins in human thyroid pathology and autoimmunity.
2 TSH signaling
2.1 TH biosynthesis, storage, and secretion
TH synthesis starts with iodide uptake and binding of TSH to its cognate receptor (TSH-R); the TSH/TSH-R couple is the principal trophic hormone of thyrocytes [16]. TSH-R is expressed on the basolateral membrane, belongs to the G protein-coupled seven transmembrane receptor family, the same that includes luteinizing hormone (LH), chorionic gonadotropin (CG) and FSH.
2.2 TSH/TSH-R/PKC/IP3 and TSH/TSH-R/PKA/cAMP cascades
Once TSH binds to TSH-R, Gsα is coupled, adenylate cyclase is activated, leading to the formation of cAMP, and protein kinase A (PKA) is phosphorylated, activating downstream proteins in the nucleus and cytosol. This pathway regulates TH synthesis, thyroidal growth and differentiation. At elevated TSH levels, after its bond to TSH-R, Gαq/11 is stimulated and the phospholipase C-dependent inositol phosphate Ca2+/diacylglycerol cascade (leading to the generation of inositol 1,4,5-triphosphate [IP3]) activates H2O2 formation and iodination. IP3 rises the intracellular Ca2+ concentration and it is released from the endoplasmic reticulum. H2O2 is a restrictive factor in iodide oxidation and organification, and coupling reaction. In addition to the TSH/TSH-R/PKC/IP3 cascade (and agents stimulating the PI pathway), also the TSH/TSH-R/PKA/cAMP pathway activates the generation of H2O2, thanks to the cross-talk between the two pathways of TSH-R signaling [16]. Interestingly, triiodothyronine (T3) upregulates the leptin expression via PI3K in adipocytes [17].
Phospholipases also release arachidonic and linoleic acids that can be metabolized by lipoxigenase to form free radicals and lipid peroxides; these radicals and peroxides provide an inflammatory stress that can induce apoptosis. TSH, and cAMP agonists as well, protect thyrocytes from apoptosis that normally would be induced by H2O2 and other molecules. In addition to PKA, the protective effect of TSH involves PI3K, as the inhibitors of the PI3K promote apoptosis in thyroid cells [16, 18].
Several cytokines alter thyrocyte growth and function, and such cytokine effects are affected by TSH and cAMP. Once cytokine receptors in the plasma membrane are activated, they attract the cytoplasmic tyrosine kinase JAK (Janus kinase), creating docking sites for SH-2-containing proteins like the “signal transducer and activator of transcription” (STATs); TSH phosphorylates STAT-3, an action that can be blocked by inhibitors of PKC, but not by inhibitors of PKA [18].
3 Phosphatydylinositol and lymphocytes
3.1 The regulation of PIP3 cascade
Deletion in different members of the PtdIns signaling cascade leads to defects in natural killer (NK) cell repertoire expression and effector functions, in mouse models [19]. NK cells are determinant against autoimmunity, cancer and infection.
PI3K regulate various aspects of lymphocyte behavior. After the involvement of antigen receptor, PI3K lead to the formation of 3-phosphorylated inositol lipid products, that function as membrane targeting signals for different proteins included in the generation of multiprotein complexes (called “signalosomes”), and in the formation of immune synapse. Class IA PI3K is the main subgroup in B-cells, and if lost leads to strong defects in development and antigen reactivity, while in T-cells, both class IA and IB PI3K are involved in development and immune function. PI3K modulates the function of effector and regulatory (Tregs) T-cells, and in mice with PI3K-deficient T-cells these diverse functions cause unforeseen autoimmune phenotypes [13].
Tregs prevent autoimmune and inflammatory disorders. The definition of the signal transduction pathways determinant for Tregs development and function is still going on. T cell receptor (TCR), interleukin-2 receptor (IL-2R), and co-stimulatory receptor signaling have a key role in function of Tregs. After the stimulation of TCR, IL-2R, and CD28, the PI3K-regulated pathway is activated and leads to T cell activation, proliferation, and cell survival. The two phosphatidylinositol phosphatases SHIP and PTEN negatively regulate the activation of the PIP3 cascade. Different mouse models deficient for the molecules involved in PIP3 cascade propose that impairment of PIP3 signaling can dysregulate immune responses and, sometimes, autoimmunity [14]. Moreover, PI3K controls chemokine responsivity and antigen-driven changes in lymphocyte trafficking. PI3K signaling is a complex network of interactions, that need to be compensated correctly in order to guarantee the right cell responses and to maintain immune homeostasis [13].
Functionally silencing autoreactive B-cells that have eluded central tolerance checkpoints is a mechanism against autoimmune processes through the use of intravenous immunoglobulins that, in ex vivo stimulated human B cells, suppressed PI3K signaling, that is known to play a central role in determining B cell fate [15].
3.2 Inositol poly-phosphatases and their targets in T cell
Recently chemical and genetic data have shown that the inositol poly-phosphatases are determinant for the effector and regulatory functions of the T cell compartment. SHIP1 and SHIP1/2 (the 5′-inositol poly-phosphatases) can deviate PI(3,4,5)P3 to the infrequent but strong signaling phosphoinositide species PI(3,4)P2 and thus these SHIP1/2, and the inositol polyphosphate 4-phosphatase type I and type II enzymes (INPP4A and INPP4B) that deplete PI(3,4)P2 may amplify or inhibit effectors of PI3K signaling that are selectively recruited and activated by PI(3,4)P2. Pharmaceutical manipulation of these enzymes for therapeutic purposes can be potentially efficient in disease settings where T cell function is a key in vivo target [20]. Mice genetically-deficient for the B isoform of the inositol 1,4,5-trisphosphate 3-kinase (or Itpkb) have a severe defect in thymocytes differentiation and thus lack peripheral T cells. These Itpkb-deficient peripheral T cells have also an increased capacity to secrete cytokines upon stimulation [21].
Moreover, antiphospholipid antibodies are measurable in about 5% of healthy subjects. Besides lupus anticoagulant, anticardiolipin, and β2-glycoprotein (the major antiphospholipid antibodies), other autoantibodies of the antiphospholipid antibody syndrome (APLAS), are against phosphatidic acid, phosphatidylcholine, phosphatidylserine, phosphatidylinositol, annexin V, and phosphatidylethanolamine. Antiphosphatydylinositol antibodies can hamper the intracellular PI3K signaling [22].
4 Myo-inositol and cytokines
Autoimmune thyroid diseases (AITD) are T cell-mediated organ-specific autoimmune disorders, resulting from a dysregulation of the immune system leading to an immune attack on the thyroid gland; in fact, the common pathological feature of AITD is the presence of lymphocyte infiltrates into the gland [23, 24].
The central role of chemokines and cytokines in the pathogenesis of organ specific and sytemic autoimmune disorders, and AITD, have been reported [25,26,27]. In thyroidal tissues, recruited T helper 1 (Th1) lymphocytes can induce a higher secretion of IFN-γ and TNF-α, that stimulates chemokine (C-X-C motif) ligand 10 (CXCL10) (the prototype of the IFN-γ-inducible Th1 chemokines) release from thyrocytes; this leads to an amplification feedback loop, initiates and perpetuates the autoimmune process [28,29,30].
Studies have shown that cytokines modulate Myo-Ins in thyroid cells. A first study investigated the action of IFN-γ on the production of inositol phosphates and intracellular Ca2+ mobilization in primary cultures of human thyrocytes using the fluorescent Ca2+ indicator fura-2. IFN-γ increased the production of inositol mono-, bis-, and trisphosphates and caused a dose-dependent increase in intracellular Ca2+. The tyrosine protein kinase inhibitor, genistein, inhibited the production of inositol phosphates and the IFN-γ-induced increase of Ca2+, with no effects on ATP, suggesting that the mobilization of intracellular Ca2+ and the production of inositol phosphates are determinant for the effect of IFN-γ in human thyroid cells [31, 32].
Whether Myo-Ins might be able to modulate chemokines production in thyroid cells remains to be elucidated.
5 Myo-inositol in autoimmune thyroiditis and hypothyroidism
A double-blind randomized controlled trial has been conducted by Nordio et al. [33] in order to evaluate the efficacy of Myo-Ins and selenium (Se) combination in patients with subclinical hypothyroidism. They enrolled 48 women having subclinical hypothyroidism and elevated serum anti-thyroglobulin (AbTg) antibodies levels (> 350 IU/ml). Patients were randomly divided in group A including twenty-four subjects treated daily with oral 83 μg Se in soft gel capsule; and in group B with twenty-four patients taking a combined treatment Myo-Ins 600 mg plus 83 μg Se (oral tablets, for 6 months). Therefore TSH, anti-thyroid peroxidase (AbTPO) antibodies, AbTg, Myo-Ins, and Se plasma concentrations were measured showing the favorable effect of the therapy with Se in patients affected by subclinical hypothyroidism, that is significantly ameliorated by the combination with Myo-Ins. In group B a significant decline (31%) of TSH levels has been observed (4.4 ± 0.9 vs 3.1 ± 0.6 μIU/ml, P < 0.01), whereas no changes were seen in group A. AbTPO and AbTg levels significantly decreased in both groups. AbTg levels lower than the threshold have been found in eleven patients of group B, in treatment with Myo-Ins plus Se, vs three patients of the group A. Thyroid ultrasonography showed a normalized echogenicity in these patients [33].
Another study by Morgante et al. [34] investigated on the prevalence of subclinical thyroid dysfunctions in infertile polycystic ovary syndrome (PCOS) patients and also evaluated if insulin sensitizers in insulin resistant PCOS patients could ameliorate thyroid function, upon 6 months of treatment. A significant high prevalence of subclinical thyroid dysfunction has been observed in PCOS patients, overall in those overweight, obese and with insulin resistance (IR). A treatment of 6 months with insulin sensitizers (containing Inositol) decreased TSH levels in insulin resistant PCOS patients, in a significant manner.
More recently, one hundred and sixty-eight patients with Hashimoto's thyroiditis (HT) and TSH levels between 3 and 6 μIU/ml were evaluated by Nordio et al. [35]. Patients were randomly divided into 2 groups; in one group they were treated with Myo-Ins + Se and in the other with only Se. Treatment with Myo-Ins + Se leads to a significative decrease of TSH, AbTPO and AbTg levels, and also to an improvement of thyroid hormones and personal wellbeing [35].
Moreover we have conducted a study enrolling twenty-one Caucasian patients with newly diagnosed euthyroid chronic autoimmune thyroiditis (AT), treated with Myo-Ins plus Se tablets (600 mg/83 μg, assumed twice/day for 6 months). We have observed a reduction of TSH levels after the treatment. Therefore this leads to hypothesize that the combined treatment is effective in reducing the risk to develop hypothyroidism in individuals affected by AITD. We also found that anti-thyroid autoantibodies decreased after treatment, as well as CXCL10 levels, thus confirming the immune-modulatory effect exerted by the treatment [36].
Myo-Ins exerts a favorable effect on TSH thanks to its biological role in the TSH hormone signaling. It is indeed able to regulate the H2O2-mediated iodination [37] and the impairment of inositol-depended TSH signaling cascade can lead to TSH resistance, and hypothyroidism [12]. Therefore, the treatment with Myo-Ins may rise the second messenger, and improve TSH sensitivity.
IFN-γ-inducible protein 10 (IP-10), also known as CXCL10, binds to the chemokine (C-X-C motif) receptor 3 (CXCR3), promoting the pathogenesis of different autoimmune diseases, systemic (such as systemic lupus erythematosus, systemic sclerosis, mixed cryoglobulinemia, or Sjogren syndrome), or organ specific (such as Graves’ disease and Graves’ Ophthalmopathy, Type 1 diabetes) [28, 38, 39].
IFN-γ stimulates CXCL10 secretion through CD4+, CD8+, and NK, also by thyrocytes. Elevated CXCL10 levels in peripheral fluids are, then, a marker of Th1 orientated immune response. CXCL10 serum levels are high in patients affected by AT, particularly in those having a hypoechoic ultrasonographic pattern, that is a sign of a more severe lymphomonocytic infiltration, and in patients with hypothyroidism. Hence, CXCL10 may be a marker of a more aggressive and stronger inflammatory response in the thyroid gland, which causes thyroid damage and thyroid dysfunction [40,41,42,43,44,45,46,47].
A study evaluated if blood mononuclear cells (PBMC) from HT and control women were protected from in vitro H2O2-induced oxidative stress upon treatment with antioxidants. H2O2 alone reduced PBMC proliferation, and it decreased furtherly and dose-dependently in either group, particularly with Myo-Ins + Se in HT. Vitality was reduced by H2O2 alone in controls and in the HT patients, but it was rescued by the three additions. The Comet score was rised above baseline in controls and in HT women by H2O2 alone. In either group, genotoxicity was contrasted dose-dependently by each addition. Chemokines levels were increased by H2O2 alone, and more in HT women than in controls. These concentrations were reduced dose-dependently (often under baseline) by each addition in either group, in particular with Myo-Ins + Se (up to about −80% of baseline). The evaluated antioxidants exert favorable effects on PBMC exposed in vitro to H2O2-induced oxidative stress both in controls and HT women, and the association Myo-Ins + Se was the most effective [48].
The immune-modulatory effect exerted by the combination of Myo-Ins and Se on CXCL10 suggests that they are capable of modulating the Th1 immune response, then these findings prompt to investigate on autoimmune diseases associated with the predominant Th1 immune response; the mechanisms need to be further explored [49, 50].
Moreover more recently Nordio et al. [51] carried out a retrospective, observational study enrolling 642 patients with suspected hypothyroidism undergoing ultrasound. In the analysis only patients having subclinical hypothyroidism or TSH levels borderline associated to thyroid nodules defined as class I and II were taken into account. The Authors concluded that a reduction of the size, number and elasticity score of thyroid nodules and of TSH levels was observed in patients with subclinical hypothyroidism after treatment with Myo-Ins plus Se [51].
6 Conclusion
Myo-inositol (Myo-Ins) plays an important role in thyroid function and autoimmunity. Myo-Ins is the precursor for the synthesis of phosphoinositides, is involved in the phosphatidylinositol (PtdIns) signal transduction cascade, and plays a decisive role in several cellular processes.
Several mouse models deficient for the molecules involved in the PIP3 pathway suggest that impairment of PIP3 signaling leads to dysregulation of immune responses and, in some cases, autoimmunity.
Clinical studies have shown that after treatment with Myo-Ins + Se, TSH levels significantly declined in patients with subclinical hypothyroidism due to autoimmune thyroiditis. The treatment was accompanied by a decline of antithyroid autoantibodies levels. Furthermore, after treatment serum CXCL10 declined, confirming the immune-modulatory effect of Myo-Ins. Additional research is necessary in larger population, to evaluate the effect on the quality of life, and to study the mechanism at the basis of chemokines modulation.
References
Murthy PP. Structure and nomenclature of inositol phosphates, phosphoinositides, and glycosylphosphatidylinositols. Subcell Biochem. 2006;39:1–19.
Hartig T. Ueber das Klebermehl. Bot Zeitung. 1855;13:881.
Clements RS Jr, Darnell B. Myo-inositol content of common foods: development of a high-myo-inositol diet. Am J Clin Nutr. 1980;33:1954–67.
Hooper NM. Glycosyl-phosphatidylinositol anchored membrane enzymes. Clin Chim Acta. 1997;266:3–12.
Di Paolo G, De Camilli P. Phosphoinositides in cell regulation and membrane dynamics. Nature. 2006;443:651–7.
Berridge MJ. Inositol trisphosphate and diacylglycerol as second messengers. Biochem J. 1984;220:345–60.
Downes CP. Twenty-fifth Colworth medal lecture. The cellular functions of myo-inositol. Biochem Soc Trans. 1989;17:259–68.
Downes CP, Macphee CH. Myo-inositol metabolites as cellular signals. Eur J Biochem. 1990;193:1–18.
Field JB, Ealey PA, Marshall NJ, Cockcroft S. Thyroid-stimulating hormone stimulates increases in inositol phosphates as well as cyclic AMP in the FRTL-5 rat thyroid cell line. Biochem J. 1987;247:519–24.
Corvilain B, Laurent E, Lecomte M, Vansande J, Dumont JE. Role of the cyclic adenosine 3′,5′-monophosphate and the phosphatidylinositol-Ca2+ cascades in mediating the effects of thyrotropin and iodide on hormone synthesis and secretion in human thyroid slices. J Clin Endocrinol Metab. 1994;79:152–9.
Song Y, Driessens N, Costa M, De Deken X, Detours V, Corvilain B, et al. Roles of hydrogen peroxide in thyroid physiology and disease. J Clin Endocrinol Metab. 2007;92:3764–73.
Grasberger H, Van Sande J, Hag-Dahood Mahameed A, Tenenbaum-Rakover Y, Refetoff S. A familial thyrotropin (TSH) receptormutation provides in vivo evidence that the inositol phosphates/Ca2+ cascade mediates TSH action on thyroid hormone synthesis. J Clin Endocrinol Metab. 2007;92:2816–20.
Fruman DA, Bismuth G. Fine tuning the immune response with PI3K. Immunol Rev. 2009;228:253–72.
Kashiwada M, Lu P, Rothman PB. PIP3 pathway in regulatory T cells and autoimmunity. Immunol Res. 2007;39:194–224.
Séïté JF, Goutsmedt C, Youinou P, Pers JO, Hillion S. Intravenous immunoglobulin induces a functional silencing program similar to anergy in human B cells. J Allergy Clin Immunol. 2014;133:181–8.e1–9.
Kopp P. Thyroid hormone synthesis. In: Braverman LE, Cooper DS, editors. Werner and Ingbar’s the thyroid: a fundamental and clinical text. 10th ed. Philadelphia: Wolters Kluwer Lippincott Williams & Wilkins; 2013. p. 48–74.
de Oliveira M, Luvizotto Rde A, Olimpio RM, De Sibio MT, Conde SJ, Biz Rodrigues Silva C, et al. Triiodothyronine increases mRNA and protein leptin levels in short time in 3T3-L1 adipocytes by PI3K pathway activation. PLoS One. 2013;8:e74856.
Spaulding SW. Biological actions of thyrotropin. In: Braverman LE, Utiger RD, editors. Werner and Ingbar’s the thyroid: a fundamental and clinical text. 9th ed. Philadelphia: Lippincott Williams & Wilkins; 2005. p. 183–97.
Gumbleton M, Kerr WG. Role of inositol phospholipid signaling in natural killer cell biology. Front Immunol. 2013;4:47.
Srivastava N, Sudan R, Kerr WG. Role of inositol poly-phosphatases and their targets in T cell biology. Front Immunol. 2013;4:288.
Pouillon V, Maréchal Y, Frippiat C, Erneux C, Schurmans S. Inositol 1,4,5-trisphosphate 3-kinase B (Itpkb) controls survival, proliferation and cytokine production in mouse peripheral T cells. Adv Biol Regul. 2013;53:39–50.
Belilos E, Carsons S. Antiphospholipid syndrome. http://emedicine.medscape.com/article/ 333221-overview. Accessed Sept 2018.
Romagnani S. The Th1/Th2 paradigm and allergic disorders. Allergy. 1998;53:12–5.
Orgiazzi J. Thyroid autoimmunity. Presse Med. 2012;41:e611–25.
Antonelli A, Fallahi P, Ferrari SM, Pupilli C, d'Annunzio G, Lorini R, et al. Serum Th1 (CXCL10) and Th2 (CCL2) chemokine levels in children with newly diagnosed type 1 diabetes: a longitudinal study. Diabet Med. 2008;25:1349–53.
Antonelli A, Ferri C, Fallahi P, Cazzato M, Ferrari SM, Sebastiani M, et al. Clinical and subclinical autoimmune thyroid disorders in systemic sclerosis. Eur J Endocrinol. 2007;156:431–7.
Antonelli A, Ferri C, Fallahi P, Ferrari SM, Frascerra S, Carpi A, et al. Alpha-chemokine CXCL10 and beta-chemokine CCL2 serum levels in patients with hepatitis C-associated cryoglobulinemia in the presence or absence of autoimmune thyroiditis. Metabolism. 2008;57:1270–7.
Antonelli A, Ferrari SM, Giuggioli D, Ferrannini E, Ferri C, Fallahi P. Chemokine (C-X-C motif) ligand (CXCL)10 in autoimmune diseases. Autoimmun Rev. 2014;13:272–80.
Antonelli A, Fallahi P, Delle Sedie A, Ferrari SM, Maccheroni M, Bombardieri S, et al. High values of Th1 (CXCL10) and Th2 (CCL2) chemokines in patients with psoriatic arthtritis. Clin Exp Rheumatol. 2009;27:22–7.
Antonelli A, Ferrari SM, Frascerra S, Galetta F, Franzoni F, Corrado A, et al. Circulating chemokine (CXC motif) ligand (CXCL)9 is increased in aggressive chronic autoimmune thyroiditis, in association with CXCL10. Cytokine. 2011;55:288–93.
Kung AW, Lau KS, Wong NS. Interferon-gamma increases intracellular calcium and inositol phosphates in primary human thyroid cell culture. Endocrinology. 1995;136:5028–33.
Kung AW, Lau KS. Gamma-interferon activates a nuclear protein that binds to the gamma-interferon activation site of the thyroglobulin gene. J Mol Endocrinol. 1998;20:293–8.
Nordio M, Pajalich R. Combined tretament with Myo-inositol and selenium ensures euthyroidism in subclinical hypothyroidism patients with autoimmune thyroiditis. J Thyroid Res. 2013;2013:424163.
Morgante G, Musacchio MC, Orvieto R, Massaro MG, De Leo V. Alterations in thyroid function among the different polycystic ovary syndrome phenotypes. Gynecol Endocrinol. 2013;29:967–9.
Nordio M, Basciani S. Myo-inositol plus selenium supplementation restores euthyroid state in Hashimoto’s patients with subclinical hypothyroidism. Eur Rev Med Pharmacol Sci. 2017;21(Suppl 2):51–9.
Ferrari SM, Fallahi P, Di Bari F, Vita R, Benvenga S, Antonelli A. Myo-inositol and selenium reduce the risk of developing overt hypothyroidism in patients with autoimmune thyroiditis. Eur Rev Med Pharmacol Sci. 2017;21(Suppl 2):36–42.
Ohye H, Sugawara M. Dual oxidase, hydrogen peroxide and thyroid diseases. Exp Biol Med (Maywood). 2010;235:424–33.
Antonelli A, Ferrari SM, Corrado A, Di Domenicantonio A, Fallahi P. Autoimmune thyroid disorders. Autoimmun Rev. 2015;14:174–80.
Fallahi P, Ferrari SM, Ruffilli I, Elia G, Biricotti M, Vita R, et al. The association of other autoimmune diseases in patients with autoimmune thyroiditis: review of the literature and report of a large series of patients. Autoimmun Rev. 2016;15:1125–8.
Antonelli A, Fallahi P, Delle Sedie A, Ferrari SM, Maccheroni M, Bombardieri S, et al. High values of alpha (CXCL10) and beta (CCL2) circulating chemokines in patients with psoriatic arthritis, in presence or absence of autoimmune thyroiditis. Autoimmunity. 2008;41:537–42.
Antonelli A, Ferrari SM, Mancusi C, Mazzi V, Pupilli C, Centanni M, et al. Interferon-α, −β and -γ induce CXCL11 secretion in human thyrocytes: modulation by peroxisome proliferator-activated receptor γ agonists. Immunobiology. 2013;218:690–5.
Antonelli A, Ferrari SM, Frascerra S, Pupilli C, Mancusi C, Metelli MR, et al. CXCL9 and CXCL11 chemokines modulation by peroxisome proliferator-activated receptor-alpha agonists secretion in Graves’ and normal thyrocyte. J Clin Endocrinol Metab. 2010;95:E413–20.
Antonelli A, Ferri C, Fallahi P, Ferrari SM, Frascerra S, Sebastiani M, et al. High values of CXCL10 serum levels in patients with hepatitis C associated mixed cryoglobulinemia in presence or absence of autoimmune thyroiditis. Cytokine. 2008;42:137–43.
Antonelli A, Ferrari SM, Fallahi P, Frascerra S, Piaggi S, Gelmini S, et al. Dysregulation of secretion of CXC alpha-chemokine CXCL10 in papillary thyroid cancer: modulation by peroxisome proliferator-activated receptor-gamma agonists. Endocr Relat Cancer. 2009;16:1299–311.
Antonelli A, Ferrari SM, Corrado A, Ferrannini E, Fallahi P. CXCR3, CXCL10 and type 1 diabetes. Cytokine Growth Factor Rev. 2014;25:57–65.
Antonelli A, Ferrari SM, Frascerra S, Di Domenicantonio A, Nicolini A, Ferrari P, et al. Increase of circulating CXCL9 and CXCL11 associated with euthyroid or subclinically hypothyroid autoimmune thyroiditis. J Clin Endocrinol Metab. 2011;96:1859–63.
Fallahi P, Ferri C, Ferrari SM, Corrado A, Sansonno D, Antonelli A. Cytokines and HCV-related disorders. Clin Dev Immunol. 2012;2012:468107.
Benvenga S, Vicchio T, Di Bari F, Vita R, Fallahi P, Ferrari SM, et al. Favorable effects of myo-inositol, selenomethionine or their combination on the hydrogen peroxide-induced oxidative stress of peripheral mononuclear cells from patients with Hashimoto's thyroiditis: preliminary in vitro studies. Eur Rev Med Pharmacol Sci. 2017;21(Suppl 2):89–101.
Alon R, Shulman Z. Chemokine triggered integrin activation and actin remodeling events guiding lymphocyte migration across vascular barriers. Exp Cell Res. 2011;317:632–41.
Cantrell D. Signaling in lymphocyte activation. Cold Spring Harb Perspect Biol. 2015;7:a018788.
Nordio M, Basciani S. Evaluation of thyroid nodule characteristics in subclinical hypothyroid patients under a myo-inositol plus selenium treatment. Eur Rev Med Pharmacol Sci. 2018;22:2153–9.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Research involving human participants
This article does not contain any studies with human participants or animals performed by any of the authors.
Rights and permissions
About this article
Cite this article
Fallahi, P., Ferrari, S.M., Elia, G. et al. Myo-inositol in autoimmune thyroiditis, and hypothyroidism. Rev Endocr Metab Disord 19, 349–354 (2018). https://doi.org/10.1007/s11154-018-9477-9
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11154-018-9477-9