Abstract
Gonadotropins, the action of which is mediated at the level of their gonadal receptors, play a key role in sexual development, reproductive functions and in metabolism. The involvement of the gonadotropins and their receptor genotypes on reproductive function are widely studied. A large number of gonadotropins and their receptors gene polymorphisms are known, but the only one considerable as a clear, absolute genetic marker of reproductive features or disfunctions is the FSHR Asn680Ser polymorphism, since it modulates ovarian response to FSH. The aim of these studies would to be the prediction of the genetic causes of sex-related diseases to enable a customized clinical setting based on individual response of patients undergoing gonadotropin stimulation. In this review we discuss the latest information about the effects of polymorphisms of the gonadotropins and their receptor genes on reproductive functions of both male and female, and discuss their patho-physiological implications.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
1.1 Physiological and molecular aspects
The follicle-stimulating hormone (FSH) and luteinizing hormone (LH) are glycoproteins produced by the anterior lobe of the pituitary gland under gonadotropin releasing hormone (GnRH) pulsatile stimulation. In primates, placental choriogonadotropin (hCG) shows LH-like activity, but the two hormones differ in their half-life, in a carboxyterminal peptide of 24 aminoacids, present in hCG but absent in LH, and in glycosilation features. Glycoprotein hormones are heterodymers consisting of a common α subunit and a specific β subunit which determines receptor specificity. The thyroid-stimulating hormone (TSH) is a glycoprotein which shares the same structure with the gonadotropins, a common α subunits and a specific β subunit, but TSH acts on the thyroid gland stimulating the secretion of the hormones thyroxine (T4) and triiodothyronine (T3). FSH, LH and hCG act on the gonads and are essential for reproduction and sexual development. In males, FSH regulates maturation of the seminiferous tubules and spermatogenesis and induces Sertoli cells to secrete inhibin B and to produce androgen binding protein. In addition, the function of LH is to stimulate the production of testosterone by the Leydig cells. In females FSH stimulates the maturation of the follicular ovarian cells and production of estrogens, while LH induces the theca interna cells to produce high levels of androgens and leads the maturation of the follicle, the ovulation and the maintenance of the corpus luteum, contributing to the progress of ovarian cycle. hCG is the typical pregnancy hormone secreted by the trophoblast and placenta in a continuous fashion and its main functions are to rescue the corpus luteum of pregnancy from atresia, prepare the endometrium for the implantation of the embryo and promote fetal testicular testosterone production. These hormones act by binding their receptors localized in the gonads, the FSH receptor (FSHR) and LH/hCG receptor (LHCGR) [1, 2]. Together with thyroid-stimulating hormone receptor (TSHR), FSHR and LHCGR belong to the superfamily of G protein-coupled receptors (GPCRs), complex transmembrane rhodopsin-like proteins characterized by seven hydrophobic helices inserted in the plasmalemma (TMD) and by intracellular (IMD) and extracellular domains (ECD) of variable dimensions depending on the type of ligand. The large ECD contains nine leucine-rich repeats (LRR) flanked by N- and C-terminal cysteine-rich regions. To allow proper signal transduction, the binding of the hormone β-subunit to ECD is required, although additional contact sites in TM domain were identified [2]. The ICD is coupled to a Gs protein and, upon hormone binding, initiates signal transduction by the protein kinase A/cAMP pathway that ultimately leads to the specific biological effects of the gonadotropins [1–5].
1.2 Genes structure
The human FSHB is a single-copy gene of 4262 base pairs located on chromosome 11p13. The LHB and hCGB subunit genes span over seven gene clusters, one for LHB and six for hCGB, which have probably evolved from a common ancestral gene by duplication of a LHB gene copy [6, 7]. They are located in contiguity in a shared genomic region 45165 base pairs-long at position 19q13.32 [8]. The whole cluster consists of seven homologous genes: one LHB gene and six CGB genes [9]. The structural similarity of the glycoprotein heterodimeric hormones and TSH forecasted the homology in the extracellular domain of their receptors [2]. Furthermore, the sequence identity within the group of glycoprotein hormone receptors is relatively high, around 47–50%, and their genes are positioned in close proximity on chromosome 2p21, except the TSHR gene which is located on chromosome 14 [10]. A similarity in the genomic structure of both receptor genes was also observed. The FSHR gene consists of ten exons and nine introns. Exons 1–9 encode the extracellular domain, while exon 10 encodes the transmembrane and intracellular domains and the C-terminal portion of the extracellular domain [1, 11]. The LHCGR gene contains 11 exons and 10 introns. The first 10 exons and part of the last exon encode the extracellular domain of the receptor while the transmembrane and intracellular domains are encoded by the remaining part of the last exon [12]. These similarities suggest a possible phylogenetic relationship between FSHR and LHCGR [2].
1.3 The polymorphisms
The response to glycoprotein hormones can vary depending on sequence variations of the receptor genes and, in case of familial diseases, underlying mutations are genomic and inherited [13]. A study approach to test response to gonadotropins is to assess genetic variations for association to medical conditions. If an association is present, a particular variant will be seen more often than expected by chance in a population carrying the trait. Such analyses are based on a chromosomal property called linkage disequilibrium (LD). LD refers to the observation that in the general population, two DNA variants that are located close to each other tend to be observed together more frequently than two variants that are located further apart. Variants may be single-base changes, known as single-nucleotide polymorphisms (SNPs), or they may occur as insertions or deletions of one base or more [14]. SNPs differ from gene mutations for their frequency in the sample population. Indeed, a genetic variation is considered a SNP when it occurs in a frequency higher than 1%. In designing and interpreting association studies, it is necessary to understand that the human genome can be subdivided in haplotype blocks. Haplotypes are particular combinations of alleles observed in a population, and are determined by the SNP combination in a given genome sequence. Thus, haplotype blocks are sizable regions historically poor in recombination events and within which only a few common haplotypes are observed [15]. Therefore, haplotype blocks could be used as marker regions in genome-wide association studies as they are mapped in the HapMap online database (http://hapmap.ncbi.nlm.nih.gov) [16, 17].
Others common tools widely used in association studies are the National Center for Biotechnology Information (NCBI) SNPs database (http://www.ncbi.nlm.nih.gov) and the Center for Human and Clinical Genetics copy number variations (http://www.humgen.nl/SNP_databases.html), in which the SNPs are registered following a specific nomenclature. For example, in the NCBI database the FSHR and LHCGR genes count more than 2,000 SNPs but only a few are considered common SNPs (Fig. 1) due to their high frequency worlwide in populations of different ethnicity. An example of common SNP is the Adenine-to-Guanine nucleotidic substitution at position 2039 (A2039G; NCBI database acronym: rs6166) respect to the transcription start site in the FSHR gene. It is a non-synonymous substitution since it determines an Asparagine-to-Serine change at position 680 of the aminoacid chain. Asn680Ser has a minor allele frequency (MAF) of about 40% in caucasian population [18] and is associated to a difference in the FSH response in vivo [19]. The Asn680Ser polymorphism of FSHR is in strong LD with another polymorphism, Thr307Ala, and they often segregate together in the allelic combination Thr307-Asn680 and Ala307-Ser680, which are equally distributed in Caucasians according to mendelian laws [20]. Other allelic combinations are more rare and vary according to ethnicity of the populations, as shown in the NCBI SNPs database. The preference of analyzing one SNP rather than another has no biological reasons but depends on individual choice.
In this review we discuss the pathophysiological implications of the gonadotropins and their receptor genes polymorphisms on reproductive functions.
2 Female fertility and infertility
2.1 Menstrual cycle, oligo-amenorrhea and amenorrhea
2.1.1 The FSHR Asn680Ser polymorphism
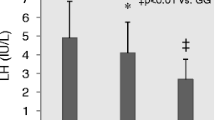
The normal ovarian cycle is controlled by the secretion of gonadotropins from the pituitary gland, the activity of which is modulated by the feedback control of the ovarian hormones. Follicular maturation begins with the monthly recruitment of a cohort of follicles at the antral stage and requires elevated serum FSH concentrations to trigger the growth and differentiation of granulosa cells, which regulate oocyte maturation [21]. The temporary nature of the FSH peak determines atresia of most of these follicles and usually only the one with the lowest FSH recruitment threshold can grow and becomes the Graafian follicle, acquiring LH resposnsiveness and undergoing ovulation [22]. In controlled ovarian hyperstimulation for assisted reproduction, the concept of a discrete FSH threshold implies that is necessary to reach a distinct serum FSH concentration allowing follicular growth, for the time necessary to avoid that most follicles become atretic [22–24]. Since the biological response to FSH depends on the FSHR expressed on granulosa cells [19, 25], several studies have focused on the influence of this receptor on menstrual cycle dynamics [19, 26, 27]. To date, the existence of some common polymorphisms which are important in determining the response to FSH stimulation is a well established concept, despite in most cases, for example the FSHR Asn680Ser polymorphism, the molecular mechanism whereby a SNP causes the phenotype is not known [28]. Indeed, women with homozygous FSHR Ser680 genotype require a higher number of FSH ampoules in ovarian hyperstimulation, compared to the homozygous Asn680 carriers [19]. These data suggests that the FSHR Ser680 genotype is less sensitive to the FSH action in vivo, compared to the FSHR Asn680 genotype. Subsequent studies in ovulatory and anovulatory women confirmed this observation [29]. Basal serum FSH levels were observed to be higher in FSHR Ser680 than Asn680 carriers during the luteo-follicular transition in normo-ovulatory women, reflecting the tendency of Ser680 genotypes to be a factor of major “resistance” to FSH stimulation and explaining its association with a higher ovarian treshold of the gonadotropin [26]. In this women, no difference in the levels of estradiol, inhibin B and in growth velocities of the dominant follicle has been observed but they show a clearly different pattern of ovarian secretion during the intercycle transition phase, compared to Asn680 carriers. The decreased negative feedback of luteal secretion to the pituitary gland triggers an earlier rise in FSH levels and provokes a duration of the menstrual cycle of about 3 days longer in homozygous Ser680 carriers [26, 27]. In summary, the homozygous FSHR Ser680 genotype results in a higher ovarian threshold for FSH, decreased negative feedback to the pituitary gland and longer menstrual cycle [26]. Therefore, it is plausible that Ser680 homozygous genotype can also influence the duration of fertile age, since the timing of puberty and probably also of menopause can be due to genetic features [30, 31]. In fact, the homozygosis for FSHR Asn680 allelic variant has been associated to a slightly delayed age of menarche in Italian women, but the same study did not find any correlation with menopausal age, excluding that FSHR gene may be a predictive factor for this parameter [32]. Studies in large populations of different ethnicity are missing, however, and the way the FSHR Ser680 polymorphism influences the duration of fertile age is not known.
2.1.2 The FSHR G-29A polymorphism
Recently, a retrospective study performed in only 86 Indian women found a FSHR polymorphism located in the 5′ untranslated region of the gene (G/A at position −29) to be related with primary amenhorrea [33]. These patients are characterized by significantly higher FSH serum levels and in this group the frequency distribution of −29G genotype was significantly higher compared to a control group of subjects without primary or secondary amenorrhea. This study is based on a too small number of patients to allow any conclusion about the role of the −29 SNP on FSHR function. Overall, the current literature is rather consistent in showing the association of the FSHR Asn680Ser polymorphism with menstrual cycle features. Other SNPs located in genes involved in the hypothalamic-pituitary-ovarian axis downstream to gonadotropins genes, such as the Estrogen receptors (ESR1 and ESR2), the enzyme aromatase (CYP19A1) or the 17β-hydroxysteroid dehydrogenase (17HSD), have been studied, and could modulate ovarian response [34] as well similarly the FSHB polymorphism G-211T (rs10835638), which affects serum FSH levels in men [35], might be involved, although its effects in women have not been studied so far.
2.2 Ovulation induction and controlled ovarian stimulation (COS)
2.2.1 FSHR polymorphisms in COS
Ovarian response could be defined as the endocrine and follicular reaction to stimulation [36]. It is of high clinical relevance to identify predictive factors of ovarian response that will enable clinicians to identify the best schemes of ovulation induction and ovarian stimulation, in order to optimize pregnancy rate without complications and to avoid failure of the clinical treatment. Although some predictive approaches have been designed, such as ovarian monitoring by ultrasonography or screening for endocrine and genetic characteristics, in practice the parameters of treatment are still entrust to the clinical experience and personal feelings of the treating physician [36]. A precise, individualized ovarian stimulation treatment designed on genetic predictive factors is still missing, despite the knowledge provided by the literature showing some correlations between SNPs in FSHR and LHB genes and ovarian function [19, 37–42]. Asn680Ser is the only FSHR polymorphism supported by a sufficient number of consistent studies which identified an association with ovarian function. It is well established that the homozygous Ser680 genotype results in a higher dose of FSH to achieve an equal peak of estradiol in the homozygous Asn680 or in the heterozygous women [19]. These data were corroborated by a study in women undergoing controlled ovarian hyperstimulation which demonstrated that an equal dose of FSH results in a significantly lower level of serum estradiol in homozygous Ser680 women, compared to Asn680 homozygous carriers [43]. No difference in number of follicles, retrieved oocytes, fertilization rate or cumulative embryo score were observed between the two groups. Whether the Ser680 polymorphism has any effect on pregnancy rate is currently not known, since different studies have obtained contradictory results, with pregnancy rates in homozygous Asn680 women undergoing IVF reported to be higher [40] or lower [44] than homozygous Ser680 carriers. However, none of these studies had the statistical power necessary to arrive to conclusive results.
The FSHR polymorphism G/A at position −29 could modulate ovarian response to FSH [45]. Indeed, a study found that women homozygous for the A genotype undergoing controlled ovarian hyperstimulation and IVF required the highest dose of exogenous FSH. Also, in these women the levels of estradiol concentration measured before hCG administration and the number of pre-ovulatory follicles and of retrieved oocytes were lower compared to the other genotypes, suggesting an association between the SNP and a poor ovarian response [45]. However, this study was performed in only 50 patients and replication in a wider population group is needed to establish the response related to the polymorphism at position −29. To date, only the studies about Asn680Ser FSHR gene polymorphism provided a consistent association with ovarian function, suggesting that the SNPs in exon 10 could be used as marker to predict differences of the response to FSH stimulation in women with normal ovarian function [46].
2.2.2 The LHCGR gene
Regarding the LHCGR gene polymorphisms (Fig. 2), the most frequent SNP, consisting in the presence or absence of two amino acids in position 18 of exon 1 [47] has been associated with breast cancer possibly related to higher, but not demonstrated, estrogen levels [48]. Despite LH being a major regulator of steroid hormone production, a clear correlation between this polymorphism and modifications of the ovarian activity was never found [49].
2.2.3 The CGB genes
Possible modulators of some pregnancy features could be the hCGB genes. A study aimed to compare the expression of individual CGB genes during the normal pregnancy and in cases of recurrent miscarriages and ectopic pregnancy [50]. Despite high DNA sequence similarity between individual CGB subunit coding genes, a high variation in expression levels exists, both among the genes themselves as well as among the individuals. The individual variation could be caused by polymorphic variation of the CGB genes. On the other hand, an extremely high diversity in the expression level of the LHB/CGB genes has been identified, probably favored by the spread of polymorphisms by gene conversion between highly similar genes [50]. In fact, in two European population, from Estonia and from Finland, two polymorphisms in intron 2 of CGB5 and CGB8 genes have been associated with a significant protective effect from miscarriage [51]. Also, a balanced, biallelic expression of CGB5 and CGB8 genes is fundamental to allow a normal, uncomplicated pregnancy [52]. Regarding the CGB5 gene, the monoallelic expression of maternal alleles and hemimethylated gene promoters were identified among Estonian women subject to miscarriage events, which may be due to a methylation allelic polymorphism in CGB5 promoter leading to expressional silencing of paternal alleles and increasing susceptibility to pregnancy loss, thus suggesting that primate-specific CGB5 gene may be imprinted. [52]. Therefore, methylation allelic polymorphisms in the placenta may lead to failure of pregnancy maybe altering the placental-maternal interface [52], but further studies are needed to fully understand its molecular mechanism.
2.3 Ovarian hyperstimulation syndrome (OHSS)
2.3.1 Association with FSHR genotype
Ovarian hyperstimulation syndrome (OHSS) is a disorder related to ovarian stimulation occurring during assisted reproduction treatment (ART). The basis of OHSS is mainly identified in an exagerated ovarian response to gonadotropin administration, but other factors such as low body mass index, young age, high estradiol or interleukins levels, renin-angiotensin system activation and vascular endothelial growth factor (VEGF) expression could be associated to OHSS [53]. A relatively low degree of ovarian hyperstimulation is considered normal in women undergoing ovulation induction and mild OHSS occurs with an incidence between 3 and 6% of all stimulated cycles, even though it is not a condition with clinical relevance. Severe OHSS occurs in about 0.1–5% of all cycles [54, 55] and its clinical feature mainly consists in increased permeability of the ovarian capillary vessels resulting in transfer of fluids into the abdominal cavity [53]. An ovarian hypersensitivity to gonadotropins has been suggested but the clear association of OHSS with high hormone concentrations is not constant and has not been demonstrated. A few studies addressed the association between OHSS and polymorphisms in FSHR or LHCGR genes since they are direct targets of gonadotropins, but no consistent correlation was found. Since some cross-interaction between FSHR and heterologous ligands of the pituitary glycoprotein hormone family at high concentrations has been suggested [56–58], the pathogenesis of OHSS could indeed be associated to FSHR-mediated signals. In fact, some rare activating mutations of FSHR gene result in a predisposition to OHSS [58, 59]. However, mutations in the FSHR are rare in OHSS [60] and polymorphisms could rather be involved. For example, the Asn680 FSHR genotype has been associated with severity of iatrogenic OHSS in a study performed on 37 French patients undergoing IVF, while the Ser680 variant occurred significantly more often in women which develop iatrogenic OHSS [61]. In another study, the FSHR polymorphism Ala307 was associated with risk of iatrogenic OHSS and with low amount of FSH required for ovarian stimulation among 50 Indian patients undergoing IVF [62]. However, the number of patients investigated so far is too limited to draw any conclusion and a clear correlation between the FSHR genotype polymorphisms and the risk of OHSS has not been demonstrated [57, 60]. The current scientific opinion substains the hypothesis that a combination of several other factors such as VEGF, the renin-angiotensin system and interleukins may have a strategic role in the pathogenesis of OHSS, but the exact mechanism remains unclear [53, 63, 64].
2.4 Polycistic ovary syndrome (PCOS)
2.4.1 The FSHR and LHCGR polymorphisms
PCOS is an endocrine disorder affecting about the 10% of women during reproductive age associated to hyperandrogenism, chronic anovulation, abnormal menstrual cycle, and polycystic ovaries detected by ultrasonography. In women who conceive successfully after treatment, an increased risk of complications during pregnancy as well as neonatal complications exist [14, 65]. Despite a large number of studies investigating the genetic features related to PCOS, the key factors involved in its pathogenesis are still unidentified [14]. To date, intriguing results have been obtained. The study of the allele frequency of Thr307Ala and Asn680Ser revealed that the G919/A2039 (Ala307/Asn680) haplotype of the FSHR is significantly linked with PCOS and could be a risk factor for the disease, although none of the FSHR polymorphisms individually analyzed was observed to be in clear association with the pathology [66]. Numerous comparisons between PCOS patients and controls showed no significant correlation in the distribution of FSHR allelic variants Ala307Thr and Asn680Ser in different ethnic populations from different countries, such as Singapore Chinese [67], Japanese [37], Dutch [68], Italian [69], Turkish [70], Caucasian [71] and Han Chinese women from Shangai [66]. The only significant association between PCOS and Asn680Ser was found in Korean women [72], however it was never independently confirmed. In summary, actual results suggest that FSHR polymorphisms are not clearly related to PCOS, regardless of the ethnic background but a risk haplotype could exists. As in normoovulatory women, also in PCOS patients the Ser680 allele is associated with significantly higher levels of FSH and LH and with hyperandrogenism [71]. Moreover, a retrospective study performed on 193 PCOS patients found that the homozygous Ser680 carriers have a double chance to develope resistance to clomiphene citrate compared to the Asn680 carriers [73]. It follows that ovarian response and the severity of PCOS are influenced by this polymorphism in exon 10 but the SNPs of FSHR cannot be used as markers to predict the predisposition to PCOS since no consistent clear-cut association could be identified [14, 71]. As far as the combination of FSHR and LHCGR polymorphisms is concerned, a recent study indicated that the analyzed LHCGR SNPs are not related to PCOS and that the disease features may depend only from the FSHR SNPs [71]. A correlation between LHCGR gene polymorphisms and PCOS has never been found so far and LHCGR is not considered as a potential marker for PCOS.
2.4.2 Association between FSHB genotype and PCOS
While the possible correlation between the SNPs in exon 10 of FSHR gene and PCOS is still widely investigated, little is known about the involvement of polymorphisms of the FSHB gene (Fig. 3). About a decade ago, an association between PCOS and a polymorphism in exon 3 of the gene (C76T) was found in women with obesity [74]. This polymorphism does not lead to any amino acid change but it could be in linkage with an unknown mutation located in a near gene or cause a cumulative effect on FSH action, interacting with other, unobserved mutations in the FSHB gene [75]. However, the association in Chinese women between PCOS and the C76T polymorphism was not confirmed, implying that it is not a marker gene for PCOS.
2.4.3 The LHB gene variant V-LH
Concerning the LHB gene, a variant named V-LH consisting in the double amino acid change Trp8Arg and Ile15Thr in the protein [76] was discovered in Finland [76, 77]. It introduces an extra glycosylation site in its aminoacidic chain [78]. These characteristics change the immunological and biological properties of the molecule which, for example, can no longer be recognized by a specific antibody because the corresponding epitope present only in the LHα/β dimer is changed [77]. Moreover, the V-LH shows lower circulatory half time and bioactivity in vivo [3, 79] compensated by a linkage mechanism with some SNPs in the promoter region which flattens these differences in vitro. Indeed, the in vitro activity of mutant LHB promoter is about 40% higher than that of the “normal” LH promoter gene and the intrinsic bioactivity of V-LH is about 30% higher than that of LH [79]. In fact, women with the V-LH variant were less likely to report ovarian cysts, more likely to report infertility, and have higher early follicular phase LH concentrations compared with LH wild type carriers [80]. To assess the role of the V-LH in female fertility, its frequency was analyzed in groups of PCOS patients from Finland, The Netherlands, United Kingdom, and United States [81]. The frequency of V-LH was lower in obese PCOS patients than healthy women and nonobese PCOS patients, suggesting that V-LH somehow protects obese women from developing symptomatic PCOS. Further, independent studies are needed to give a solid confirmation to this observation, but V-LH may be a genetic target to allow the discrimination between individuals with high and low risks of PCOS, especially in obese women [81].
2.4.4 Other genes
In the future more information about the genetic causes of PCOS will derive from studies focused on the interactions between polymorphisms located in different genes involved in the whole hypothalamic-pituitary-gonadal axis. Indeed, recent studies have identified several genes that might be relevant in PCOS pathogenesis [14], such as anti-Müllerian hormone [82, 83]; gonadotropin-releasing hormone [71], aromatase [84], androgen receptors [85], 17β-hydroxysteroid dehydrogenase type 5 and type 6 [86], interleukins [87, 88], vascular endothelial growth factor [89] or genes involved in folliculogenesis [90].
2.5 Premature ovarian failure (POF)
2.5.1 FSHR genotype is associated with the feature of the disease
POF is a disorder characterized by the occurrence of amenorrhea before the age of 40 years due to the loss of ovarian function [91]. Approximately 1% of women under the age of 40 and 0.1% under the age of 30 years are affected by the disease, characterized by the presence of amenorrhea for at least 4 months, high serum FSH levels (comparable to those measured during the age of menopause), and hypoestrogenism [92, 93]. Since a few years is back in use the term “premature ovarian insufficiency” (POI), a more accurate term than POF as it includes a wider range of ovarian conditions that differ from the healthy ovary activity but which does not exclude an intermittent recovery of the follicular maturation and pregnancy [94, 95]. The causes of POF can be very diverse and they are to be found among phenomena of genetic familiarity, autoimmune conditions, abnormal X-chromosome, autoimmunity, iatrogenesis or idiopathic origins [96, 97]. Genetic studies on the causes of the disease rely on the identification of mutations or polymorphisms in several target genes directed by the mechanism that leads to the disorder. Research performed by a genome-wide approach suggested the possible involvement of SNPs in the gonadotropins and their receptors in the pathogenesis of POF [95, 98, 99]. In the NCBI database the studies that aim to evaluate a correlation between genes and POF are focused on FSHR, FSHB, LHCGR and LHB, but other genes involved in the pituitary-gonadal axis are the target of many studies and due to its activity on ovarian function, the possible correlation between FSHR gene mutations and ovarian failure has been investigated [100]. Also recently, an intriguing experiment performed in FSHR knockout mice confirms its link to POF. These mice are sterile due to the block of folliculogenesis at the primary stage and display the classical POF phenotype with high levels of serum FSH and low estrogen levels, but after intra-ovarian injection of an adenovirus expressing a copy of human FSHR they undergo restoration of folliculogenesis with increase in follicle number and estrogen serum levels, decrease in FSH serum levels, FSHR mRNA expression in the ovaries and responsiveness to FSH [101]. Besides, also the genes involved in the negative feedback control of FSH, such as inhibin subunit genes, could be good candidates. Indeed, a functional mutation in the Inhibin α subunit gene resulting in FSH increase, follicle depletion and POF has already been described [102]. Despite these experimental evidences, some meta-analysis studies including 157 cases and 633 controls revealed that the two most popular FSHR polymorphisms are not associated to POF in humans [37, 66, 67, 93, 100, 103, 104] and may be only associated with the feature of the disease [105]. It was observed that the Ala307Thr polymorphism may be linked to a precocious occurrence of the last menstruation in Brazilian women affected by POF, since the Ala307 carrier subjects show onset of the clinical features at the age of about 33.3 ± 7.1 years while the Thr307 carrier at 28.6 ± 11.4 years (p = 0.04) [105], but these data must be confirmed by further evidence. Up to now, only a few rare inactivating mutations in the FSHR gene can be correlated to POF, e.g. C566T [100]. This mutation was observed mainly in the Finnish population, probably due to a founder effect, and causes the Ala189Val aminoacid exchange, leading to ovarian failure due to resistant ovary. The Ala189Val mutation determines the suppression of the cell membrane folding of the receptor causing the abolition of cAMP response and FSH signal transduction pathway impairment [103, 106].
2.6 Endometriosis
2.6.1 No consistent association between endometriosis and the genotype of gonadotropin genes exists
Endometriosis is a disorder that affects 5–10% of women of reproductive age and can impair fertility [107]. The disease is characterized by the growth of endometrial cells outside the uterus and has a strong genetic component which prompted a large number of association studies in the investigation of a wide variety of polymorphisms [108, 109]. The most studied target genes possibly linked to endometriosis are genes of inflammatory mediators, metabolic enzymes, genes regulating vascular function and tissue remodelling and genes involved in sex hormone activity, especially estrogen receptor, progesterone receptor and androgen receptor. However, the results obtained from studies of association between endometriosis and any genes are often inconsistent or in conflict because they are sporadic, conducted on a limited sample and are not independently confirmed across ethnic barriers [109]. For example, a G1502A mutation of the LHB subunit gene was found in only two infertile women with endometriosis in a sample of the Singapore Chinese population consisting of 212 healthy fertile women, 40 infertile women with menstrual disorders, polycystic ovary syndrome, and endometriosis and 12 women with idiopathic infertility [110]. This mutation results in the amino acid substitution of Ser102Gly and, although the authors suggest that it may be associated to endometriosis in some women, no further evidence or in vitro validation has been provided. A recent work performed on Brazilian women found that the LHB G1502A polymorphism may be involved in the predisposition to a mild endometriosis-associated infertility, but the same authors conclude that due to the lack of independent confirmation, it might be only a coincidental finding [111].
Lastly, a recent study suggested that the homozygous FSHR polymorphism Asn680 may have a protective effect on the development of endometriosis, inducing a higher activity of aromatase than the homozygous FSHR Ser680 or eterozygous in Taiwanese Chinese women [112]. In summary, there is no convincing evidence to link gonadotropin genes to endometriosis but a role of the FSHR gene cannot be excluded.
3 Male fertility and infertility
3.1 Spermatogenesis and male infertility
3.1.1 The FSHR polymorphisms in male fertility
FSH is required for normal function of Sertoli cells and gametogenesis in men. Thus, the proper functioning of the FSHR-mediated signaling is required to maintain the normal testicular response to FSH. FSHR mutations could lead to phenotipic effects as abnormalities in spermatogenesis or infertility, depending on the severity of signalling cascade impairment [113]. Since the FSHR genotype is responsible for some differences in the FSH dose needed for stimulation in women [19, 38] and in transcriptional activity in vitro [114], several studies have been conducted to verify possible differences in men. However, the common FSHR polymorphism Asn680Ser showed no effect on serum FSH levels and other clinical parameters in men [18, 115], leading to a discrepancy between the two genders which still remains unexplained. In men, several investigations on the distribution of G-29A, A919G and A2039G FSHR SNPs were made, producing various and sometimes contradictory results [18, 116–118] and suggesting that some polymorphisms lead to testicular dysfunction only when in association with a particular genetic background or environmental factors. A convincing result was obtained from a study which compared the presence in association of the three SNPs G-29A, A919G and A2039G in 345 German men with nonobstructive azoospermia and 186 controls with normal spermatogenesis [116]. No correlation between serum FSH levels and FSHR allele was found and the A-29 allele had higher frequency of distribution than the G-29 allele both in controls and in patients. Moreover, the combination of G-29A, Thr307Ala and Asn680Ser SNPs form four discrete haplotypes occurring in ten combinations with significant different frequency between controls and azoospermic men. The A-29/Ala307/Ser680 and the G-29/Thr307/Asn680 alleles are differently distributed in normal and azoospermic men and might represents genetic factors contributing to phenotypic expression of spermatogenetic impairment regardless serum FSH levels [116]. One possible explanation could be that the transcriptional activity of the FSHR promoter might be influenced by the SNP located in the core-promoter region at position −29 in such a way that FSH becomes less “efficient” when the Ala307/Ser680 receptor isoform is expressed and more “efficient” in the presence of the Thr307/Asn680 isoform. These observations are corroborated by a recent meta-analysis study which combined the previous data from Ahda and colleagues [116] with the new results obtained in 150 azoospermic Estonian men and 208 normozoospermic controls [117]. It was confirmed that the G-29/A919/A2039 (G-29/Thr307/Asn680) haplotype is more prevalent in normozoospermic than in azoospermic men. Moreover, the normozoospermic men carrying at least one minor A allele in position −29 had a smaller mean testicular volume compared to the G-29 homozygous normozoospermic men [117]. Lastly, a study performed in 270 Turkish infertile men and 240 proven fathers confirmed that FSHR gene polymorphisms have no direct influence on spermatogenesis and FSH serum levels [118]. However, the authors identified a difference in the distribution of the G-29/Asn680–G-29/Ser680 versus G-29/Ser680–G-29/Ser680 allelic frequency between the two groups, concluding that the FSHR haplotype is differently distributed in Turkish proven fathers and infertile men, despite the lack of association with serum FSH levels [118]. Collectively, these data suggest that the FSHR G-29/A919/A2039 allele may be a protective factor against male sterility although larger numbers are needed to obtain a clear-cut confirmation. The FSHR Ala307/Ser680 variant and the A-29 variant seem to be less responsive to FSH but the mechanism by which the FSHR haplotype affects spermatogenesis remains to be determined. In fact, the in vitro activity of the G-29A SNP was found to be both unchanged [114] and decreased [119]. Further in vivo association studies should evaluate if additional genetic factors contribute to a multigenic origin of abnormalities in spermatogenesis or infertility.
3.1.2 The FSHB genotype in male fertility
Similarly to the FSHR, the role of FSH in the regulation of spermatogenesis and testicular function is discussed [120]. Maybe due to its crucial role in the regulation of fertility, the FSHB gene sequence is highly conserved in humans [75, 121] and very few inactivating mutations were detected [75, 122–132]. Consistent with its high conservation rate, the most frequent variants of the FSHB gene are only five worlwide, but only two are carried by about 96.6% of population samples chosen in three continents [121]. It follows that association studies between polymorphisms of the FSHB gene and testicular function require large number of patients. To date, only a polymorphism located in the promoter of the FSHB gene (G-211T; rs10835638) was significantly associated with serum FSH level in men [35]. This SNP is localized in a highly conserved promoter region among the placental mammals and is predicted to harbor a transcription regulatory element, suggesting its likely contribution to the regulation of FSHB expression [133]. By a genetic association study performed on 544 Estonian healthy men, the correlation between the G allele and higher FSH serum levels was demonstrated [35]. Indeed, the G-211G genotype exhibited higher FSH levels compared with G-211T, and T-211T. Moreover, the T-211T genotype was associated with a significant decrease in the free testosterone index and testis volumes but no differences in inhibin-B and sperm parameters between the T carriers and homozygous G carriers were observed [35]. Another study performed in Estonian men found that the T allele is significantly more represented in a group of 1,029 infertile men than in the control group (n = 544) [133] and is associated to lower serum FSH levels and FSH/LH ratio [133]. These results corroborate the observation that the transcription rate of FSHB is the limiting factor to FSH production [134] and the highly conserved promoter regions of the FSHB gene have a regulatory function on the transcription of this gene [35]. In fact, the in vitro relative activity of the FSHB proximal promoter region carrying the T nucleotide was shown to be only 46–58% compared to the region carrying the G nucleotide, evaluated by luciferase assay [135]. These findings demonstrate convincingly that FSHB gene promoter variants may be involved to the modulation of testicular functions both in healthy and infertile men and could become a valid genetic marker for molecular diagnostics of male infertility and responders to FSH treatment. The interaction between the polymorphisms in the FSHB promoter and in the FSHR exon 10 remains to be investigated.
3.1.3 LHCGR and ALF genes
Testicular descent is a fundamental process that completes the development of male genitalia which may be affected by LHCGR gene defects, since the inguinoscrotal phase of testis descent is androgen-dependent and requires integrity of LHCGR [136, 137]. Whether polymorphisms in the LHCGR gene are associated with maldescensus was elucidated by a retrospective, case–control study assessing 278 patients with maldescended testes, 277 infertile men without maldescensus and 271 healthy controls [138]. No difference in testosterone levels and in the distributions of the 18insLQ (insertion of a leucine-glutamine at position 18) and Asn291Ser LHCGR polymorphisms among the three groups was found. However, the Asn312 variant (Ser312Asn polymorphism in exon 10) was significantly more frequent in controls than in men with maldescended testes and in infertile men with and without maldescensus considered together [138]. The study concluded for an association of the Ser312Asn SNP in exon 10 with the infertile phenotype, rather than with maldescensus [138]. Since, at the genomic level, a large part of LHCGR gene including exon 10 is embedded in an intronic region of the germ cell-specific ALF gene sequence, a paralogue of TFIIA-α/β-like factor gene [139] which was found to be altered in male infertility [140], it is possible that association found is linked to the molecular or physiological activity of the ALF gene rather than the LHCGR, affecting testicular function. This hypothesis requires confirmation, but suggests that ALF could be a risk factor for male infertility [138].
Concerning the LHB gene (Fig. 4), boys homozygous for a common polymorphic variant of the LHB gene with reduced bioactivity [141] are found more frequently among cryptorchids than in controls [142]. As described above, the intrinsic bioactivity of V-LH is higher than LH activity [79]. However, the short circulatory half time of V-LH makes it a biologically less active form of LH [141], leading to the speculation that it could be involved in the incidence of cryptorchidism due to its decreased activity in the perinatal period [142]. An association study was performed on 93 Finnish cryptorchid neonates and 211 healthy control to compare the frequency of V-LH genotype. Despite no difference in the frequency of distribution of the V-LH allele between the two groups, the prevalence of V-LH increased clearly with gestational age among the cryptorchid children. These findings are in agreement with the hypothesis that a sufficient LH action is necessary for testicular descent in late pregnancy, although it is not critical for normal testicular descendent. This association however, was not independently confirmed.
4 Bone metabolism and osteoporosis
4.1 FSH has not direct effects on bone
Osteoporosis is a disease affecting 18% of aging men [143] and millions of postmenopausal women worldwide and is due to an imbalance of bone turnover in favor of osteoclast-mediated bone absorption against the formation mediated by the osteoblasts [144, 145]. The regulation mechanisms of bone metabolism are closely linked to the hormonal system since the risk of bone fractures and postmenopausal osteoporosis can be reduced by estrogen replacement [146]. Some in vitro experiments suggests that estrogens inhibit osteoclast-mediated bone absorption by reducing the activation of inflammatory cytokines such as IL-1, IL-6, and TNF-α [147, 148] and by acting on bone-marrow osteoclast precursors decreasing its differentiation [149, 150]. The exact mechanism of estrogen action on the bone metabolism at cellular level remains however unclear, and estrogen deficiency alone does not fully explain the bone loss. Indeed, an in vivo experiment in knockout mice for estrogen receptors shows that only mild osteopenia occurs while knockout mice for either receptor have normal bone mass [151–153]. Moreover, ovariectomized rats are affected by profound bone loss, instead hypophysectomized rats have only partial osteopenia [154, 155], suggesting that to obtain a full decrease in bone mass following gonadectomy a normal pituitary function is required. In fact, rather than serum estrogen levels, the markers of bone turnover are best correlated to FSH in postmenopausal women [156] and in women with amenorrhea [157]. These findings provide an unexpected, diverse metabolic model in which the gonadotropins and pituitary gland activity, not declining levels of estrogens, could be strongly associated to the hypogonadal bone loss. In support to this controversial point of view some in vivo experiments show that, in hypogonadal FSHB −/− mice FSH stimulates TNF-α production from immune cells to enhance osteoblast and osteoclast formation [158]. Also, in hypogonadal FSHB −/− and FSHB +/− mice an increase in bone mineral density and cortical thickness has been demonstrated, suggesting a skeletal remodeling model affected by FSH via MEK/Erk, NF-κB, and Akt pathways FSHR-mediated [159]. The authors postulated that an FSHR or FSHB allelic insufficiency determines a loss of function in FSH signalling which protects mice from bone loss despite severe hypogonadism. This effect would be due to the inhibition of osteoclastic bone resorption caused by the deficit in circulating FSH, is observable even in the presence of normal estrogen level, indicating an estrogen-independent action of FSH on the osteoclast [159]. The authors concluded that similar mechanisms, FSH- or FSHR-mediated, may be present in humans. However, these observations are in strong contrast with the evidences that FSH and LH have their only target cells in the gonads and that postmenopausal bone loss is attributable to decreased levels of estrogen, not to a direct effect of FSH on bone metabolism [160]. In fact, an experiment performed in FSHR null mice (FORKO mouse) contradicts the FSH-dependent bone loss model [161]. In FORKO mice, an age-dependent decline in bone mineral density and bone volume over 3 months was demonstrated, which could be reversed by ovarian transplantation. Indeed, bilaterally ovariectomized FORKO mice shown reduced serum testosterone levels and decreased bone mass alike ovariectomized wild-type controls. These results indicate that ovarian estrogen and the androgen-to-estrogen ratio can alter bone turnover independently from the action of FSH [161] and this evidence has been confirmed by another, independent, in vivo experiment [162]. In transgenic hypogonadal female mice expressing human FSH (TgFSH) lacking endogenous FSH and luteinizing hormone (LH) function, a positive correlation between trabecular bone volume and ovarian-derived serum inhibin A or testosterone levels was observed. Also, ovariectomy abolished TgFSH-induced bone formation. However, FSH did not directly stimulate bone since in mouse or cultured osteoblasts or osteoclasts the FSHR mRNA was not detectable. Therefore, these findings demonstrate that FSH effects on bone require an ovary-dependent pathway, which is independent of LH activity, and does not involve direct FSH actions on bone cells [162].
4.2 FSHR polymorphisms on bone
Finally, a study aiming at evaluating the association between FSHR polymorphisms and risk of osteoporosis in postmenopausal women was performed in a group of 289 Italian patients [163]. The DNA of all patients was genotyped for the SNPs G-29A and G2039A (Asn680Ser) to evaluate the frequency of the two polymorphisms and assess a possible correlation with the bone mineral density and bone turnover markers. Surprisingly, A2039A women (homozygous Asn680 carriers) showed a lower bone mineral density measured in femoral neck and total body, lower stiffness index and higher serum levels of bone turnover markers compared to G2039G women (homozygous Ser680 carriers), suggesting that A2039A genotype is associated with higher osteoporosis risk [163]. Since this result is not dependent from serum levels of FSH, its possible biological significance may be based in the concept of major “resistance” of FSHR isoform Ser680 to FSH stimulation [26], therefore, the “more active” FSHR Asn680 variant favors bone loss in postmenopausal women due to its higher sensibility to FSH. However, this study only suggests that FSH could be indirectly involved in the bone loss mechanisms since a correlation between FSH levels and bone metabolism has never been observed in other populations. Conversely, some studies are in complete disagreement with the model which provides a direct FSH regulation of the bone mass [164–166]. The presence of direct transcriptional targets of sex steroid hormones in bone [167] and the evidence of a clear association between estrogen receptors subtypes with the features of the bone metabolism [168–175], suggest that gonadotropins and their receptors do not have a predominant role in regulating the bone turnover mechanisms. Finally, a prospective study performed on postmenopausal women treated with a GnRH agonist, demonstrated that suppression of serum FSH levels does not reduce markers of bone resorption, providing a final, strong evidence that FSH does not directly regulates bone resorption [176].
5 Cancer
5.1 Ovarian cancer
5.1.1 No strong association between ovarian cancer and FSHR polymorphisms
Ovarian cancer is the sixth most common form of female cancer in developed countries and is most lethal than the other gynecological malignancies combined. The molecules involved in cell cycle regulation are the focus of several studies as potential markers of ovarian cancer, due to their implication in the pathogenesis of most human cancers [177–179]. In addition, in the last decade the hypothesis that the stimulating effects of gonadotropin and sex-steroid hormones on cell proliferation may play a role in the development of ovarian cancer was suggested [180]. This hypothesis was corroborated by an in vitro experiment which demonstrated that FSH stimulates the growth of ovarian surface epithelium cell lines and that an overexpression of FSHR in Chinese hamster ovary (CHO) cells leads to cell proliferation [181]. In addition the expression of FSHR was higher both at transcriptional and protein levels in ovarian cancerous tissues, compared to normal tissues. Lastly, an evaluation of the gene expression profile revealed that FSH treatment induces the down-regulation of Oncostatin M, a gene with antiproliferative effect on various cancer tissues [182–185], suggesting that FSH is a risk factor for ovarian carcinogenesis [181]. Whether SNPs in the FSHR gene could be involved in ovarian cancer risk was investigated in a study performed in a Chinese population of Hong Kong examining the possible association between the Ala307Thr and Asn680Ser SNPs and different subtypes of ovarian cancer [186]. This association study was based on genotyping technique using restriction enzymes on DNA samples extracted from paraffin-embedded tissues from 202 patients with serous, mucinous, endometrioid or clear cell carcinoma and 266 controls, demonstrating an association between Ala307/Ser680 haplotype with susceptibility to ovarian cancer, especially in the serous and mucinous subtypes [186]. However, the results obtained in Asian patients do not completely match the association studies on patients of Caucasian ethnicity, suggesting that the modification of tumor risk may be affected by the ethnic origin of the patient. Indeed, in German women no significant differences in the numbers of Ala307/Ser680 haplotype carriers between two groups represented by 115 control donors and 115 patients with epithelial neoplasms including serous, mucinous, clear cell, and endometrioid histology were found [187]. On the contrary, a comparison performed in Polish population of 215 patients with ovarian cancer and 352 controls, suggested that women homozygous for Ala307 or Ser680 have a significantly higher risk for ovarian cancer [188]. This study has the limitation that the group of patients analyzed may not be a representative of whole Polish population, since they come from only two Warsaw area hospitals, implying high genetic homogeneity, and the impossibility to perform the analysis in different histological cancer subgroups. Considerable genetic difference between different ethnic groups exists, resulting in a different LD between both FSHR polymorphisms [187]. Indeed, the International HapMap Project [189] shows that the distribution of the genotypes Asn680Ser and Thr307Ala and its LD ratio is different among Utah residents with Northern and Western European ancestry (CEU), Han Chinese, Japanese and Youruba Nigerian populations. The LD ratio appears to be strong in CEU (the database most likely to represent European people), while it is less distinct in Han Chinese and the Japanese and no linkage was observed in Yoruba Nigerian population. In conclusion, genetic associations can be altered by the diverse genetic backgrounds in different populations [187].
5.2 Breast cancer
5.2.1 The LHCGR 18insLQ variant
Breast cancer is the most common cause of death among women between the ages of 40 and 55 years [190]. Exposure to endogenous and exogenous estrogens is a breast cancer risk factor since high serum hormone levels have been found in women affected by these malignancies. Moreover, also genetic variants of the hormones involved in metabolic pathways or enzymes involved in the biosynthesis and metabolism of estradiol may be low-risk factors for breast cancer. For example, variants of the aromatase gene have been associated with estrogen and progesterone receptor-mediated tumor features, as it is involved in hormone synthesis [191]. Therefore, polymorphisms in LHB or LHCGR genes could contribute to breast cancer risk by alternating the level of estrogen exposure or by an indirect pathway through the ovaries, since both genes are involved in estradiol synthesis [48] but LHCGR expression is absent in breast tumor cells [192]. In fact, the frequency of the LHB gene variants [193] and the incidence of breast cancer [194] is higher in Northern Europe than in Asian populations, leading to the speculation that they might be linked [48]. This hypothesis has been confirmed by a study performed on 266 Northern European Caucasian patients with breast cancer and 108 healthy controls, by a polymorphism genotyping approach performed using PCR-restriction fragment length. Although the LHCGR genotype does not seem to influence breast cancer risk or other clinical parameters, the ins18LQ allele was related to an earlier age of disease onset. The homozygous patients for the LHCGR 18insLQ allele were 8.3 years younger at diagnosis than LHCGR (without 18insLQ polymorphism) homozygous carriers [48]. An in vitro comparative functional analysis between LHCGR 18insLQ and LHCGR variant was performed by transfection studies [195]. The effect of 18insLQ polymorphism on the receptor function involves increased receptor sensitivity and plasma membrane expression, leading to the conclusion that the 18insLQ LHCGR variant is more active than LHCGR variant. Indeed, LHCGR 18insLQ has a 1.9 fold lower hCG ED50 and a plasma membrane expression 1.4 fold higher, measured by luciferase assay. These in vitro findings reflect a significant association between the 18insLQ LHCGR variant and a shorter disease-free survival observed in a cohort of 751 breast cancer patients, suggesting that the female carriers for this polymorphism are probably exposed to an increased estrogen exposure which affects the disease-free survival duration [195].
5.2.2 Role of the exon 10 of LHCGR in breast cancer
These observations elucidate that LHCGR variants have different bioactivity in vitro and in vivo. The biological explanation could be that the receptor variants act in a different manner on signal transduction pathway activation, as suggested by in vitro experiments [196]. Indeed, COS-7 cells transfected with a LHCGR variant lacking exon 10 (LHCGR-ex10) showed an impaired cAMP production under LH stimulation, compared to the wild type receptor expressed at equal levels on the cell surface. In respect to LHCGR, LHCGR-ex10 requires about 30 fold higher LH concentrations to stimulate signal transduction. Surprisingly, hCG stimulation did not result in any difference in cAMP production between the mutant and wild type receptor [196]. These results led to the conclusion that exon 10 of the LHCGR affects the ability of the receptor itself to activate the signal transduction pathway modulating its bioactivity and may have subtle effects on the sensitivity of LH compared to hCG [196, 197]. For these reasons, the two specific LHCGR polymorphisms Asn291Ser (rs12470652) and Ser312Asn (rs2293275) located in exon 10 are good candidate markers for association studies. Moreover, they are located near to glycosylation sites potentially increasing the possibility to affect LH or hCG action, since glycosylation plays an important role in the GPCR family for signalling regulation and physiological features of the receptor trafficking regulation [198]. However, no association between Asn291Ser or Ser312Asn and breast cancer was found in a large cohort of Dutch patients compared to healthy controls, although in vitro transfection studies revealed increased receptor sensitivity for the Ser291/Asn312 haplotype due to an altered glycosylation status [199]. Indeed, the hCG ED50 on the Ser291/Asn312 variant was increased two folds compared to other receptor variants as measured by cAMP-dependent luciferase reporter gene in transfected HEK 293 cells [199]. These results indicate that functional changes of the LHCGR signalling pathway due to exon 10 polymorphisms were observed, although no associations with breast cancer were found in vivo.
5.3 Testis cancer
5.3.1 Gonadotropins may have a role in testis cancer
With a worldwide incidence of 7.5 per 100,000, testicular cancer is the most common malignancy among white men aged between 15 and 45 years [200]. Testicular Germ Cell Tumours (TGCTs) account for approximately 95% of all testicular cancer and represent a model of a curable malignancy [201], although a clear understanding of the molecular aspects involved in carcinogenesis is still missing [202]. As the main peak of testicular cancer incidence is just after puberty [203], it is postulated that the tumor is under endocrine control although definitive evidence is lacking. As other reproductive conditions associated to TGCT, such as cryptorchidism, testicular atrophy, inguinal hernia or infertility, the tumor incidence follows evident geographic and ethnic differences [201, 204]. Polymorphisms located in genes involved in the hormonal regulation of testicular function could alter the sensitivity to hormones and be associated with the ethnic differences in the incidence [202]. Gonadotropin, androgen and oestrogen and their receptor genes are possible candidate for tumor development, and some findings corroborate this hypothesis [202]. Indeed, testis cancer development and progression are coupled to high LH and FSH levels in combination with high oestradiol and intratesticular androgen levels and lower androgen activity [204, 205], while subjects with Kallmann syndrome affected by hypogonadotrophic hypogonadism never develop the malignancy [202].
The possible association between TGCT and twelve FSHR polymorphisms has been studied for the first time in 188 Italian patients affected by the malignancy and compared to 152 controls. The results showed that Ala307/Ser680 haplotype is significantly lower in the control group, suggesting an association to a decreased risk of TGCT, alone or in combination with the G-29 allele [202]. This data strengthen the idea that the SNPs in FSH and its receptor gene may have a role in TGCT carcinogenesis but further analyses are needed to evaluate if this association does exist in other ethnic groups. The study has not been replicated so far.
6 Summary and conclusions
During the last two decades the scientific understanding about the role of polymorphisms in gonadotropin and their receptor genes and their implications in the modulation of reproductive functions and in a variety of pathological conditions has been improved. In silico technologies such as online SNPs databases or softwares for LD block analysis gave a strong contribution to the development of accurate genome-wide association studies in cohorts of reproductive anomalies or cancer patients.
To date, the common FSHR polymorphism Asn680Ser mostly in LD with Ala307Thr is a well established determinant of response to FSH. In women, the in vivo results suggests that Ser680 genotypes is a factor of major “resistance” to FSH stimulation resulting in a higher FSH serum levels, thus leading to prolonged duration of the menstrual cycle, although its role in vitro remains to be determined. This aspect needs consideration in patients undergoing COS because some women could be at risk of ovarian hyperstimulation due to a excessive stimulation by FSH. Since no consistent correlations between FSHR SNPs and non reproductive phenotypes, osteoporosis risk or cancer development were found, the role of these polymorphisms is not confirmed. Moreover, in vitro models suggests that the G-29A FSHR SNP in the core promoter region can modulate the transcriptional activity of FSHR gene and determines a less FSH-responsive phenotype, but its role in vivo is not clear because poor and discordant data were provided so far both in men and women.
In contrast to FSHR, the FSHB subunit gene carries only a few polymorphisms. The G-211T SNP in a highly conserved promoter region seems to play some role in the male. Affecting FSH serum levels, it may be involved to the modulation of testicular functions. Its role in women was not studied yet.
The LHCGR most common polymorphisms are located in exon 1 and in exon 10. They could affect the cAMP response to LH or hCG leading to a modulation of hormone activity. In women, the LHCGR genotype is associated with age of onset and disease-free survival in breast cancer patients. The LHCGR SNP in exon 10 is possibly associated with spermatogenesis features and the “infertile” phenotype in men.
The V-LH common variant has a different immunoreactivity and bioactivity in vitro. However, the V-LH in vivo shows only lower circulatory half time and bioactivity compensated by an intriguing linkage mechanism with some SNPs in the promoter region which abolish these differences in vitro. Moreover, V-LH has been associated with PCOS and higher prevalence of infertility treatments in women. No other convincing associations between this common LH variants and reproductive functions were found.
Future studies should take in consideration the complexity of the hypothalamo-pituitary gonadal axis and investigate, in adequately large populations of well selected patients with homogeneous phenotype, the interplay of the different polymorphisms in fine-tuning gonadal functions.
References
Simoni M, Gromoll J, Nieschlag E. The follicle-stimulating hormone receptor: biochemistry, molecular biology, physiology, and pathophysiology. Endocr Rev. 1997;18(6):739–73.
Ascoli M, Fanelli F, Segaloff DL. The lutropin/choriogonadotropin receptor, a 2002 perspective. Endocr Rev. 2002;23(2):141–74.
Themmen APN, Huhtaniemi IT. Mutations of gonadotropins and gonadotropin receptors: elucidating the physiology and pathophysiology of pituitary-gonadal function. Endocr Rev. 2000;21(5):551–83.
Dias JA, Van Roey P. Structural biology of human follitropin and its receptor. Arch Med Res. 2001;32(6):510–9.
Szkudlinski MW, Fremont V, Ronin C, Weintraub BD. Thyroid-stimulating hormone and thyroid-stimulating hormone receptor structure-function relationships. Physiol Rev. 2002;82(2):473–502.
Maston G, Ruvolo M. Chorionic gonadotropin has a recent origin within primates and an evolutionary history of selection. Mol Biol Evol. 2002;19(3):320–35.
Henke A, Gromoll J. New insights into the evolution of chorionic gonadotrophin. Mol Cell Endocrinol. 2008;291(1–2):11–9.
Nagirnaja L, Rull K, Uusküla L, Hallast P, Grigorova M, Laan M. Genomics and genetics of gonadotropin beta-subunit genes: unique FSHB and duplicated LHB/CGB loci. Mol Cell Endocrinol. 2010;329(1–2):4–16.
Talmadge K, Vamvakopoulos NC, Fiddes JC. Evolution of the genes for the beta subunits of human chorionic gonadotropin and luteinizing hormone. Nature. 1984;5(5946):37–40.
Fredriksson R, Lagerström MC, Lundin L, Schiöth HB. The G-protein-coupled receptors in the human genome form five main families. Phylogenetic analysis, paralogon groups, and fingerprints. Mol Pharmacol. 2003;63(6):1256–72.
Gromoll J, Pekel E, Nieschlag E. The structure and organization of the human follicle-stimulating hormone receptor (FSHR) gene. Genomics. 1996;35(2):308–11.
Atger M, Misrahi M, Sar S, Le Flem L, Dessen P, Milgrom E. Structure of the human luteinizing hormone-choriogonadotropin receptor gene: unusual promoter and 5′ non-coding regions. Mol Cell Endocrinol. 1995;111(2):113–23.
Nordhoff V, Gromoll J, Simoni M. Constitutively active mutations of G protein-coupled receptors: the case of the human luteinizing hormone and follicle-stimulating hormone receptors. Arch Med Res. 1999;30(6):501–9.
Simoni M, Tempfer CB, Destenaves B, Fauser BCJM. Functional genetic polymorphisms and female reproductive disorders: Part I: polycystic ovary syndrome and ovarian response. Hum Reprod Update. 2008;14(5):459–84.
Gabriel SB, Schaffner SF, Nguyen H, Moore JM, Roy J, Blumenstiel B, et al. The structure of haplotype blocks in the human genome. Science. 2002;296(5576):2225–9.
Barrett JC, Fry B, Maller J, Daly MJ. Haploview: analysis and visualization of LD and haplotype maps. Bioinformatics. 2005;21(2):263–5.
Barrett JC. Haploview: Visualization and analysis of SNP genotype data. Cold Spring Harb Protoc. 2009 Ott;2009(10):pdb.ip71.
Simoni M, Gromoll J, Höppner W, Kamischke A, Krafft T, Stähle D, et al. Mutational analysis of the follicle-stimulating hormone (FSH) receptor in normal and infertile men: identification and characterization of two discrete FSH receptor isoforms. J Clin Endocrinol Metab. 1999;84(2):751–5.
Perez Mayorga M, Gromoll J, Behre HM, Gassner C, Nieschlag E, Simoni M. Ovarian response to follicle-stimulating hormone (FSH) stimulation depends on the FSH receptor genotype. J Clin Endocrinol Metab. 2000;85(9):3365–9.
Gromoll J, Simoni M. Follicle-stimulating-hormone receptor and twinning. Lancet. 2001;20(9251):230. author reply 231–232.
Hodgen GD. The dominant ovarian follicle. Fertil Steril. 1982;38(3):281–300.
Baird DT. A model for follicular selection and ovulation: lessons from superovulation. J Steroid Biochem. 1987;27(1–3):15–23.
Brown JB. Pituitary control of ovarian function—concepts derived from gonadotrophin therapy. Aust N Z J Obstet Gynaecol. 1978;18(1):46–54.
Schipper I, Hop WCJ, Fauser BCJM. The Follicle-Stimulating Hormone (FSH) threshold/window concept examined by different interventions with exogenous FSH during the follicular phase of the normal menstrual cycle: duration, rather than magnitude, of FSH increase affects follicle development. J Clin Endocrinol Metab. 1998;83(4):1292–8.
Zheng W, Magid M, Kramer E, Chen Y. Follicle-stimulating hormone receptor is expressed in human ovarian surface epithelium and fallopian tube. Am J Pathol. 1996;148(1):47–53.
Greb RR, Grieshaber K, Gromoll J, Sonntag B, Nieschlag E, Kiesel L, et al. A common single nucleotide polymorphism in exon 10 of the human follicle stimulating hormone receptor is a major determinant of length and hormonal dynamics of the menstrual cycle. J Clin Endocrinol Metab. 2005;90(8):4866–72.
Gromoll J, Simoni M. Genetic complexity of FSH receptor function. Trends Endocrinol Metab. 2005;16(8):368–73.
Nordhoff V, Sonntag B, von Tils D, Götte M, Schüring AN, Gromoll J, et al. Effects of the FSH receptor gene polymorphism p.N680S on cAMP and steroid production in cultured primary human granulosa cells. Reprod Biomed Online [Internet]. 2011 Mag 12 [citato 2011 Lug 29]; Available from: http://www.ncbi.nlm.nih.gov/pubmed/21680247
Loutradis D, Vlismas A, Drakakis P, Antsaklis A. Pharmacogenetics in ovarian stimulation—current concepts. Ann N Y Acad Sci. 2008;1127:10–9.
Meyer JM, Eaves LJ, Heath AC, Martin NG. Estimating genetic influences on the age-at-menarche: a survival analysis approach. Am J Med Genet. 1991;39(2):148–54.
Kaprio J, Rimpelä A, Winter T, Viken RJ, Rimpelä M, Rose RJ. Common genetic influences on BMI and age at menarche. Hum Biol. 1995;67(5):739–53.
Zerbetto I, Gromoll J, Luisi S, Reis FM, Nieschlag E, Simoni M, et al. Follicle-stimulating hormone receptor and DAZL gene polymorphisms do not affect the age of menopause. Fertil Steril. 2008;90(6):2264–8.
Achrekar SK, Modi DN, Meherji PK, Patel ZM, Mahale SD. Follicle stimulating hormone receptor gene variants in women with primary and secondary amenorrhea. J Assist Reprod Genet. 2010;27(6):317–26.
Sowers MR, Jannausch ML, McConnell DS, Kardia SR, Randolph JF. Menstrual cycle markers of ovarian aging and sex steroid hormone genotypes. Am J Med. 2006;119(9 Suppl 1):S31–43.
Grigorova M, Punab M, Ausmees K, Laan M. FSHB promoter polymorphism within evolutionary conserved element is associated with serum FSH level in men. Hum Reprod. 2008;23(9):2160–6.
Fauser BCJM, Diedrich K, Devroey P. Predictors of ovarian response: progress towards individualized treatment in ovulation induction and ovarian stimulation. Hum Reprod Update. 2008;14(1):1–14.
Sudo S, Kudo M, Wada S, Sato O, Hsueh AJW, Fujimoto S. Genetic and functional analyses of polymorphisms in the human FSH receptor gene. Mol Hum Reprod. 2002;8(10):893–9.
de Castro F, Ruiz R, Montoro L, Pérez-Hernández D, Sánchez-Casas Padilla E, Real LM, et al. Role of follicle-stimulating hormone receptor Ser680Asn polymorphism in the efficacy of follicle-stimulating hormone. Fertil Steril. 2003;80(3):571–6.
de Castro F, Morón FJ, Montoro L, Galán JJ, Hernández DP, Padilla ES, et al. Human controlled ovarian hyperstimulation outcome is a polygenic trait. Pharmacogenetics. 2004;14(5):285–93.
Jun JK, Yoon JS, Ku S, Choi YM, Hwang KR, Park SY, et al. Follicle-stimulating hormone receptor gene polymorphism and ovarian responses to controlled ovarian hyperstimulation for IVF-ET. J Hum Genet. 2006;51(8):665–70.
Loutradis D, Patsoula E, Minas V, Koussidis GA, Antsaklis A, Michalas S, et al. FSH receptor gene polymorphisms have a role for different ovarian response to stimulation in patients entering IVF/ICSI-ET programs. J Assist Reprod Genet. 2006;23(4):177–84.
Alviggi C, Clarizia R, Pettersson K, Mollo A, Humaidan P, Strina I, et al. Suboptimal response to GnRHa long protocol is associated with a common LH polymorphism. Reprod Biomed Online. 2009;18(1):9–14.
Behre HM, Greb RR, Mempel A, Sonntag B, Kiesel L, Kaltwasser P, et al. Significance of a common single nucleotide polymorphism in exon 10 of the follicle-stimulating hormone (FSH) receptor gene for the ovarian response to FSH: a pharmacogenetic approach to controlled ovarian hyperstimulation. Pharmacogenet Genomics. 2005;15(7):451–6.
Klinkert ER, te Velde ER, Weima S, van Zandvoort PM, Hanssen RGJM, Nilsson PR, et al. FSH receptor genotype is associated with pregnancy but not with ovarian response in IVF. Reprod Biomed Online. 2006;13(5):687–95.
Achrekar SK, Modi DN, Desai SK, Mangoli VS, Mangoli RV, Mahale SD. Poor ovarian response to gonadotrophin stimulation is associated with FSH receptor polymorphism. Reprod Biomed Online. 2009;18(4):509–15.
Ochsenkühn R, von Schönfeldt V, Simoni M. FSH receptor polymorphisms and ovarian function. Texte intégral de l’article Printable version FSH receptor polymorphisms and ovarian function. MT/médecine de la reproduction, gynécologie et endocrinologie. 2009;11(4):265–70.
Richter-Unruh A, Martens JWM, Verhoef-Post M, Wessels HT, Kors WA, Sinnecker GHG, et al. Leydig cell hypoplasia: cases with new mutations, new polymorphisms and cases without mutations in the luteinizing hormone receptor gene. Clin Endocrinol (Oxf). 2002;56(1):103–12.
Powell BL, Piersma D, Kevenaar ME, van Staveren IL, Themmen APN, Iacopetta BJ, et al. Luteinizing hormone signaling and breast cancer: polymorphisms and age of onset. J Clin Endocrinol Metab. 2003;88(4):1653–7.
Themmen APN. An update of the pathophysiology of human gonadotrophin subunit and receptor gene mutations and polymorphisms. Reproduction. 2005;130(3):263–74.
Rull K, Laan M. Expression of beta-subunit of HCG genes during normal and failed pregnancy. Hum Reprod. 2005;20(12):3360–8.
Rull K, Nagirnaja L, Ulander V, Kelgo P, Margus T, Kaare M, et al. Chorionic gonadotropin beta-gene variants are associated with recurrent miscarriage in two European populations. J Clin Endocrinol Metab. 2008;93(12):4697–706.
Uusküla L, Rull K, Nagirnaja L, Laan M. Methylation allelic polymorphism (MAP) in chorionic gonadotropin beta5 (CGB5) and its association with pregnancy success. J Clin Endocrinol Metab. 2011;96(1):E199–207.
Nastri CO, Ferriani RA, Rocha IA, Martins WP. Ovarian hyperstimulation syndrome: pathophysiology and prevention. J Assist Reprod Genet. 2010;27(2–3):121–8.
Delvigne A, Rozenberg S. Epidemiology and prevention of ovarian hyperstimulation syndrome (OHSS): a review. Hum Reprod Update. 2002;8(6):559–77.
Vlahos NF, Gregoriou O. Prevention and management of ovarian hyperstimulation syndrome. Ann N Y Acad Sci. 2006;1092:247–64.
Nappi RG, Di Naro E, D’Aries AP, Nappi L. Natural pregnancy in hypothyroid woman complicated by spontaneous ovarian hyperstimulation syndrome. Am J Obstet Gynecol. 1998;178(3):610–1.
Michaelson-Cohen R, Altarescu G, Beller U, Reens R, Halevy-Shalem T, Eldar-Geva T. Does elevated human chorionic gonadotropin alone trigger spontaneous ovarian hyperstimulation syndrome? Fertil Steril. 2008;90(5):1869–74.
Dieterich M, Bolz M, Reimer T, Costagliola S, Gerber B. Two different entities of spontaneous ovarian hyperstimulation in a woman with FSH receptor mutation. Reprod Biomed Online. 2010;20(6):751–8.
Rodien P, Beau I, Vasseur C. Ovarian hyperstimulation syndrome (OHSS) due to mutations in the follicle-stimulating hormone receptor. Ann Endocrinol (Paris). 2010;71(3):206–9.
Kerkelä E, Skottman H, Friden B, Bjuresten K, Kere J, Hovatta O. Exclusion of coding-region mutations in luteinizing hormone and follicle-stimulating hormone receptor genes as the cause of ovarian hyperstimulation syndrome. Fertil Steril. 2007;87(3):603–6.
Daelemans C, Smits G, de Maertelaer V, Costagliola S, Englert Y, Vassart G, et al. Prediction of severity of symptoms in iatrogenic ovarian hyperstimulation syndrome by follicle-stimulating hormone receptor Ser680Asn polymorphism. J Clin Endocrinol Metab. 2004;89(12):6310–5.
Achrekar SK, Modi DN, Desai SK, Mangoli VS, Mangoli RV, Mahale SD. Follicle-stimulating hormone receptor polymorphism (Thr307Ala) is associated with variable ovarian response and ovarian hyperstimulation syndrome in Indian women. Fertil Steril. 2009;91(2):432–9.
Chen S, Chou C, Lin C, Lee H, Wu J, Lu H, et al. Signal mechanisms of vascular endothelial growth factor and interleukin-8 in ovarian hyperstimulation syndrome: dopamine targets their common pathways. Hum Reprod. 2010;25(3):757–67.
Peitsidis P, Agrawal R. Role of vascular endothelial growth factor in women with PCO and PCOS: a systematic review. Reprod Biomed Online. 2010;20(4):444–52.
Norman RJ, Dewailly D, Legro RS, Hickey TE. Polycystic ovary syndrome. Lancet. 2007;370(9588):685–97.
Du J, Zhang W, Guo L, Zhang Z, Shi H, Wang J, et al. Two FSHR variants, haplotypes and meta-analysis in Chinese women with premature ovarian failure and polycystic ovary syndrome. Mol Genet Metab. 2010;100(3):292–5.
Tong Y, Liao WX, Roy AC, Ng SC. Absence of mutations in the coding regions of follicle-stimulating hormone receptor gene in Singapore Chinese women with premature ovarian failure and polycystic ovary syndrome. Horm Metab Res. 2001;33(4):221–6.
Laven JSE, Mulders AGMGJ, Suryandari DA, Gromoll J, Nieschlag E, Fauser BCJM, et al. Follicle-stimulating hormone receptor polymorphisms in women with normogonadotropic anovulatory infertility. Fertil Steril. 2003;80(4):986–92.
Orio F, Ferrarini E, Cascella T, Dimida A, Palomba S, Gianetti E, et al. Genetic analysis of the follicle stimulating hormone receptor gene in women with polycystic ovary syndrome. J Endocrinol Invest. 2006;29(11):975–82.
Unsal T, Konac E, Yesilkaya E, Yilmaz A, Bideci A, Ilke Onen H, et al. Genetic polymorphisms of FSHR, CYP17, CYP1A1, CAPN10, INSR, SERPINE1 genes in adolescent girls with polycystic ovary syndrome. J Assist Reprod Genet. 2009;26(4):205–16.
Valkenburg O, Uitterlinden AG, Piersma D, Hofman A, Themmen APN, de Jong FH, et al. Genetic polymorphisms of GnRH and gonadotrophic hormone receptors affect the phenotype of polycystic ovary syndrome. Hum Reprod. 2009;24(8):2014–22.
Gu B, Park J, Baek K. Genetic variations of follicle stimulating hormone receptor are associated with polycystic ovary syndrome. Int J Mol Med. 2010;26(1):107–12.
Overbeek A, Kuijper EAM, Hendriks ML, Blankenstein MA, Ketel IJG, Twisk JWR, et al. Clomiphene citrate resistance in relation to follicle-stimulating hormone receptor Ser680Ser-polymorphism in polycystic ovary syndrome. Hum Reprod. 2009;24(8):2007–13.
Tong Y, Liao WX, Roy AC, Ng SC. Association of AccI polymorphism in the follicle-stimulating hormone beta gene with polycystic ovary syndrome. Fertil Steril. 2000;74(6):1233–6.
Lamminen T, Jokinen P, Jiang M, Pakarinen P, Simonsen H, Huhtaniemi I. Human FSH beta subunit gene is highly conserved. Mol Hum Reprod. 2005;11(8):601–5.
Nilsson C, Jiang M, Pettersson K, Iitiä A, Mäkelä M, Simonsen H, et al. Determination of a common genetic variant of luteinizing hormone using DNA hybridization and immunoassays. Clin Endocrinol (Oxf). 1998;49(3):369–76.
Pettersson K, Ding YQ, Huhtaniemi I. An immunologically anomalous luteinizing hormone variant in a healthy woman. J Clin Endocrinol Metab. 1992;74(1):164–71.
Suganuma N, Furui K, Kikkawa F, Tomoda Y, Furuhashi M. Effects of the mutations (Trp8→Arg and Ile15→Thr) in human luteinizing hormone (LH) beta-subunit on LH bioactivity in vitro and in vivo. Endocrinology. 1996;137(3):831–8.
Jiang M, Pakarinen P, Zhang FP, El-Hefnawy T, Koskimies P, Pettersson K, et al. A common polymorphic allele of the human luteinizing hormone beta-subunit gene: additional mutations and differential function of the promoter sequence. Hum Mol Genet. 1999;8(11):2037–46.
Cramer DW, Petterson KS, Barbieri RL, Huhtaniemi IT. Reproductive hormones, cancers, and conditions in relation to a common genetic variant of luteinizing hormone. Hum Reprod. 2000;15(10):2103–7.
Tapanainen JS, Koivunen R, Fauser BCJM, Taylor AE, Clayton RN, Rajkowa M, et al. A new contributing factor to polycystic ovary syndrome: the genetic variant of luteinizing hormone. J Clin Endocrinol Metab. 1999;84(5):1711–5.
Kevenaar ME, Laven JSE, Fong SL, Uitterlinden AG, de Jong FH, Themmen APN, et al. A functional anti-mullerian hormone gene polymorphism is associated with follicle number and androgen levels in polycystic ovary syndrome patients. J Clin Endocrinol Metab. 2008;93(4):1310–6.
van Houten ELAF, Themmen APN, Visser JA. Anti-Müllerian hormone (AMH): regulator and marker of ovarian function. Ann Endocrinol (Paris). 2010;71(3):191–7.
Xita N, Lazaros L, Georgiou I, Tsatsoulis A. CYP19 gene: a genetic modifier of polycystic ovary syndrome phenotype. Fertil Steril. 2010;94(1):250–4.
Laisk T, Haller-Kikkatalo K, Laanpere M, Jakovlev U, Peters M, Karro H, et al. Androgen receptor epigenetic variations influence early follicular phase gonadotropin levels. Acta Obstet Gynecol Scand. 2010;89(12):1557–63.
Ke L, Che Y, Cao Y, Wu X, Hu Y, Sun H, et al. Polymorphisms of the HSD17B6 and HSD17B5 genes in Chinese women with polycystic ovary syndrome. J Womens Health (Larchmt). 2010;19(12):2227–32.
Mu Y, Liu J, Wang B, Wen Q, Wang J, Yan J, et al. Interleukin 1 beta (IL-1β) promoter C [−511] T polymorphism but not C [+3953] T polymorphism is associated with polycystic ovary syndrome. Endocrine. 2010;37(1):71–5.
Yang Y, Qiao J, Li M. Association of polymorphisms of interleukin-18 gene promoter region with polycystic ovary syndrome in Chinese population. Reprod Biol Endocrinol. 2010;8:125.
Vural P, Küskü-Kiraz Z, Doğru-Abbasoğlu S, Cil E, Karadağ B, Akgül C, et al. Vascular endothelial growth factor −2578 A/C, −460 T/C and +405 G/C polymorphisms in polycystic ovary syndrome. Eur J Obstet Gynecol Reprod Biol. 2009;147(1):57–60.
Sproul K, Jones MR, Mathur R, Azziz R, Goodarzi MO. Association study of four key folliculogenesis genes in polycystic ovary syndrome. BJOG. 2010;117(6):756–60.
Coulam CB, Adamson SC, Annegers JF. Incidence of premature ovarian failure. Obstet Gynecol. 1986;67(4):604–6.
Rebar RW, Connolly HV. Clinical features of young women with hypergonadotropic amenorrhea. Fertil Steril. 1990;53(5):804–10.
Conway GS. Premature ovarian failure. Br Med Bull. 2000;56(3):643–9.
Welt CK. Primary ovarian insufficiency: a more accurate term for premature ovarian failure. Clin Endocrinol (Oxf). 2008;68(4):499–509.
De Vos M, Devroey P, Fauser BCJM. Primary ovarian insufficiency. Lancet. 2010;376(9744):911–21.
Woad KJ, Watkins WJ, Prendergast D, Shelling AN. The genetic basis of premature ovarian failure. Aust N Z J Obstet Gynaecol. 2006;46(3):242–4.
Persani L, Rossetti R, Cacciatore C, Bonomi M. Primary ovarian insufficiency: X chromosome defects and autoimmunity. J Autoimmun. 2009;33(1):35–41.
van Dooren MF, Bertoli-Avellab AM, Oldenburg RA. Premature ovarian failure and gene polymorphisms. Curr Opin Obstet Gynecol. 2009;21(4):313–7.
Christin-Maitre S, Tachdjian G. Genome-wide association study and premature ovarian failure. Ann Endocrinol (Paris). 2010;71(3):218–21.
Aittomäki K, Lucena JL, Pakarinen P, Sistonen P, Tapanainen J, Gromoll J, et al. Mutation in the follicle-stimulating hormone receptor gene causes hereditary hypergonadotropic ovarian failure. Cell. 1995;82(6):959–68.
Ghadami M, El-Demerdash E, Salama SA, Binhazim AA, Archibong AE, Chen X, et al. Toward gene therapy of premature ovarian failure: intraovarian injection of adenovirus expressing human FSH receptor restores folliculogenesis in FSHR(−/−) FORKO mice. Mol Hum Reprod. 2010;16(4):241–50.
Shelling AN, Burton KA, Chand AL, van Ee CC, France JT, Farquhar CM, et al. Inhibin: a candidate gene for premature ovarian failure. Hum Reprod. 2000;15(12):2644–9.
Rannikko A, Pakarinen P, Manna PR, Beau I, Misrahi M, Aittomäki K, et al. Functional characterization of the human FSH receptor with an inactivating Ala189Val mutation. Mol Hum Reprod. 2002;8(4):311–7.
Conway GS, Conway E, Walker C, Hoppner W, Gromoll J, Simoni M. Mutation screening and isoform prevalence of the follicle stimulating hormone receptor gene in women with premature ovarian failure, resistant ovary syndrome and polycystic ovary syndrome. Clin Endocrinol (Oxf). 1999;51(1):97–9.
Vilodre LC, Kohek MBF, Spritzer PM. Screening of follicle-stimulating hormone receptor gene in women with premature ovarian failure in southern Brazil and associations with phenotype. J Endocrinol Invest. 2008;31(6):552–7.
Doherty E, Pakarinen P, Tiitinen A, Kiilavuori A, Huhtaniemi I, Forrest S, et al. A Novel mutation in the FSH receptor inhibiting signal transduction and causing primary ovarian failure. J Clin Endocrinol Metab. 2002;87(3):1151–5.
Bulun SE. Endometriosis. N Engl J Med. 2009;360(3):268–79.
Falconer H, D’Hooghe T, Fried G. Endometriosis and genetic polymorphisms. Obstet Gynecol Surv. 2007;62(9):616–28.
Tempfer CB, Simoni M, Destenaves B, Fauser BCJM. Functional genetic polymorphisms and female reproductive disorders: part II—endometriosis. Hum Reprod Update. 2009;15(1):97–118.
Liao WX, Roy AC, Chan C, Arulkumaran S, Ratnam SS. A new molecular variant of luteinizing hormone associated with female infertility. Fertil Steril. 1998;69(1):102–6.
Mafra FA, Bianco B, Christofolini DM, Souza AMB, Zulli K, Barbosa CP. Luteinizing hormone beta-subunit gene (LHbeta) polymorphism in infertility and endometriosis-associated infertility. Eur J Obstet Gynecol Reprod Biol. 2010;151(1):66–9.
Wang H, Cheng B, Wu H, Yen C, Liu C, Chao A, et al. A mutant single nucleotide polymorphism of follicle-stimulating hormone receptor is associated with a lower risk of endometriosis. Fertil Steril [Internet]. 2010 Set 2 [citato 2010 Dic 9];Available from: http://www.ncbi.nlm.nih.gov/pubmed/20817169
Layman LC, McDonough PG. Mutations of follicle stimulating hormone-beta and its receptor in human and mouse: genotype/phenotype. Mol Cell Endocrinol. 2000;161(1–2):9–17.
Wunsch A, Ahda Y, Banaz-Yaşar F, Sonntag B, Nieschlag E, Simoni M, et al. Single-nucleotide polymorphisms in the promoter region influence the expression of the human follicle-stimulating hormone receptor. Fertil Steril. 2005;84(2):446–53.
Asatiani K, Gromoll J, Eckardstein SV, Zitzmann M, Nieschlag E, Simoni M. Distribution and function of FSH receptor genetic variants in normal men. Andrologia. 2002;34(3):172–6.
Ahda Y, Gromoll J, Wunsch A, Asatiani K, Zitzmann M, Nieschlag E, et al. Follicle-stimulating hormone receptor gene haplotype distribution in normozoospermic and azoospermic men. J Androl. 2005;26(4):494–9.
Lend AK, Belousova A, Haller-Kikkatalo K, Punab M, Poolamets O, Peters M, et al. Follicle-stimulating hormone receptor gene haplotypes and male infertility in estonian population and meta-analysis. Syst Biol Reprod Med. 2010;56(1):84–90.
Balkan M, Gedik A, Akkoc H, Izci Ay O, Erdal ME, Isi H, et al. FSHR single nucleotide polymorphism frequencies in proven fathers and infertile men in Southeast Turkey. J Biomed Biotechnol. 2010;2010:640318.
Nakayama T, Kuroi N, Sano M, Tabara Y, Katsuya T, Ogihara T, et al. Mutation of the follicle-stimulating hormone receptor gene 5′-untranslated region associated with female hypertension. Hypertension. 2006;48(3):512–8.
Plant TM, Marshall GR. The functional significance of FSH in spermatogenesis and the control of its secretion in male primates. Endocr Rev. 2001;22(6):764–86.
Grigorova M, Rull K, Laan M. Haplotype structure of FSHB, the beta-subunit gene for fertility-associated follicle-stimulating hormone: possible influence of balancing selection. Ann Hum Genet. 2007;71(Pt 1):18–28.
Matthews CH, Borgato S, Beck-Peccoz P, Adams M, Tone Y, Gambino G, et al. Primary amenorrhoea and infertility due to a mutation in the beta-subunit of follicle-stimulating hormone. Nat Genet. 1993;5(1):83–6.
Layman L, Lee E, Peak D, Namnoum A, Vu K, Van Lingen B, et al. Delayed puberty and hypogonadism caused by mutations in the follicle-stimulating hormone β-subunit gene. N Engl J Med. 1997;337(9):607–11.
Lindstedt G, Nyström E, Matthews C, Ernest I, Janson PO, Chatterjee K. Follitropin (FSH) deficiency in an infertile male due to FSHβ gene mutation. A syndrome of normal puberty and virilization but under-developed testicles with azoospermia, low FSH but high lutropin and normal serum testosterone concentrations. Clin Chem Lab Med. 1998;36(8):663–5.
Phillip M, Arbelle JE, Segev Y, Parvari R. Male hypogonadism due to a mutation in the gene for the beta-subunit of follicle-stimulating hormone. N Engl J Med. 1998;338(24):1729–32.
Clark AD, Layman LC. Analysis of the Cys82Arg mutation in follicle-stimulating hormone beta (FSHbeta) using a novel FSH expression vector. Fertil Steril. 2003;79(2):379–85.
Huhtaniemi I. Mutations affecting gonadotropin secretion and action. Horm Res. 2003;60 Suppl 3:21–30.
Giltay JC, Deege M, Blankenstein RA, Kastrop PMM, Wijmenga C, Lock TTWT. Apparent primary follicle-stimulating hormone deficiency is a rare cause of treatable male infertility. Fertil Steril. 2004;81(3):693–6.
Berger K, Souza H, Brito VN, d’Alva CB, Mendonca BB, Latronico AC. Clinical and hormonal features of selective follicle-stimulating hormone (FSH) deficiency due to FSH beta-subunit gene mutations in both sexes. Fertil Steril. 2005;83(2):466–70.
Lofrano-Porto A, Casulari LA, Nascimento PP, Giacomini L, Naves LA, da Motta LDC, et al. Effects of follicle-stimulating hormone and human chorionic gonadotropin on gonadal steroidogenesis in two siblings with a follicle-stimulating hormone beta subunit mutation. Fertil Steril. 2008;90(4):1169–74.
Ciccone NA, Xu S, Lacza CT, Carroll RS, Kaiser UB. Frequency-dependent regulation of follicle-stimulating hormone beta by pulsatile gonadotropin-releasing hormone is mediated by functional antagonism of bZIP transcription factors. Mol Cell Biol. 2010;30(4):1028–40.
Kottler M, Chou Y, Chabre O, Richard N, Polge C, Brailly-Tabard S, et al. A new FSHbeta mutation in a 29-year-old woman with primary amenorrhea and isolated FSH deficiency: functional characterization and ovarian response to human recombinant FSH. Eur J Endocrinol. 2010;162(3):633–41.
Grigorova M, Punab M, Poolamets O, Kelgo P, Ausmees K, Korrovits P, et al. Increased prevalance of the −211 T allele of follicle stimulating hormone (FSH) beta subunit promoter polymorphism and lower serum FSH in infertile men. J Clin Endocrinol Metab. 2010;95(1):100–8.
Miller WL, Shafiee-Kermani F, Strahl BD, Huang H. The nature of FSH induction by GnRH. Trends Endocrinol Metab. 2002;13(6):257–63.
Hoogendoorn B, Coleman SL, Guy CA, Smith K, Bowen T, Buckland PR, et al. Functional analysis of human promoter polymorphisms. Hum Mol Genet. 2003;12(18):2249–54.
Knobil E, Neill JD. Knobil and Neill’s physiology of reproduction. Gulf Professional Publishing; 2006.
Toppari J, Kaleva M, Virtanen HE, Main KM, Skakkebaek NE. Luteinizing hormone in testicular descent. Mol Cell Endocrinol. 2007;269(1–2):34–7.
Simoni M, Tüttelmann F, Michel C, Böckenfeld Y, Nieschlag E, Gromoll J. Polymorphisms of the luteinizing hormone/chorionic gonadotropin receptor gene: association with maldescended testes and male infertility. Pharmacogenet Genomics. 2008;18(3):193–200.
Catena R, Argentini M, Martianov I, Parello C, Brancorsini S, Parvinen M, et al. Proteolytic cleavage of ALF into alpha- and beta-subunits that form homologous and heterologous complexes with somatic TFIIA and TRF2 in male germ cells. FEBS Lett. 2005;579(16):3401–10.
Huang M, Wang H, Li J, Zhou Z, Du Y, Lin M, et al. Involvement of ALF in human spermatogenesis and male infertility. Int J Mol Med. 2006;17(4):599–604.
Lamminen T, Huhtaniemi I. A common genetic variant of luteinizing hormone; relation to normal and aberrant pituitary-gonadal function. Eur J Pharmacol. 2001;414(1):1–7.
Kaleva M, Virtanen H, Haavisto A, Main K, Skakkebaek NE, Huhtaniemi I, et al. Does variant luteinizing hormone (V-LH) predispose to improper testicular position in late pregnancy? Pediatr Res. 2005;58(3):447–50.
Martin AC. Osteoporosis in men: a review of endogenous sex hormones and testosterone replacement therapy. J Pharm Pract. 2011;24(3):307–15.
Zaidi M, Alam AS, Shankar VS, Bax BE, Bax CM, Moonga BS, et al. Cellular biology of bone resorption. Biol Rev Camb Philos Soc. 1993;68(2):197–264.
Manolagas SC, Jilka RL. Bone marrow, cytokines, and bone remodeling. Emerging insights into the pathophysiology of osteoporosis. N Engl J Med. 1995;332(5):305–11.
Lindsay R. Hormones and bone health in postmenopausal women. Endocrine. 2004;24(3):223–30.
Roggia C, Gao Y, Cenci S, Weitzmann M, Toraldo G, Isaia G, et al. Up-regulation of TNF-producing T cells in the bone marrow: a key mechanism by which estrogen deficiency induces bone loss in vivo. Proc Natl Acad Sci USA. 2001;98(24):13960–5.
Cenci S, Toraldo G, Weitzmann M, Roggia C, Gao Y, Qian W, et al. Estrogen deficiency induces bone loss by increasing T cell proliferation and lifespan through IFN-γ-induced class II transactivator. Proc Natl Acad Sci USA. 2003;100(18):10405–10.
Shevde N, Bendixen A, Dienger K, Pike J. Estrogens suppress RANK ligand-induced osteoclast differentiation via a stromal cell independent mechanism involving c-Jun repression. Proc Natl Acad Sci USA. 2000;97(14):7829–34.
Srivastava S, Toraldo G, Weitzmann M, Cenci S, Ross F, Pacifici R. Estrogen decreases osteoclast formation by down-regulating receptor activator of NF-κB ligand (RANKL)-induced JNK activation. J Biol Chem. 2001;276(12):8836–40.
McCauley L, Tözüm T, Rosol T. Estrogen receptors in skeletal metabolism: Lessons from genetically modified models of receptor function. Crit Rev Eukaryot Gene Expr. 2002;12(2):89–100.
Windahl S, Andersson G, Gustafsson J. Elucidation of estrogen receptor function in bone with the use of mouse models. Trends Endocrinol Metab. 2002;13(5):195–200.
Lindberg M, Alatalo S, Halleen J, Mohan S, Gustafsson J, Ohlsson C. Estrogen receptor specificity in the regulation of the skeleton in female mice. J Endocrinol. 2001;171(2):229–36.
Yeh J, Chen M, Aloia J. Ovariectomy-induced high turnover in cortical bone is dependent on pituitary hormone in rats. Bone. 1996;18(5):443–50.
Yeh J, Chen M, Aloia J. Effects of 17β-estradiol administration on cortical and cancellous bone of ovariectomized rats with and without hypophysectomy. Bone. 1997;20(5):413–20.
Sowers M, Greendale G, Bondarenko I, Finkelstein J, Cauley J, Neer R, et al. Endogenous hormones and bone turnover markers in pre- and perimenopausal women: SWAN. Osteoporos Int. 2003;14(3):191–7.
Devleta B, Adem B, Senada S. Hypergonadotropic amenorrhea and bone density: new approach to an old problem. J Bone Miner Metab. 2004;22(4):360–4.
Iqbal J, Sun L, Kumar TR, Blair HC, Zaidi M. Follicle-stimulating hormone stimulates TNF production from immune cells to enhance osteoblast and osteoclast formation. Proc Natl Acad Sci USA. 2006;103(40):14925–30.
Sun L, Peng Y, Sharrow AC, Iqbal J, Zhang Z, Papachristou DJ, et al. FSH directly regulates bone mass. Cell. 2006;125(2):247–60.
Baron R. FSH versus estrogen: who’s guilty of breaking bones? Cell Metab. 2006;3(5):302–5.
Gao J, Tiwari-Pandey R, Samadfam R, Yang Y, Miao D, Karaplis AC, et al. Altered ovarian function affects skeletal homeostasis independent of the action of follicle-stimulating hormone. Endocrinology. 2007;148(6):2613–21.
Allan CM, Kalak R, Dunstan CR, McTavish KJ, Zhou H, Handelsman DJ, et al. Follicle-stimulating hormone increases bone mass in female mice. Proc Natl Acad Sci USA. 2010;107(52):22629–34.
Rendina D, Gianfrancesco F, De Filippo G, Merlotti D, Esposito T, Mingione A, et al. FSHR gene polymorphisms influence bone mineral density and bone turnover in postmenopausal women. Eur J Endocrinol. 2010;163(1):165–72.
Seibel MJ, Dunstan CR, Zhou H, Allan CM, Handelsman DJ. Sex steroids, not FSH, influence bone mass. Cell. 2006;127(6):1079. author reply 1080–1081.
Castelo-Branco C, León M, Durán M, Balasch J. Follicle-stimulating hormone does not directly regulate bone mass in human beings: evidence from nature. Fertil Steril. 2008;90(6):2211–6.
Rouach V, Katzburg S, Koch Y, Stern N, Somjen D. Bone loss in ovariectomized rats: dominant role for estrogen but apparently not for FSH. J Cell Biochem. 2011;112(1):128–37.
Krum SA. Direct transcriptional targets of sex steroid hormones in bone. J Cell Biochem. 2011;112(2):401–8.
Rauhio A, Uusi-Rasi K, Kunnas T, Nikkari ST, Kannus P, Sievänen H. Estrogen receptor-1 genotype is associated with bone structure in premenopausal obese women. Maturitas [Internet]. 2011 Gen 7 [citato 2011 Gen 28]; Available from: http://www.ncbi.nlm.nih.gov/pubmed/21216543
Zhang X, Dai J, Long Y, Wu H, Li X, Ding Y. Correlation of estrogen receptor alpha gene polymorphisms and bone mineral density in Chinese women with chronic periodontitis. Chin Med J. 2010;123(22):3262–7.
Erdogan MO, Yıldız H, Artan S, Solak M, Taşcıoğlu F, Dündar U, et al. Association of estrogen receptor alpha and collagen type I alpha 1 gene polymorphisms with bone mineral density in postmenopausal women. Osteoporos Int [Internet]. 2010 Giu 8 [citato 2011 Gen 28]; Available from: http://www.ncbi.nlm.nih.gov/pubmed/20532479
Harsløf T, Husted LB, Carstens M, Stenkjaer L, Langdahl BL. Genotypes and haplotypes of the estrogen receptor genes, but not the retinoblastoma-interacting zinc finger protein 1 gene, are associated with osteoporosis. Calcif Tissue Int. 2010;87(1):25–35.
Kim SY, Kim HH, Nam CM, Kim HC, Suh I, Kang BY. Association of estrogen receptor-alpha gene polymorphism with pathogenesis of osteoporosis in Korean vegetarian men. Med Princ Pract. 2010;19(3):200–5.
Ichikawa S, Koller DL, Padgett LR, Lai D, Hui SL, Peacock M, et al. Replication of previous genome-wide association studies of bone mineral density in premenopausal American women. J Bone Miner Res. 2010;25(8):1821–9.
Jeedigunta Y, Bhoomi Reddy PR, Kolla VK, Munshi A, Ananthapur V, Narasimulu G, et al. Association of estrogen receptor alpha gene polymorphisms with BMD and their affect on estradiol levels in pre- and postmenopausal women in south Indian population from Andhra Pradesh. Clin Chim Acta. 2010;411(7–8):597–600.
Elfassihi L, Giroux S, Bureau A, Laflamme N, Cole DE, Rousseau F. Association with replication between estrogen-related receptor gamma (ESRRgamma) polymorphisms and bone phenotypes in women of European ancestry. J Bone Miner Res. 2010;25(4):901–11.
Drake MT, McCready LK, Hoey KA, Atkinson EJ, Khosla S. Effects of suppression of follicle-stimulating hormone secretion on bone resorption markers in postmenopausal women. J Clin Endocrinol Metab. 2010;95(11):5063–8.
D’Andrilli G, Giordano A, Bovicelli A. Epithelial ovarian cancer: the role of cell cycle genes in the different histotypes. Open Clin Cancer J. 2008;2:7–12.
Yamano T, Morii E, Arai I, Takada T, Kubota K, Sato M, et al. Diagnosis of primary versus metastatic ovarian adenocarcinoma using p53 gene mutation analysis. Int J Clin Oncol. 2010;15(6):621–5.
Zagouri F, Dimopoulos MA, Bournakis E, Papadimitriou CA. Molecular markers in epithelial ovarian cancer: their role in prognosis and therapy. Eur J Gynaecol Oncol. 2010;31(3):268–77.
Risch HA. Hormonal etiology of epithelial ovarian cancer, with a hypothesis concerning the role of androgens and progesterone. J Natl Cancer Inst. 1998;90(23):1774–86.
Ji Q, Liu PI, Chen PK, Aoyama C. Follicle stimulating hormone-induced growth promotion and gene expression profiles on ovarian surface epithelial cells. Int J Cancer. 2004;112(5):803–14.
Brown TJ, Lioubin MN, Marquardt H. Purification and characterization of cytostatic lymphokines produced by activated human T lymphocytes. Synergistic antiproliferative activity of transforming growth factor beta 1, interferon-gamma, and oncostatin M for human melanoma cells. J Immunol. 1987;139(9):2977–83.
Horn D, Fitzpatrick WC, Gompper PT, Ochs V, Bolton-Hansen M, Zarling J, et al. Regulation of cell growth by recombinant oncostatin M. Growth Factors. 1990;2(2–3):157–65.
Mosley B, De Imus C, Friend D, Boiani N, Thoma B, Park LS, et al. Dual oncostatin M (OSM) receptors. Cloning and characterization of an alternative signaling subunit conferring OSM-specific receptor activation. J Biol Chem. 1996;271(51):32635–43.
Douglas AM, Goss GA, Sutherland RL, Hilton DJ, Berndt MC, Nicola NA, et al. Expression and function of members of the cytokine receptor superfamily on breast cancer cells. Oncogene. 1997;14(6):661–9.
Yang CQ, Chan KYK, Ngan HYS, Khoo US, Chiu PM, Chan QKY, et al. Single nucleotide polymorphisms of follicle-stimulating hormone receptor are associated with ovarian cancer susceptibility. Carcinogenesis. 2006;27(7):1502–6.
Heubner M, Riemann K, Otterbach F, Kimmig R, Kasimir-Bauer S, Siffert W, et al. The haplotype of two FSHR polymorphisms in ovarian cancer—a potential role of ethnology in risk modification. Gynecol Oncol. 2009;112(3):486–9.
Ludwig AH, Murawska M, Panek G, Timorek A, Kupryjanczyk J. Androgen, progesterone, and FSH receptor polymorphisms in ovarian cancer risk and outcome. Endocr Relat Cancer. 2009;16(3):1005–16.
The International HapMap Project. Nature. 2003;426(6968):789–96.
Harris JR, Lippman ME, Veronesi U, Willett W. Breast cancer (1). N Engl J Med. 1992;327(5):319–28.
Martin A, Weber BL. Genetic and hormonal risk factors in breast cancer. J Natl Cancer Inst. 2000;92(14):1126–35.
Kuijper TM, Ruigrok-Ritstier K, Verhoef-Post M, Piersma D, Bruysters MWP, Berns EMJJ, et al. LH receptor gene expression is essentially absent in breast tumor tissue: implications for treatment. Mol Cell Endocrinol. 2009;302(1):58–64.
Nilsson C, Pettersson K, Millar RP, Coerver KA, Matzuk MM, Huhtaniemi IT. Worldwide frequency of a common genetic variant of luteinizing hormone: an international collaborative research. Fertil Steril. 1997;67(6):998–1004.
Key TJ, Verkasalo PK, Banks E. Epidemiology of breast cancer. Lancet Oncol. 2001;2(3):133–40.
Piersma D, Berns E, Verhoef-Post M, Uitterlinden A, Braakman I, Pols H, et al. A common polymorphism renders the luteinizing hormone receptor protein more active by improving signal peptide function and predicts adverse outcome in breast cancer patients. J Clin Endocrinol Metab. 2006;91(4):1470–6.
Müller T, Gromoll J, Simoni M. Absence of exon 10 of the human luteinizing hormone (LH) receptor impairs LH, but not human chorionic gonadotropin action. J Clin Endocrinol Metab. 2003;88(5):2242–9.
Piersma D, Verhoef-Post M, Berns E, Themmen A. LH receptor gene mutations and polymorphisms: An overview. Mol Cell Endocrinol. 2007;260–262:282–6.
Saito Y, Tetsuka M, Yue L, Kawamura Y, Maruyama K. Functional role of N-linked glycosylation on the rat melanin-concentrating hormone receptor 1. FEBS Lett. 2003;533(1–3):29–34.
Piersma D, Verhoef-Post M, Look MP, Uitterlinden AG, Pols HAP, Berns EMJJ, et al. Polymorphic variations in exon 10 of the luteinizing hormone receptor: functional consequences and associations with breast cancer. Mol Cell Endocrinol. 2007;276(1–2):63–70.
Rapley EA, Nathanson KL. Predisposition alleles for testicular germ cell tumour. Curr Opin Genet Dev. 2010;20(3):225–30.
Horwich A, Shipley J, Huddart R. Testicular germ-cell cancer. Lancet. 2006;367(9512):754–65.
Ferlin A, Pengo M, Selice R, Salmaso L, Garolla A, Foresta C. Analysis of single nucleotide polymorphisms of FSH receptor gene suggests association with testicular cancer susceptibility. Endocr Relat Cancer. 2008;15(2):429–37.
Oosterhuis JW, Looijenga LHJ. Testicular germ-cell tumours in a broader perspective. Nat Rev Cancer. 2005;5(3):210–22.
Rajpert-De Meyts E. Developmental model for the pathogenesis of testicular carcinoma in situ: genetic and environmental aspects. Hum Reprod Update. 2006;12(3):303–23.
Garolla A, Ferlin A, Vinanzi C, Roverato A, Sotti G, Artibani W, et al. Molecular analysis of the androgen receptor gene in testicular cancer. Endocr Relat Cancer. 2005;12(3):645–55.
Acknowledgements
This work was supported by Fondazione Cassa di Risparmio di Modena and by a grant of the Italian Ministry of University and Research (FIRB IDEAS 2008 RBID08777T).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Casarini, L., Pignatti, E. & Simoni, M. Effects of polymorphisms in gonadotropin and gonadotropin receptor genes on reproductive function. Rev Endocr Metab Disord 12, 303–321 (2011). https://doi.org/10.1007/s11154-011-9192-2
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11154-011-9192-2