Abstract
The modern world is plagued with expanding epidemics of diseases related to metabolic dysfunction. The factors that are driving obesity, diabetes, cardiovascular disease, hypertension, and dyslipidemias (collectively termed metabolic syndrome) are usually ascribed to a mismatch between the body’s homeostatic nutrient requirements and dietary excess, coupled with insufficient exercise. The environmental obesogen hypothesis proposes that exposure to a toxic chemical burden is superimposed on these conditions to initiate or exacerbate the development of obesity and its associated health consequences. Recent studies have proposed a first set of candidate obesogens (diethylstilbestrol, bisphenol A, phthalates and organotins among others) that target nuclear hormone receptor signaling pathways (sex steroid, RXR–PPARγ and GR) with relevance to adipocyte biology and the developmental origins of health and disease (DOHaD). Perturbed nuclear receptor signaling can alter adipocyte proliferation, differentiation or modulate systemic homeostatic controls, leading to long-term consequences that may be magnified if disruption occurs during sensitive periods during fetal or early childhood development.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Chronic disruption of the body’s energy balance equation—caloric intake versus expenditure—is the central driving mechanism that promotes obesity. With the exception of specific instances of obesity arising from genetic causes, a multitude of biological and behavioral factors affect energy balance. These factors interact with an individual’s genetic heritage to expose the risk for, and modify the severity of obesity. Hence, an explanation for the rapid advance in worldwide obesity rates observed over the last decades has been offered in terms of the body’s inability to adequately compensate for modern lifestyle choices with its excessive calories and inadequate physical activity. The “thrifty genotype” hypothesis [1] first proposed to account for modern day increases in disorders of metabolism, e.g. type 2 diabetes, summarizes the concept that genetic traits established to weather unpredictable food supplies in our harsher evolutionary history are a poor fit for the modern obesogenic environment. Rapid accumulation of energy stores and other thrifty phenotypic adaptations were hedges against the unknown.
But something else has changed in the modern world—the chemical environment to which we are exposed has been significantly altered compared to what existed prior to World War 2. A large and ever increasing number of synthetic chemical products permeate the diet and environment and human exposure to these is unavoidable. Whereas humans have evolved over the years to tolerate and metabolize natural products found in the diet, they may be unable to handle the expanded and exotic repertoire of functional groups on molecules not usually encountered in nature. Exposure to these xenobiotic compounds raises concerns that they may interfere with normal physiological processes, for instance as endocrine disruptors targeting those hormonal signaling pathways activated by small lipophilic ligands [2, 3].
The “environmental obesogen” hypothesis proposes that a subset of environmental pollutants disrupts normal development or interferes with the body’s homeostatic controls. Exposure to these obesogens initiates or exacerbates obesity through mis-regulation of critical pathways involved in adipogenesis, lipid metabolism, or energy balance. This review highlights recent findings that provide supporting evidence for chemical exposure models as contributing factors in the epidemic of obesity and associated metabolic syndrome diseases. Emphasis is given to ligand activators of nuclear receptors involved in adipose biology, specifically the sex steroid, RXR–PPARγ, and glucocorticoid receptors, where the link between exposure level and altered gene regulation is the most direct and suspected to be causal. This does not preclude the existence or relevance of other molecular targets, for instance those modifying neuroendocrine functions or behavior. Rather, we focus on research areas where the mechanistic understanding is sufficient to begin evaluating specific disease indicators and epidemiological risk in relation to the levels of exposure encountered in the environment.
2 Environment–gene interactions in the initiation of obesity
The quality and quantity of nutrition is an environmental variable on both daily and longer-term scales. Therefore, adaptive mechanisms to accommodate this fluctuating supply are essential to maintain homeostatic control over the body’s energy requirements. Gastrointestinal, hormonal, and metabolic signals from the major energy storage and metabolic organs (adipose, liver, and muscle) are centrally integrated in the brain through a mesh of feedback networks to effect compensatory changes on food intake, metabolic efficiency, activity, and resource partitioning. Estimates of the daily variability in caloric intake and expenditure are respectively 25 % and 8 % [4]. This suggests that adaptive responses in expenditure are less able to compensate for a prolonged significant supply side surplus. Deposition and mobilization of fat stores plays an integral role in the body’s response to buffer against these challenges. Identifying the factors that influence the normal range of this adaptive response to modify fat storage in individuals is the focus of intensive research. Of special interest to obesity research are altered mechanisms that directly or indirectly impart long-term changes on the overall metabolic set point affecting adipose tissue biology. Such mechanisms include epigenetic changes in regulation of key metabolic enzymes, lesions in endocrine organs, and recruitment or differentiation of cells into the adipocyte lineage, since these are likely to misdirect the body’s regulatory efforts disproportionately compared to an initial triggering event. The “developmental origins of health and disease” (DOHaD) paradigm proposes that fetal and perinatal stages of development represent periods of heightened sensitivity for the establishment of persistent changes to the individual’s adaptive physiology. In the case of metabolic diseases, integration of environmental signals such as maternal nutritional status, stress, and xenobiotic exposure, are misinterpreted and acted upon to program the body for a worldview that is biologically out of synchrony with the reality of a nutrient rich modern world.
Indeed considerable evidence illustrates that maternal nutritional status via the fetal environment is a strong prognostic risk indicator for adult onset metabolic diseases [5–7]. Low birth weight as an measure of suboptimal fetal development and subsequent early adipogenic catch-up growth are associated with metabolic syndrome disease states including type 2 diabetes, cardiovascular disease, hypertension and dyslipidemias [8]. Chronic stress, whether it is physical or psychological, can also elicit similar responses. For example, studies with macaque monkeys show that early life stress results in enhanced visceral fat deposition and elevated incidences of metabolic syndrome phenotypes [9, 10]. Might exposure to toxic chemical agents lead to the same results if the insult intersects with common metabolic regulatory or stress response pathways?
Prenatal maternal smoking represents the proof-of-concept for an environmental toxic chemical insult that promotes obesity following exposure during a sensitive developmental window. Multiple studies showed that the adjusted odds ratio for obesity is elevated between 1.5–2.0 in children born to mothers who smoked during pregnancy and that the risk persists through adulthood [11–13]. Smoking before or after pregnancy does not carry the same risk, indicating that exposure during fetal development is the critical component to elicit persistent changes. Therefore, the existence of other toxicants that are able to trigger or exacerbate obesity is a provocative and relevant question to address.
3 Environmental obesogens and nuclear hormone receptors
The term “environmental obesogen” can be considered as an extension of endocrine disruptor research. Endocrine disrupting chemicals (EDCs) are compounds present in the environment that have the ability to mimic or block the natural action of endogenous hormones in the body (reviewed in [14, 15]). Irreversible changes in patterns of gene expression (organizational effects) are expected to be more common with developmental exposure, whereas activational responses, that are largely reversible with cessation of exposure, will likely predominate at post-developmental stages. To date, most examples of EDCs studied have acted through the nuclear hormone receptor (NRs) superfamily, with the majority of the work focused on aspects of dysfunctional estrogenic signaling in reproductive functions. Members of the NR transcription factor superfamily function as concentration dependent sensors of cognate ligands to coordinate gene regulation of key developmental and homeostatic hormone signaling pathways. A common feature of NR ligands is their small size and lipophilicity. Not surprisingly, many EDCs share these same characteristics. Examples can be found among persistent organochlorine pollutants (DDT, PCBs), plasticizers (phthalates), pharmaceutical and agrochemicals (atrazine) and synthetic/natural hormones (diethylstilbesterol (DES)/birth control pills) in effluent waste water [14]. The overall conserved mechanism of action between NR members has naturally extended the investigation of EDCs beyond the effects on reproductive fitness via estrogenic, anti-estrogenic, or anti-androgenic models to include additional phenotypes. Receptors such as the peroxisome proliferator activated receptors (PPARs), thyroid receptor (TR), liver X receptor (LXR), farnesoid X receptor (FXR), estrogen related receptors (ERRs), retinoid X receptors (RXRs) and glucocorticoid receptor (GR) have come under increased scrutiny as potential targets for mis-regulation in metabolic conditions including obesity.
4 Perturbed estrogenic signaling in obesity
Androgens and estrogens are tightly regulated players in the establishment and maintenance of normal adipose tissue and its functions. As a result, the introduction of hormone mimics is expected to profoundly alter the overall integration of regulatory mechanisms between the major organs. Sex hormone signaling in adipose tissue is usually seen in the context of its anti-adipogenic effects in adults. Sex steroids, in conjunction with growth hormone, have general fat mobilizing properties that are counteracted by the lipid accumulating hormones cortisol and insulin. Disturbances in this balance favoring the latter can account for increased fat deposition in a multitude of physiological and clinical conditions, including genetic disturbances (Cushing’s syndrome), polycystic ovary syndrome, GH-deficiency, menopause, aging, alcoholism and depression [16]. Additional complexity arises from the sexually dimorphic nature of adipose distribution and depot specific responsiveness to sex steroid signaling in humans [17, 18]. Although women exhibit both a greater number and size of adipocytes with a consequent higher percent of total body weight as fat following puberty, this adipose mass is principally distributed as subcutaneous fat that carries a lower risk for health complications. The accretion of abdominal fat in women, the prominent risk factor for metabolic diseases, is generally delayed until changes during menopause lower estrogen levels and alter the estrogen–androgen ratio [19]. Estrogen hormone replacement therapy in postmenopausal women, or ovariectomy in rodent models, can normalize these adipose changes [20, 21]. Similar conclusions were reached in developmental genetic models of impaired estrogen receptor signaling. Thus, the FSH receptor knockout (FORKO) [22], aromatase knockout (ArKO) [23] and estrogen receptor alpha knockout (αERKO) [24] mouse models all exhibit a substantial gain in overall adiposity as a result of both increased adipocyte hypertrophy and hyperplasia. Major targets for the action of estrogens in adipocytes include reduction in lipogenesis via direct inhibition of adipocyte lipoprotein lipase expression [25] and altered sensitivity to hormone-sensitive lipase [26]. Effects on hypothalamic signaling that centrally regulate feeding and energy expenditure are well known [19, 24]. Hepatic control over triglyceride and fatty acid homeostasis is also implicated [22, 27]. It would seem from these data that endocrine disrupting chemicals that act as anti-estrogens would most likely exhibit obesogenic effects. Nevertheless, there are examples of compounds with estrogen receptor (ER) agonist activity that behave as obesogens under specific conditions or when exposure occurs during sensitive developmental windows.
4.1 Diethylstilbestrol (DES)
Between the 1940–1980s, the synthetic estrogen diethylstilbestrol was prescribed to women for estrogen deficient states as hormone replacement therapy and to an estimated 2–8 million pregnant women at risk of miscarriage. Subsequent studies established the long-term endocrine disrupting consequences of DES exposure for multiple generations. DES exposed mothers have an increased risk of developing breast cancer, whereas DES daughters display a high incidence of reproductive tract abnormalities, cervical and vaginal neoplasias, infertility and autoimmune disorders; DES sons also exhibit increased health risks. A prenatal mouse exposure model recapitulates many of these alterations [28]. Consistent with its estrogenic activity, DES doses between 10–100 μg kg−1 day−1 result in a depressed birth weight that is subsequently maintained. Surprisingly though, a dose of 1 μg kg−1 day−1 did not alter birth weight. Rather it was associated with a significant rise in adult body weight [29]. New data from Newbold et al. [30] now demonstrate that high doses of DES (1 mg kg−1 day−1) administered between postnatal day 1–5 (during the period of adipocyte differentiation), cause an initial body weight reduction, followed by a period of “catch-up” growth around puberty and a sustained increase in adult body weight. This increase was associated with a higher percent body fat and preceded by elevated serum levels of adipokines and triglycerides. Hence, it appears that the pro- or anti-adipogenic effects of this estrogenic insult may depend both on the time of exposure and on non-monotonic aspects of the dose–response curve.
4.2 Bisphenol A (BPA)
Bisphenol A was also first identified in the 1940s as a synthetic estrogen but it never found widespread use with the advent of more potent agonists. Instead, the ubiquitous presence of BPA in the environment results from its use as the monomer in polycarbonate plastic and epoxy resins used to line food cans. Surface damage leads to progressive deterioration and leaching of BPA from these substrates. Prenatal exposure of mice in range of 100 μg kg−1 day−1 to 1.2 mg kg−1 day−1 was found to increase postnatal growth [31, 32]. In vitro studies also demonstrated the ability of BPA to synergize with insulin to promote proliferation and differentiation of 3T3-L1 preadipocytes [33]. Recent data suggests that the ability to promote adipocyte differentiation may be independent of the action of ER signaling. GLUT4 mediated glucose uptake into 3T3-F442A cells is enhanced by BPA but not blocked by ER antagonists [34]. In addition, triglyceride accumulation and adipocyte differentiation marker expression could be blocked by an inhibitor of phosphatidylinositol 3-kinase leading to downregulation of Akt kinase activity [35].
4.3 Phytoestrogens and weak estrogenic agonists
Plant derived phytoestrogens such as genistein and daidzein found in soy products have significant estrogenic activity and generally mimic estrogen action on adipogenesis and lipogenesis. Genistein reverses the increase in fat accumulation observed in ovariectomized mice and postmenopausal women given dietary supplementation [36–39]. This effect is not observed in αERKO animals, providing evidence that the mode of action is receptor mediated. Nevertheless, a small number of studies make contradictory observations regarding its anti-adipogenic effects on bone marrow derived cells [40, 41]. Dang et al. report that low doses of genistein exerted the expected estrogenic activity and promoted osteogenic differentiation of primary bone marrow derived cells and the cell line KS483. High doses, however, favored adipogenic differentiation through a proposed activation of PPARγ signaling [41]. A recent feeding study in mice also highlights gender specific and dose-dependent differences. Genistein given to males at nutritional doses promoted increased epididymal and renal fat pad size whereas higher doses in both sexes mimicked the decrease in adipose weight observed with estradiol [42]. Although estrogens are predicted to be anti-adipogenic, estrogenic alkylphenols that lead to adipocyte proliferation in 3T3-L1 cells have also been described [43]. On balance, adult exposure to environmental estrogens appears likely to promote anti-adipogenic effects with the caveat that in certain instances compound-specific interaction with other non-estrogenic signaling pathways or depot specific adipogenic effects may result. In contrast, prenatal or early perinatal exposure to estrogenic compounds is likely to be pro-adipogenic.
4.4 Organotins
Introduction of organotins into the environment began in the 1960s with their widespread use to control marine mollusks by inclusion as antifouling agents in ship paints. The potent biocidal properties of organotins extended their uses to the production of high value food crops and in industrial processes that required anti-fungal agents. Organotins are used extensively in the manufacture of polyolefin plastics (PVC) as a heat stabilizer during polymerization [44]. Research over the past 30 years has established the profound endocrine disrupting properties of organotins in multiple species. In vertebrates, hepatic-, neuro- and immunocytotoxicity are prevalently described effects [45]. In female marine gastropod mollusks, a causal link has been made between the incidence of imposex (the abnormal induction of male sex characteristics) with consequent reproductive failure and exposure to environmentally relevant doses of tributyltin [46, 47]. Masculinization effects of TBT exposure have also been described in two fish species [48, 49].
Inhibition of aromatase, the key cytochrome P450 enzyme required for the conversion of androgens to estrogens, has been proposed as one molecular target for the action of organotins in both invertebrates and vertebrates [50–52]. Effective inhibition of aromatase by organotins occurs in the micromolar range when measured in vitro [50, 53]. Organotins such as tributyltin (TBT) and triphenyltin (TPT) are therefore predicted to exhibit anti-estrogenic activity by inhibiting the production of estradiol and altering levels and ratios of sex steroids. Whether organotins perturb aromatase function through direct enzyme inhibition or, as more recently proposed through transcriptional regulation via RXR–PPARγ [54–56] (described in detail in Section 5.2), a general adipogenic effect, or depot specific remodeling may result. Indeed recent data from the groups of Nishikawa, Inadera, and from our laboratory confirm the ability of TBT to act as an adipogenic agonist in both cell culture and in vivo models [57–59]. In both Xenopus laevis and murine developmental exposure experiments, low doses of TBT resulted in respectively ectopic gonadal adipocyte formation or increased epididymal fat mass with age [59]. As reviewed next, a proposed anti-estrogenic action for tributyltins, e.g. via aromatase inhibition, is not the only relevant molecular mechanism that can account for these effects. Rather it may be synergistic with potent activation of additional nuclear receptor pathways involved in adipocyte differentiation that are already stimulated at nanomolar levels of exposure.
5 Perturbed RXR–PPARγ signaling in obesity
An understanding of the developmental factors controlling adipocyte number and differentiation remains as yet incompletely described. In vitro preadipocyte cell models together with genetic data, however, have identified some of the master regulators able to effectively regulate preadipocyte proliferation and promote terminal adipocyte differentiation [61]. Among these, the peroxisome proliferator-activated receptor gamma (PPARγ) is crucial. Loss of PPARγ function is incompatible with formation of differentiated cells in the adipocyte lineage [62]. Acting as a metabolic sensor for a variety of dietary and metabolic small fatty acid ligands, PPARγ integrates the homeostatic control over energy, lipid and glucose metabolism. PPARγ forms functional transcription factor complexes with retinoid X receptors (RXRα, β, γ). RXRs act as common heterodimeric partners to a wider range of other nuclear hormone receptors. Importantly, the RXR–PPARγ complex can be transcriptionally activated by both PPARγ and RXR-specific ligands. Such receptor heterodimers are said to be permissive. Activation of PPARγ leads to feed-forward changes in gene expression that favor adipocyte differentiation and energy storage. Thus, PPARγ can aptly be described as the ultimate “thrifty” gene [63]. Variations in PPARγ activity observed in natural allelic variants or provoked by differential activation with receptor agonists or antagonists illustrate a spectrum of metabolic consequences. Constitutive activation seen in the PPARγ Pro115Gln variant promotes obesity and insulin resistance [64]; a hypomorphic variant Pro12Ala lowers body mass, improves insulin sensitivity and lipid profiles [65]. Similarly pharmacological activation by PPARγ agonists, such as the thiazolidinedione drugs used to treat diabetic hyperglycemia, improves insulin sensitivity but promotes weight gain [66, 67]; partial agonists lower glucose effectively but are less adipogenic [68, 69]. Hence, persistent activation of PPARγ signaling through nutrient overload or endocrine disrupting agonists is predicted to be obesogenic.
5.1 Phthalates
Widespread introduction of phthalates started in the 1950s with their use as plasticizing agents to soften polyvinyl chloride products. In addition, numerous common household products contain a variety of phthalate components and their ubiquitous presence in the population from chronic exposure (75% of U.S. population has measurable levels) has raised concern over their endocrine disrupting properties. Certain phthalates are able to perturb testicular steroid hormone synthesis and male reproductive system development [70–72]. Measured phthalate concentrations in urine and breast milk have been associated with genital changes and altered reproductive hormone levels in boys [73, 74]. Since androgen deprivation, as induced for instance in prostate cancer therapy, can result in elevations in total fat, serum glucose and a higher prevalence of metabolic syndrome [75, 76], a recent study by Stahlhut et al. [77] drawing a link between specific urinary phthalate metabolites to increased waist circumference and insulin resistance suggests an obesogenic outcome via an anti-androgenic pathway. Prior data, however, also suggests that this phenotype may be mediated through the ability of a subset of phthalates to act as agonists of PPARs [78, 79]. Micromolar ligand activation of PPARγ and associated adipocyte differentiation of 3T3-L1 cells is observed with phthalates including mono-ethyl-hexyl phthalate (MEHP) and mono-benzyl phthalate (MBzP) among others. Recent data describe the ability of MEHP to differentially recruit PPARγ coactivators and modulate a subset of PPARγ inducible genes [80]. It is therefore reasonable to hypothesize that phthalates, with their widespread contamination of the general population, can be linked to persistent unregulated stimulation of PPARγ and induction of a thrifty adipogenic response.
5.2 Organotins
As noted above, organotins such as tributyltin (TBT) can produce potent masculinization effects in sensitive species. Although imposex in vertebrates has only been observed in fish and not in mammals, the underlying endocrine disrupting mechanisms may share common elements but manifest themselves according to the limits imposed by species-specific differences in physiology. Recent studies have uncovered a novel molecular site of action that may provide a coherent explanation for disparate low dose effects across species and explain the ability of organotins to act as environmental obesogens in vertebrates. An earlier series of studies detected a convergent promoter-specific transcriptional inhibition between TBT and synthetic RXR–PPARγ ligands on the regulation of human aromatase in a granulosa cell line [54–56]. More recently, independent in vitro and cell based nuclear receptor screens of high priority environmental pollutants identified a group of organotins, including TBT and TPT, as agonistic ligands for the retinoid X receptors (RXRs), PPARγ and a number of additional permissive RXR-heterodimeric partners [57, 59]. Consistent with results for human cells, TBT and RXR–PPARγ ligands coordinately downregulated amphibian gonadal aromatase expression [59]. Furthermore, the natural RXR ligand, 9-cis retinoic acid, induced imposex in marine mollusks via its invertebrate homolog [81]. Transcriptional mis-regulation by organotins via inappropriate nuclear receptor activation may represent a unifying principle for many of its low dose endocrine disrupting and toxic effects. Mammalian receptor affinities (Kds) and transcriptional activation (EC50s) for tributyltin (TBT) are in the range of 5–12 nM for RXRs and 10–30 nM for PPARγ [59], making this dual agonist a potent mediator for RXR–PPARγ signaling at environmentally relevant levels. In line with the known pharmacology of these receptors, 3T3-L1 cells were efficiently differentiated into mature adipocytes by TBT and in vivo expression patterns of key murine lipogenic RXR–PPARγ target genes in liver and adipose showed coordinate responses. Enhanced adipose lipid accumulation and hepatic steatosis were also observed in mouse neonates following prenatal exposure, suggesting over-stimulated lipogenesis. Altered fatty acid metabolism and lipid accumulation in response to TBT has recently been reported in ramshorn snails, extending this phenotype to invertebrates [60]. At 2 months of age, epididymal fat pad mass in males was greater but neither sex was statistically different in total body weight compared to controls [59]. Measurements beyond 6 months, however, suggest a trend towards greater body weight in both sexes after neonatal TBT exposure (Grün and Blumberg, unpublished data). These observations are reminiscent of the findings described previously for other endocrine disrupting agents, e.g. DES, where changes in body weight and composition may not become exposed until later life unless they meet discreet dose and exposure windows.
6 Perturbed glucocorticoid signaling in obesity
Stress has been described as a state of disharmony or threatened homeostasis. Metabolic syndrome diseases can equally be described as a state of deranged metabolic homeostasis with obesity a result of the body’s efforts to reestablish equilibrium over energy balance. Therefore, it is not coincidental that stress, or the inability to adequately respond to stress, plays a significant role in the development of these disease states. Stressors (nutritional status, pregnancy complications, psychosocial factors, toxic exposure etc.) experienced during fetal development or early childhood can result in long-term changes in central stress response pathways. Epigenetic imprinting is one mechanism that establishes permanent alterations. Effects on the glucocorticoid receptor (GR) promoter and on GR sensitive target genes have been documented in response to behavioral stress or exogenous chemical exposure (dexamethasone, benz[a]pyrene) [84–87]. Without balanced GR action on key enzymes of glucose, lipid, amino acid metabolism, as well as adipokine and inflammatory factors, the adaptive stress response inadequately compensates for physiological changes. Changes in the hypothalamic–pituitary–adrenal axis (HPA) that lead to over-stimulated production, or perturbed metabolism of cortisol and glucocorticoid receptor signaling have been implicated as contributing factors in the development of obesity [88, 89]. In addition to systemic adrenal production, localized control over glucocorticoid signaling can be influenced in peripheral tissues by the inter-conversion between cortisol (active) and cortisone (inactive) GR ligands, mediated respectively by the activities of 11β-hydroxysteroid dehydrogenase (11β-HSD) types 1 and 2. The presence of glucocorticoids in adipose tissues is deleterious. Thus, transgenic overexpression of 11β-HSD1 increases cortisol levels and results in visceral obesity, glucose intolerance and insulin resistance [90]. It is reasonable to expect that exposure to environmental chemicals that can directly stimulate the HPA axis, activate GR, alter GR function, or elevate the peripheral levels of endogenous GR ligands may be contributing factors for metabolic complications. Indeed, an extensive and chemically diverse group of compounds have been reported that serve as examples of environmental agents able to elevate or depress glucocorticoid signaling (reviewed in [91]).
7 Localized perturbation through 11β-HSD inhibition
In light of the candidate environmental obesogens already discussed, a number of recent observations make synergistic glucocorticoid disruption probable, especially for those candidates like organotins with broad mechanisms of toxicity. Metabolic inactivation of active glucocorticoids is essential to prevent renal hypertensive effects [92] and placental clearance protects the fetus from elevated maternal glucocorticoid levels that would otherwise result in intrauterine growth retardation [93]. Specific downregulation of placental 11β-HSD2 function under conditions of maternal nutrient deprivation provides a link between low birth weight and metabolic syndrome. The organotin TBT was recently shown to directly inhibit 11β-HSD2 (the inactivating enzyme), thereby providing a mechanism for augmenting hypertensive effects and disrupting fetal glucocorticoid status as seen with other 11β-HSD inhibitors [94]. These include licorice-derived glycyrrhizin, its synthetic derivatives (sweeteners) [95, 96] and dithiocarbamate chemicals. Dithiocarbamates are widely found in cosmetics, pesticides, and rubber products. Inhibition of 11β-HSD2 by dithiocarbamates and organotins is reported to be mediated through critical cysteine residues [94, 97]. Both 11β-HSD isoforms exhibit sensitivity to RXR–NR transcriptional regulation, opening the possibility of further disruption by organotin regulation of NR function. Placental type 2 expression is downregulated by RXR–PPARδ activation [98], whereas RXR–PPARα, RXR–PPARγ and RXR–LXR heterodimers downregulate the type 1 isoform [99–101]. Tissue specific effects on both glucocorticoid clearance and peripheral reactivation are predicted to follow exposure.
7.1 H–P–A Perturbation
Aside from modulation of peripheral 11β-HSD activity in specific organs such as placenta and adipose, environmental obesogens might also target more central aspects of the stress response mediated by the H–P–A axis via transcriptional misregulation of NR sensitive target genes in neural tissues or through site specific toxicity that create lesions and permanently alter integrative functions. Given the potent ligand agonist properties of certain organotins, e.g. TBT, transcriptional effects as a result of inappropriate activation of RXR-heterodimers can be reasonably proposed. In addition other organotin species with shorter alkyl chains, e.g. trimethyl (TMT) and triethyltin (TET), may exert their influence through yet a different mechanism. These species are poor transcriptional activators of RXR and its heterodimers [59]. They are highly neurotoxic, however, creating lesions primarily in pyramidal neurons of the hippocampus that may impact glucocorticoid dependent feedback on the HPA axis. Adrenalectomy exacerbated, whereas glucocorticoid supplementation protected against this loss [102]. Taken together, these results strongly suggest that at least some of the endocrine disrupting actions of organotins are mediated by altered glucocorticoid homeostasis that is known to impact the initiation and progression of obesity and its metabolic sequelae.
8 Conclusions
It seems clear that a chemical origin for the obesity epidemic as first postulated by Baillie-Hamilton [103] has sufficient merit to warrant more intensive investigation. Only in this way can we determine the overall significance of xenobiotic exposure as a contributing risk factor for obesity in addition to the well-known dietary and lifestyle considerations. Sufficient examples exist of pharmaceuticals (thiazolidinediones, atypical anti-psychotics, antihistamines, antidepressants, anticonvulsants) that result in progressive, undesirable body weight gain with pharmacological administration. Therefore, obesity as a consequence of exposure to mixtures of environmental pollutants with obesogenic properties is not unexpected a priori. Furthermore, considering that organotins and phthalates came into wide use in the 1960s, it can also be predicted that obesity as a result of exposure to these chemicals would become manifest in the 1980s and afterward. This corresponds closely to the rise in the epidemic of obesity and metabolic syndrome diseases. The difficulty in determining the validity of this hypothesis rests in the uncertainty of extrapolations of biological effects to low dose or intermittent exposure where observable metrics are apparent only after long periods and in the presence of multiple confounding parameters. What is necessary to address these issues is the identification of specific obesogenic compounds with clear-cut molecular targets, a mechanistic understanding of their action, and corroborating data from relevant animal models and human studies. In this context, epidemiological data can be evaluated properly for exposure risk and clinical health significance.
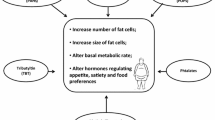
Much progress has been made toward meeting these criteria. The examples cited above (e.g. DES, BPA, phthalates, organotins, dithiocarbamates) reflect those candidates where experimental data has provided a mechanistic basis of action and detrimental effects from environmental exposure are relevant to a large percentage of the population. Figure 1 provides a brief schematic summary for their possible molecular sites of action. Frequently, candidate environmental obesogens exhibit pleiotropic effects on multiple organ systems. This is not unexpected if they function either as direct agonists or disrupt endogenous ligand levels of nuclear receptors that are ubiquitously involved in regulating metabolic homeostasis. As highlighted for organotins the ability to efficiently activate RXRs or significantly disrupt other NR pathways broadens the potential to disrupt metabolic regulation beyond just adipose tissues. Besides its role in adipogenesis, RXR–PPARγ signaling is central also to transcriptional regulation in muscle, liver and macrophages that impact disposition of glucose and triglycerides and regulate inflammatory cytokines. Furthermore, RXR acts as the heterodimeric partner for an extensive set of nuclear receptors relevant to metabolic regulation, including thyroid hormone receptor (TR), vitamin D receptor, steroid and xenobiotic receptor (SXR/PXR), farnesoid X receptor (FXR) and liver X receptor (LXR). These heterodimers are involved in many aspects of energy regulation, steroid metabolism, cholesterol and lipid homeostasis (reviewed in [82, 83]). Hence, heterodimeric NR combinations sensitive to permissive transactivation by RXR-specific ligands, e.g. TBT, are molecular targets that warrant further investigation, not just in adipogenesis, but also for involvement in a wider variety of metabolic disease states, e.g. hypertension, diabetes, artherosclerosis and non-alcoholic steatohepatitis.
Environmental obesogens and NR pathways. The schematic depicts a limited set of candidate obesogens and potential molecular targets in the initiation and progression of obesity. Maternal smoking during pregnancy establishes a long term propensity for increased risk of metabolic syndrome most likely through altered programming of neuroendocrine functions. Above micromolar levels, pollutant organotins e.g. TBT (red circles), exhibit general cytotoxicity resulting in apoptosis; certain short chain organotins e.g. TMT (orange triangles) can evoke specific lesions through selective neurotoxicity in a subset of hippocampal neurons that interact with central H–P–A regulation. Micromolar levels of TBT also targets intracellular enzymes relevant to metabolism of NR steroidal ligands e.g. glucocorticoid inactivation is potentially perturbed via inhibition of 11β-hydroxysteroid dehydrogenase type 2; likewise inhibition of cytochrome P450s, such as aromatase, is expected to result in altered ratios of estrogens (E) and androgens (T) with effects on developmental programming and fat mobilization. Under some circumstances developmental exposure to the synthetic estrogen receptor agonist DES (green squares) or other environmental estrogenic compounds disturb body weight gain and fat distribution. At nanomolar levels, the organotin TBT is a potent dual agonist for retinoid X receptors (RXRs) and a number of permissive heterodimeric partners, including PPARγ; phthalates (blue hexagons) target PPARγ at micromolar levels. RXR–PPARγ signaling effectively promotes preadipocyte differentiation, affects glucose and triglyceride disposition and impacts a wide variety of key adipogenic genes in multiple target organs. Additional activation of permissive RXR-heterodimer combinations by TBT or secondary effects on classical NR steroidal ligands via modulation of RXR-heterodimer sensitive targets e.g. 11β-HSD and aromatase, broadens the potential for synergistic metabolic misregulation and promotion of obesogenic phenotypes. Overall, exposure to environmental obesogens, especially during development or early-life, is envisioned to magnify long-term effects on metabolic homeostasis through mechanisms such as altered fetal programming of neuroendocrine pathways (H–P–A axis), hormonal imprinting of key metabolic enzymes and recruitment or excessive proliferation of cells in the adipose lineage
The discovery of novel obesogens will no doubt accelerate with the continued improvement in knowledge about adipose biology and mechanisms of obesity. A better understanding of the factors required to establish adipocyte proliferation, differentiation, and depot specific differences during development will be particularly valuable. A recent published study has begun to address this deficiency by identifying several developmental genes strongly predictive of adipose depot specificity and body adiposity [104]. The bias towards activators of nuclear receptors is in part historical, but also reflective of the fact that these intracellular mediators are activated by high affinity endogenous ligands with EC50 values in the range between nanomolar to micromolar. Pharmacological activation by environmental hormone mimics necessitates similar affinities or persistent exposure to increase the probability for disruption from dilute non-point source contamination, where the total sustained dose received is likely limited to levels below milligram per kilogram body weight. With worldwide production values for phthalates, bisphenol A and organotins climbing to exceed 100,000 tons/year, however, the introduction of large quantities of potential rogue hormone receptor activators entering the environment should not ignored.
Abbreviations
- RXR:
-
retinoic X receptor
- PPARγ:
-
peroxisome proliferatpr activated receptor gamma
- LXR:
-
liver X receptor
- FXR:
-
farnesoid X receptor
- GR:
-
glucocorticoid receptor
- ER:
-
estrogen receptor
- BPA:
-
bisphenol A
- DES:
-
diethylstilbestrol
- TBT:
-
tributyltin chloride
- TPT:
-
triphenyltin chloride
- EDC:
-
endocrine disrupting chemicals
- TZD:
-
thiazolidinedione
References
Neel JV. Diabetes mellitus: a “thrifty” genotype rendered detrimental by “progress”? Am J Hum Genet 1962;14:353–62
Nilsson R. Endocrine modulators in the food chain and environment. Toxicol Pathol 2000;28(3): 420–31
Waring RH, Harris RM. Endocrine disrupters: a human risk? Mol Cell Endocrinol 2005;244(1–2):2–9
Jebb SA. Energy intake and body weight. In: Fairburn CG, Brownwell KD, editors. Eating disorder and obesity. New York: Guildford; 2002. p. 37–42.
Barker DJ. Fetal origins of coronary heart disease. BMJ 1995;311(6998):171–4.
Hales CN, Barker DJ. The thrifty phenotype hypothesis. Br Med Bull 2001;60:5–20.
Yajnik C. Interactions of perturbations in intrauterine growth and growth during childhood on the risk of adult-onset disease. Proc Nutr Soc 2000;59(2):257–65.
Cottrell EC, Ozanne SE. Developmental programming of energy balance and the metabolic syndrome. Proc Nutr Soc 2007;66(2):198–206.
Shively CA, Clarkson TB. Regional obesity and coronary artery atherosclerosis in females: a non-human primate model. Acta Med Scand Suppl 1988;723:71–8.
Kaufman D, Banerji MA, Shorman I, Smith EL, Coplan JD, Rosenblum LA, et al. Early-life stress and the development of obesity and insulin resistance in juvenile bonnet macaques. Diabetes 2007;56(5):1382–6.
Power C, Jefferis BJ. Fetal environment and subsequent obesity: a study of maternal smoking. Int J Epidemiol 2002;31(2):413–9.
Al Mamun A, Lawlor DA, Alati R, O’Callaghan MJ, Williams GM, Najman JM. Does maternal smoking during pregnancy have a direct effect on future offspring obesity? Evidence from a prospective birth cohort study. Am J Epidemiol 2006;164(4):317–25.
Oken E, Huh SY, Taveras EM, Rich-Edwards JW, Gillman MW. Associations of maternal prenatal smoking with child adiposity and blood pressure. Obes Res 2005;13(11):2021–8.
McLachlan JA. Environmental signaling: what embryos and evolution teach us about endocrine disrupting chemicals. Endocr Rev 2001;22(3):319–41.
Crews D, McLachlan JA. Epigenetics, evolution, endocrine disruption, health, and disease. Endocrinology 2006;147(6 Suppl):S4–10.
Bjorntorp P. The regulation of adipose tissue distribution in humans. Int J Obes Relat Metab Disord 1996;20(4):291–302.
Butte NF, Hopkinson JM, Wong WW, Smith EO, Ellis KJ. Body composition during the first 2 years of life: an updated reference. Pediatr Res 2000;47(5): 578–85.
Taylor RW, Jones IE, Williams SM, Goulding A: Body fat percentages measured by dual-energy X-ray absorptiometry corresponding to recently recommended body mass index cutoffs for overweight and obesity in children and adolescents aged 3–18 y. Am J Clin Nutr 2002;76(6):1416–21.
Wade GN, Gray JM, Bartness TJ. Gonadal influences on adiposity. Int J Obes 1985;9(Suppl 1):83–92.
Mohamed MK, Abdel-Rahman AA. Effect of long-term ovariectomy and estrogen replacement on the expression of estrogen receptor gene in female rats. Eur J Endocrinol 2000;142(3):307–14.
Haarbo J, Marslew U, Gotfredsen A, Christiansen C. Postmenopausal hormone replacement therapy prevents central distribution of body fat after menopause. Metabolism 1991;40(12):1323–6.
Danilovich N, Babu PS, Xing W, Gerdes M, Krishnamurthy H, Sairam MR. Estrogen deficiency, obesity, and skeletal abnormalities in follicle-stimulating hormone receptor knockout (FORKO) female mice. Endocrinology 2000;141(11):4295–308.
Murata Y, Robertson KM, Jones ME, Simpson ER. Effect of estrogen deficiency in the male: the ArKO mouse model. Mol Cell Endocrinol 2002;193(1–2):7–12.
Heine PA, Taylor JA, Iwamoto GA, Lubahn DB, Cooke PS. Increased adipose tissue in male and female estrogen receptor-alpha knockout mice. Proc Natl Acad Sci U S A 2000;97(23):12729–34.
Homma H, Kurachi H, Nishio Y, Takeda T, Yamamoto T, Adachi K, et al. Estrogen suppresses transcription of lipoprotein lipase gene. Existence of a unique estrogen response element on the lipoprotein lipase promoter. J Biol Chem 2000;275(15):11404–11.
Palin SL, McTernan PG, Anderson LA, Sturdee DW, Barnett AH, Kumar S. 17Beta-estradiol and anti-estrogen ICI:compound 182,780 regulate expression of lipoprotein lipase and hormone-sensitive lipase in isolated subcutaneous abdominal adipocytes. Metabolism 2003;52(4):383–8.
Jones ME, Thorburn AW, Britt KL, Hewitt KN, Wreford NG, Proietto J, et al. Aromatase-deficient (ArKO) mice have a phenotype of increased adiposity. Proc Natl Acad Sci U S A 2000;97(23):12735–40.
Newbold R. Cellular and molecular effects of developmental exposure to diethylstilbestrol: implications for other environmental estrogens. Environ Health Perspect 1995;103(Suppl 7):83–7.
Newbold RR, Padilla-Banks E, Snyder RJ, Jefferson WN. Developmental exposure to estrogenic compounds and obesity. Birth Defects Res A Clin Mol Teratol 2005;73(7):478–80.
Newbold RR, Padilla-Banks E, Snyder RJ, Phillips TM, Jefferson WN. Developmental exposure to endocrine disruptors and the obesity epidemic. Reprod Toxicol 2007;23(3):290–6.
Howdeshell KL, Hotchkiss AK, Thayer KA, Vandenbergh JG, vom Saal FS. Exposure to bisphenol A advances puberty. Nature 1999;401(6755):763–4.
Rubin BS, Murray MK, Damassa DA, King JC, Soto AM. Perinatal exposure to low doses of bisphenol A affects body weight, patterns of estrous cyclicity, and plasma LH levels. Environ Health Perspect 2001;109(7):675–80.
Masuno H, Kidani T, Sekiya K, Sakayama K, Shiosaka T, Yamamoto H, et al. Bisphenol A in combination with insulin can accelerate the conversion of 3T3-L1 fibroblasts to adipocytes. J Lipid Res 2002;43(5):676–84.
Sakurai K, Kawazuma M, Adachi T, Harigaya T, Saito Y, Hashimoto N, et al. Bisphenol A affects glucose transport in mouse 3T3-F442A adipocytes. Br J Pharmacol 2004;141(2):209–14.
Masuno H, Iwanami J, Kidani T, Sakayama K, Honda K. Bisphenol a accelerates terminal differentiation of 3T3-L1 cells into adipocytes through the phosphatidylinositol 3-kinase pathway. Toxicol Sci 2005;84(2):319–27.
Naaz A, Yellayi S, Zakroczymski MA, Bunick D, Doerge DR, Lubahn DB, et al. The soy isoflavone genistein decreases adipose deposition in mice. Endocrinology 2003;144(8):3315–20.
Kim HK, Nelson-Dooley C, Della-Fera MA, Yang JY, Zhang W, Duan J, et al. Genistein decreases food intake, body weight, and fat pad weight and causes adipose tissue apoptosis in ovariectomized female mice. J Nutr 2006;136(2):409–14.
Goodman-Gruen D, Kritz-Silverstein D. Usual dietary isoflavone intake and body composition in postmenopausal women. Menopause 2003;10(5):427–32.
Wu J, Oka J, Tabata I, Higuchi M, Toda T, Fuku N, et al. Effects of isoflavone and exercise on BMD and fat mass in postmenopausal Japanese women: a 1-year randomized placebo-controlled trial. J Bone Miner Res 2006;21(5):780–9.
Heim M, Frank O, Kampmann G, Sochocky N, Pennimpede T, Fuchs P, et al. The phytoestrogen genistein enhances osteogenesis and represses adipogenic differentiation of human primary bone marrow stromal cells. Endocrinology 2004;145(2):848–59.
Dang ZC, Audinot V, Papapoulos SE, Boutin JA, Lowik CW. Peroxisome proliferator-activated receptor gamma (PPARgamma ) as a molecular target for the soy phytoestrogen genistein. J Biol Chem 2003;278(2):962–7.
Penza M, Montani C, Romani A, Vignolini P, Pampaloni B, Tanini A, et al. Genistein affects adipose tissue deposition in a dose-dependent and gender-specific manner. Endocrinology 2006;147(12):5740–51.
Masuno H, Okamoto S, Iwanami J, Honda K, Shiosaka T, Kidani T, et al. Effect of 4-nonylphenol on cell proliferation and adipocyte formation in cultures of fully differentiated 3T3-L1 cells. Toxicol Sci 2003;75(2):314–20.
Appel KE. Organotin compounds: toxicokinetic aspects. Drug Metab Rev 2004;36(3–4):763–86.
Boyer IJ. Toxicity of dibutyltin, tributyltin and other organotin compounds to humans and to experimental animals. Toxicology 1989;55(3):253–98.
Blaber SJM. The occurrence of a penis-like outgrowth behind the right tentacle in spent females of Nucella lapillus. Proc Malacol Soc Lond 1970;39:231–3.
Matthiessen P, Gibbs P. Critical appraisal of the evidence for tributyltin-mediated endocrine disruption in mollusks. Environ Toxicol Chem 1998;17:37–43.
Shimasaki Y, Kitano T, Oshima Y, Inoue S, Imada N, Honjo T. Tributyltin causes masculinization in fish. Environ Toxicol Chem 2003;22(1):141–4.
McAllister BG, Kime DE. Early life exposure to environmental levels of the aromatase inhibitor tributyltin causes masculinisation and irreversible sperm damage in zebrafish (Danio rerio). Aquat Toxicol 2003;65(3):309–16.
Heidrich DD, Steckelbroeck S, Klingmuller D. Inhibition of human cytochrome P450 aromatase activity by butyltins. Steroids 2001;66(10):763–9.
Cooke GM. Effect of organotins on human aromatase activity in vitro. Toxicol Lett 2002;126(2):121–30.
Horiguchi T. Masculinization of female gastropod mollusks induced by organotin compounds, focusing on mechanism of actions of tributyltin and triphenyltin for development of imposex. Environ Sci 2006;13(2):77–87.
Lo S, Allera A, Albers P, Heimbrecht J, Jantzen E, Klingmuller D, et al. Dithioerythritol (DTE) prevents inhibitory effects of triphenyltin (TPT) on the key enzymes of the human sex steroid hormone metabolism. J Steroid Biochem Mol Biol 2003;84(5):569–76.
Mu YM, Yanase T, Nishi Y, Takayanagi R, Goto K, Nawata H. Combined treatment with specific ligands for PPARgamma:RXR nuclear receptor system markedly inhibits the expression of cytochrome P450arom in human granulosa cancer cells. Mol Cell Endocrinol 2001;181(1–2):239–48.
Mu YM, Yanase T, Nishi Y, Waseda N, Oda T, Tanaka A, et al. Insulin sensitizer, troglitazone, directly inhibits aromatase activity in human ovarian granulosa cells. Biochem Biophys Res Commun 2000;271(3):710–3.
Saitoh M, Yanase T, Morinaga H, Tanabe M, Mu YM, Nishi Y, et al. Tributyltin or triphenyltin inhibits aromatase activity in the human granulosa-like tumor cell line KGN. Biochem Biophys Res Commun 2001;289(1):198–204.
Kanayama T, Kobayashi N, Mamiya S, Nakanishi T, Nishikawa J. Organotin compounds promote adipocyte differentiation as agonists of the peroxisome proliferator-activated receptor gamma/retinoid X receptor pathway. Mol Pharmacol 2005;67(3):766–74.
Inadera H, Shimomura A. Environmental chemical tributyltin augments adipocyte differentiation. Toxicol Lett 2005;159(3):226–34.
Grun F, Watanabe H, Zamanian Z, Maeda L, Arima K, Chubacha R, et al. Endocrine disrupting organotin compounds are potent inducers of adipogenesis in vertebrates. Mol Endocrinol 2006;20(9):2141–55.
Janer G, Navarro JC, Porte C. Exposure to TBT increases accumulation of lipids and alters fatty acid homeostasis in the ramshorn snail Marisa cornuarietis. Comp Biochem Physiol C Toxicol Pharmacol 2007, in press.
Rangwala SM, Lazar MA. Transcriptional control of adipogenesis. Annu Rev Nutr 2000;20:535–59.
Rosen ED, Sarraf P, Troy AE, Bradwin G, Moore K, Milstone DS, et al. PPAR gamma is required for the differentiation of adipose tissue in vivo and in vitro. Mol Cell 1999;4(4):611–17.
Auwerx J. PPARgamma, the ultimate thrifty gene. Diabetologia 1999;42(9):1033–49.
Ristow M, Muller-Wieland D, Pfeiffer A, Krone W, Kahn CR. Obesity associated with a mutation in a genetic regulator of adipocyte differentiation. N Engl J Med 1998;339(14):953–9.
Yen CJ, Beamer BA, Negri C, Silver K, Brown KA, Yarnall DP, et al. Molecular scanning of the human peroxisome proliferator activated receptor gamma (hPPAR gamma) gene in diabetic Caucasians: identification of a Pro12Ala PPAR gamma 2 missense mutation. Biochem Biophys Res Commun 1997;241(2):270–4.
Larsen TM, Toubro S, Astrup A. PPARgamma agonists in the treatment of type II diabetes: is increased fatness commensurate with long-term efficacy? Int J Obes Relat Metab Disord 2003;27(2):147–61.
Rubenstrunk A, Hanf R, Hum DW, Fruchart JC, Staels B. Safety issues and prospects for future generations of PPAR modulators. Biochim Biophys Acta 2007.
Rocchi S, Picard F, Vamecq J, Gelman L, Potier N, Zeyer D, et al. A unique PPARgamma ligand with potent insulin-sensitizing yet weak adipogenic activity. Mol Cell 2001;8(4):737–47.
Fukui Y, Masui S, Osada S, Umesono K, Motojima K. A new thiazolidinedione, NC-2100, which is a weak PPAR-gamma activator, exhibits potent antidiabetic effects and induces uncoupling protein 1 in white adipose tissue of KKAy obese mice. Diabetes 2000;49(5):759–67.
Bell FP. Effects of phthalate esters on lipid metabolism in various tissues, cells and organelles in mammals. Environ Health Perspect 1982;45:41–50.
Parks LG, Ostby JS, Lambright CR, Abbott BD, Klinefelter GR, Barlow NJ, et al. The plasticizer diethylhexyl phthalate induces malformations by decreasing fetal testosterone synthesis during sexual differentiation in the male rat. Toxicol Sci 2000;58(2):339–49.
Fisher JS. Environmental anti-androgens and male reproductive health: focus on phthalates and testicular dysgenesis syndrome. Reproduction 2004;127(3):305–15.
Swan SH, Main KM, Liu F, Stewart SL, Kruse RL, Calafat AM, et al. Decrease in anogenital distance among male infants with prenatal phthalate exposure. Environ Health Perspect 2005;113(8):1056–61.
Main KM, Mortensen GK, Kaleva MM, Boisen KA, Damgaard IN, Chellakooty M, et al. Human breast milk contamination with phthalates and alterations of endogenous reproductive hormones in infants three months of age. Environ Health Perspect 2006;114(2):270–6.
Braga-Basaria M, Dobs AS, Muller DC, Carducci MA, John M, Egan J, et al. Metabolic syndrome in men with prostate cancer undergoing long-term androgen-deprivation therapy. J Clin Oncol 2006;24(24):3979–83.
Sharifi N, Gulley JL, Dahut WL. Androgen deprivation therapy for prostate cancer. Jama 2005;294(2):238–44.
Stahlhut RW, van Wijgaarden E, Dye TD, Cook S, Swan SH. Concentrations of urinary phthalate metabolites are associated with increased waist circumferece and insulin resistance in adult U.S. males. Environ Health Perspect 2007;115(6):876–82.
Maloney EK, Waxman DJ. trans-Activation of PPARalpha and PPARgamma by structurally diverse environmental chemicals. Toxicol Appl Pharmacol 1999;161(2):209–18.
Hurst CH, Waxman DJ. Activation of PPARalpha and PPARgamma by environmental phthalate monoesters. Toxicol Sci 2003;74(2):297–308.
Feige JN, Gelman L, Rossi D, Zoete V, Metivier R, Tudor C, et al. The endocrine disruptor mono-ethyl-hexyl-phthalate is a selective PPARgamma modulator which promotes adipogenesis. J Biol Chem 2007;282(26):19152–66.
Nishikawa J, Mamiya S, Kanayama T, Nishikawa T, Shiraishi F, Horiguchi T. Involvement of the retinoid X receptor in the development of imposex caused by organotins in gastropods. Environ Sci Technol 2004;38(23):6271–6.
Chawla A, Repa JJ, Evans RM, Mangelsdorf DJ. Nuclear receptors and lipid physiology: opening the X-files. Science 2001;294(5548):1866–70.
Handschin C, Meyer UA. Regulatory network of lipid-sensing nuclear receptors: roles for CAR, PXR, LXR, and FXR. Arch Biochem Biophys 2005;433(2):387–96.
Weaver IC, Cervoni N, Champagne FA, D’Alessio AC, Sharma S, Seckl JR, et al. Epigenetic programming by maternal behavior. Nat Neurosci 2004;7(8):847–54.
Weaver IC, Champagne FA, Brown SE, Dymov S, Sharma S, Meaney MJ, et al. Reversal of maternal programming of stress responses in adult offspring through methyl supplementation: altering epigenetic marking later in life. J Neurosci 2005;25(47):11045–54.
Nyirenda MJ, Lindsay RS, Kenyon CJ, Burchell A, Seckl JR. Glucocorticoid exposure in late gestation permanently programs rat hepatic phosphoenolpyruvate carboxykinase and glucocorticoid receptor expression and causes glucose intolerance in adult offspring. J Clin Invest 1998;101(10):2174–81.
Csaba G, Inczefi-Gonda A. Molecules acting on receptor level at weaning, durably influence liver glucocorticoid receptors. Acta Physiol Hung 2005;92(1):33–8.
Bjorntorp P, Rosmond R: Obesity and cortisol. Nutrition 2000;16(10):924–36.
Pasquali R, Vicennati V, Cacciari M, Pagotto U. The hypothalamic–pituitary–adrenal axis activity in obesity and the metabolic syndrome. Ann NY Acad Sci 2006;1083:111–28.
Masuzaki H, Yamamoto H, Kenyon CJ, Elmquist JK, Morton NM, Paterson JM, et al. Transgenic amplification of glucocorticoid action in adipose tissue causes high blood pressure in mice. J Clin Invest 2003;112(1):83–90.
Odermatt A, Gumy C, Atanasov AG, Dzyakanchuk AA. Disruption of glucocorticoid action by environmental chemicals: potential mechanisms and relevance. J Steroid Biochem Mol Biol 2006;102(1–5):222–31.
White PC, Mune T, Agarwal AK (1997) 11 beta-Hydroxysteroid dehydrogenase and the syndrome of apparent mineralocorticoid excess. Endocr Rev 1997;18(1):135–56.
Seckl JR. Glucocorticoids, feto-placental 11 beta-hydroxysteroid dehydrogenase type 2, and the early life origins of adult disease. Steroids 1997;62(1):89–94.
Atanasov AG, Nashev LG, Tam S, Baker ME, Odermatt A. Organotins disrupt the 11beta-hydroxysteroid dehydrogenase type 2-dependent local inactivation of glucocorticoids. Environ Health Perspect 2005;113(11):1600–6.
Kageyama Y, Suzuki H, Saruta T. Glycyrrhizin induces mineralocorticoid activity through alterations in cortisol metabolism in the human kidney. J Endocrinol 1992;135(1): 147–52.
Lindsay RS, Lindsay RM, Waddell BJ, Seckl JR: Prenatal glucocorticoid exposure leads to offspring hyperglycaemia in the rat: studies with the 11 beta-hydroxysteroid dehydrogenase inhibitor carbenoxolone. Diabetologia 1996;39(11):1299–305.
Atanasov AG, Tam S, Rocken JM, Baker ME, Odermatt A. Inhibition of 11 beta-hydroxysteroid dehydrogenase type 2 by dithiocarbamates. Biochem Biophys Res Commun 2003;308(2):257–62.
Julan L, Guan H, van Beek JP, Yang K. Peroxisome proliferator-activated receptor delta suppresses 11beta-hydroxysteroid dehydrogenase type 2 gene expression in human placental trophoblast cells. Endocrinology 2005;146(3):1482–90.
Stulnig TM, Oppermann U, Steffensen KR, Schuster GU, Gustafsson JA. Liver X receptors downregulate 11beta-hydroxysteroid dehydrogenase type 1 expression and activity. Diabetes 2002;51(8):2426–33.
Hermanowski-Vosatka A, Gerhold D, Mundt SS, Loving VA, Lu M, Chen Y, et al. PPARalpha agonists reduce 11beta-hydroxysteroid dehydrogenase type 1 in the liver. Biochem Biophys Res Commun 2000;279(2):330–6.
Tomlinson JW, Moore J, Cooper MS, Bujalska I, Shahmanesh M, Burt C, et al. Regulation of expression of 11beta-hydroxysteroid dehydrogenase type 1 in adipose tissue: tissue-specific induction by cytokines. Endocrinology 2001;142(5):1982–9.
Imai H, Nishimura T, Sadamatsu M, Liu Y, Kabuto M, Kato N. Type II glucocorticoid receptors are involved in neuronal death and astrocyte activation induced by trimethyltin in the rat hippocampus. Exp Neurol 2001;171(1):22–8.
Baillie-Hamilton PF. Chemical toxins: a hypothesis to explain the global obesity epidemic. J Altern Complement Med 2002;8(2):185–92.
Gesta S, Bluher M, Yamamoto Y, Norris AW, Berndt J, Kralisch S, et al. Evidence for a role of developmental genes in the origin of obesity and body fat distribution. Proc Natl Acad Sci U S A 2006;103(17):6676–81.
Acknowledgements
Research in the authors laboratory was supported by grants from the US Environmental Protection Agency (STAR R830686) and National Institutes of Health (GM-60572) (to B.B.) and from the University of California Toxic Substance Research and Training Program (UC-37579) (to F.G.).
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Grün, F., Blumberg, B. Perturbed nuclear receptor signaling by environmental obesogens as emerging factors in the obesity crisis. Rev Endocr Metab Disord 8, 161–171 (2007). https://doi.org/10.1007/s11154-007-9049-x
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11154-007-9049-x