Abstract
The main purpose of the present study is to investigate the efficiency of the biomass waste, which is represented by grape leaf waste, for the adsorption of methylene blue textile dye from an aqueous solution after chemical treatment with phosphoric acid. The synthesized activated carbon acid-GL has been characterized using FTIR, SEM, and BET to realize the possibility of the surface for the purpose of the study. The removal of methylene blue from the aqueous medium is studied parametrically using parameters like pH solution, amount of adsorbent, MB levels, volume solution, and temperature. However, the investigation of the parameters has been provided using response surface methodology to see which factors are significant and have a strong effect on the removal of dye. The adsorption profile has followed the Langmuir model with a correlation factor (R2 = 0.9766), which indicates that it fits the experimental data from the other isotherm, like Freundlich (R2 = 0.948). Methylene blue (MB) has a maximum adsorption capacity of 28.85 mg/g on acid-GL at 20 °C and pH 7. The model of pseudo-second order provides a satisfactory fitting rate equation (R2 = 0.998) compared to other kinetics models, according to the kinetics analysis of the MB adsorption. Overall, the results suggest using grape leaves as an inexpensive, environmentally friendly adsorbent to remove basic (cationic) textile dye (MB) from water. Utilizing experimental data, RSM was able to accurately forecast the removal percentage under ideal circumstances.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Pollution has risen over the past few years as a result of numerous industrial activities. Numerous colored effluents are created and released in the textile industry, with or without adhering to the local pollution standards. Many small and medium-sized companies in Iraq and other Different dyes were employed in different countries, and when they are released into the atmosphere, it causes environmental issues. Unsuitable removal of dyes from wastewater results in the detection of toxic dyes, which can reduce the life of ecosystems [1]. Over 100,000 dyes are used globally, and 700,000 tons are manufactured each year. It was reported that 12 percent of the produced textile dyes are discarded in manufacturing and processing activities, and 10–15% of the dyes are lost during the process. Biodegradation is not possible in wastewater from the textile sector because of the wastewater’s stable and intricate chemical makeup. Assume a tiny amount of dye (less than 1 mg/L) is present in the water. If this dye is both visible and undesirable, it could interfere with sunlight transmission and disrupt the natural processes of metabolism in the water [2]. Because of their high toxicity, lack of biodegradability, and mutagenicity, some dyes are having an adverse direct or indirect effect on people, animals, and ecosystems. Acidic, cationic, direct, and dispersion dyes can cause tumors (both benign and malignant), while direct dyes can cause bladder cancer. Reactive dyes can cause asthma, skin inflammation, aggravation of the conjunctive tissue in the eye, nasal itching, and irritation of the nasal mucous membranes [3]. Methylene blue, a cationic water-soluble dye, is used in the manufacture of textiles, plastic, paper, medicines, and cosmetics [4].
Although it is non-biodegradable due to its complicated structure, it is poisonous and carcinogenic at a certain dosage and is difficult to degrade naturally.
MB must be removed from wastewater because of its negative effects on ecosystems and public health. However, different treatment methods have been applied, such as coagulation and flocculation, physical and biosorption, and electrochemical degradation [5,6,7,8]. The disadvantages of other conventional techniques, including photolysis and photocatalytic processes, include incomplete removal, significant sludge generation, and costly operation [9]. Because of its low cost, cozy design, adaptability, and ability to remove dye pollutants, adsorption is the most effective approach for getting rid of dyes out of these options [10]. In the modern era, many common, low-cost absorbents from agricultural wastes and byproducts have been used to remove color. These absorbents are incredibly affordable, environmentally benign, have excellent dye-adsorption capabilities, and are widely available. As a result, several researchers have tried to alter agricultural wastes using various treatments, including celery [11], Luffaa Egyptian peel [12], husks of rice [13], and fig leaves [14]. Utilizing agricultural waste or by-products in an environmentally sustainable way, whether they are in their natural state or have been altered, such as by the use of chemicals, acids, bases, or activated carbon. Due to their accessibility, cost, and efficiency in removing dyes, these materials can be employed instead of more expensive and traditional adsorbents. The inexpensive cost of regenerated organic material is one of these biosorbents’ key benefits [15]. The grape tree is frequently planted in the governorates of Dahuk, Erbil, and Sulaymaniyah, where the favorable soil and climate conditions allow it to flourish. The most extensively cultivated grape fruit in Iraq is a variety of the genus Vitus vinifera, also referred to as the European grape or the grape of the ancient world. For hundreds of thousands of families in various cities around Iraq, the production of grapes is a source of income and employment. Syrup, raisins, vinegar, fresh grape juice, and alcoholic beverages are all made from grapes.
The adsorption technique has many advantages over alternative methods, including an excellent potential for dye adsorption, high design and production flexibility, a simple design, and enjoyable performance [16]. The most commonly used method for removing dye from wastewater at the moment is adsorption with activated carbon [17]. Scientists have been attempting to create affordable adsorbents to remove various types of contaminants from water and wastewater over the past few years using agricultural waste. Using agricultural wastes as the primary raw material for the manufacturing of activated carbon has the benefits of being environmentally benign, renewable, and biodegradable [18]. According to certain studies, agricultural byproducts, including durian shells [19], powdered mango seed kernels [20], and grape stalks [21], can be used to create inexpensive adsorbents for dye removal from wastewater. The goal of the current study was to investigate the potential and use of grape leaves treated with phosphoric acid for the removal of MB from aqueous solutions. As we know, utilizing grape leaves for the removal of methylene blue dye is the first project to be applied in Iraq. However, application response surface methodology is used to explain the most significant factor in the present study.
The main goals of this study are: (i) to examine the effects of various parametric factors such as pH solution, amount of adsorbent, MB levels, volume solution, and temperature. (ii) to characterize the surface of the acid-GL before and after adsorption. (iii) to observe the most significant parameters through response surface methodology. (iv) to investigate the pathway of adsorption from Langmuir and Freundlich isotherm models.
Experimental
Materials used
The vineyards in the Iraqi city of Babylon provided the grape leaves needed to achieve the purpose of the present study. Grape leaves were washed in distilled water and allowed to air dry. The smashed, undersized particles from screening the dried grape leaves were used for the subsequent stages. Merck supplied the activating agent H3PO4, which was used. Adsorption studies use the basic (cationic) dye methylene blue (MB) (C16H18ClN3S) as the adsorbate model. The Chinese company Dyestuffs and Chemicals Co. sold the methylene blue dye. No additional procedures were necessary; just analytical-grade chemicals were used.
Preparation of adsorbents
Methylene blue (MB) removal from an aqueous solution using fig leaves has been described in an earlier study [14]. Following the identical procedures outlined in the prior paper, grape leaves are collected, prepared, and acid-modified. In this study, MB is taken out of synthetic solutions using acid-modified grape leaves (acid-GL).
The grape leaves were gathered when they were crisp or dry, washed vigorously with water four times to get rid of the dirt and other solids, and then dried. After being sieved through a 75-mesh filter, the grape leaves were ground in an electric grinder. The addition of 20 mL of deionized water to a beaker holding 6 g of powdered grape leaves took place. 5 mL of concentrated H3PO4 were added and vigorously mixed to ensure they thoroughly penetrated the acid. It was continuously cleaned with water while being left for 1 h. A pH meter was used to measure the pH after washing, yielding a result of 6.8. Following that, the sample was dried naturally for one night. The powder that was dry was then carbonized in a furnace for a 2 h period at 350 °C.
Preparing a stockpile solution of MB
The preparation of a 1000 mg/L methylene blue (MB) solution involves diffusing analytical-grade methylene blue (1000 mg of MB in powder form) in 1 L of double-distilled water. By diluting the stockpile combination with water (twice distilled), the various acceptable mixture strengths are arranged.
Examination of MB
A UV spectrophotometer measures the absorption of MB levels at 654 nm that are both known and unknown. The test solution levels and absorbency are then used to create a calibration curve.
Instruments and supplies
Methylene blue (99%), hydrochloric acid (38%), sodium hydroxide (96%), and phosphoric acid (85%) were provided from E. Merck Ltd. (Mumbai, India). The pH of the solutions is determined using a digital pH meter (Lovibond@Water Testing). A shaking device with temperature control was used to do the batch adsorption. Solution pH was controlled using diluted HCl and NaOH. Water that had been doubly distilled was used during the whole investigation, and each reagent was of analytical quality.
Batch mode experimental procedure
A 100 mL borosilicate conical flask filled with 25 mL of MB solution at different levels was used in the batch mode of the experiment. 0.1 mol/L HCl or 0.1 mol/L NaOH have been used to correct the pH of the starting solution. The 100 mL conical is then placed in the shaker at a rate of 200 rpm and 20 ± 2 °C, along with the necessary amount of adsorbents. At various times, the conicals are removed one by one, revealing that filtration has taken place. After that, a UV spectrophotometer is used to detect the absorbency of MB concentrations that are unknown, and the calibration curve is used to determine the concentration in “mg/L” of those unknown concentrations. Repeatedly using the experimental method in MB’s batch mode under varied operating conditions is required. Equations 1 and 2 are used to calculate the adsorption capacity (Qt) and removal % (R%).
Here V is the volume of the solution (L), m is the dosage of adsorbent (g), and C0 is the initial MB strength (mg/L). Ct is the residual concentration at time t or at saturation (mg/L). Qt is the adsorption capacity (mg/g) at various periods. R% is the portion of the MB dye solution that was removed upon adsorption.
Experimental design
RSM is a method for developing models based on evidence that is used to enhance removal % that are influenced by a variety of various input variables through mathematical and statistical approaches. RSM produces a regression model with the equation and the ideal circumstances by running the lowest amount of experiments necessary to conform to the experimental design [22]. A Box-Behnken Design software was used for the current project. This rotatable design is suitable for fitting a quadratic surface and normally performs well for process optimization [23]. To determine how operating variables affect dye removal (Y), we used five independent variables: MB concentration (A), carbon dose (B), solution volume (C), temperature (D), and shaking time (E). Box-Behnken design was used to execute 46 runs for each of the six variables.
Table 1 comprises low level (-1), central (0), and high level (+ 1) components, as well as the levels and range of variables that affect the removal efficiency of MB. Through the statistical analysis of the P-value and F-value of the regression coefficients (P 0.05), analysis of variance (ANOVA) was performed to investigate the influence of the input components on the results. Additionally, the coefficient of determination (R2) and adjusted coefficient of determination (R2adj.) were used to report on the validity of the model. In order to illustrate how separate elements interact with one another and how that interaction affects the response, a contour response plot has been devised.
Results and discussions
Determination of point of zero charge (pHPZC)
Point of zero charge (pHpzc) for GL was determined by adding sodium chloride (0.1 M, 50 mL) and, under certain conditions, adjusting the solution’s initial pH value (2–11). After that, 1 g/L of the dosage is added to each conical (0.25 L) and shakes for 24 h at irregular intervals. The meeting point of the plot of pH (pHf–pHi) vs. pHi and line of pH 0 (shown in Fig. S1) is used to determine the pHpzc point. The exterior of the adsorbent becomes negatively charged at pH > pHpzc, favoring the adsorption of basic dyes [24]. For MB adsorption, the point of zero charge for acid-GL is 6.3.
Characterization of acid-GL
Acid-GL’s external topography was created using an EVO 18 ZEISS SEM equipment at a 15 kV accelerating voltage and a 2KX magnification. The Acid-GL specimens were fixed with carbon tape, then covered in gold nanoparticles and examined in a hydrogen atmosphere. The exteriors of acid-GL, which participated in the adsorption of MB, have uneven and previous construction, as shown by the morphological representation of the adsorbents (Fig. 1, top). The structure of the carbonized grape leaves was more porous and heterogeneous as a result of ruptures caused by the phosphoric acid treatments. Surface ruptures occur after acid-GL activation treatment. When MB ions are adsorbed onto acid-GL, the adsorbent surface noticeably smoothens out after adsorption.
(top) SEM characterization for acid-GL before and after adsorption of methylene blue (12 mg/L MB, 60 min and 0.6 g adsorbent). (bottom) FTIR for the adsorbents of original-GL (pristine), acid-GL (carbonized), and acid-GL loaded with MB (loaded) under the following conditions (12 mg/L MB, 60 min and 0.6 g adsorbent)
Fourier-transform infrared spectroscopy
We produced infrared spectra of fresh and carbonized grape leaves in the 4000–500 cm−1 range using a Nicolet i35 (iD3 ATR) (FT-IR) from Thermo-Fisher Scientific. Fig. 1 (bottom) displays the outcome. In both samples, the most prevalent functional groups were present. According to recent studies [25,26,27,28], the primary functional groups O–H, C=O, and C–O appeared at around 3400–3500, 1560–1672, and 1300–1340 cm−1, for carbonized grape leaves. While the same primary peaks with heavily boarder peaks were visible at 3100–3600, 1500–1700, and 1300–1377 cm−1. Compared to the virgin sample, the peaks were sharper after carbonization. The C=O group’s peak strength was noticeably reduced after MB adsorption, and its wave number was slightly changed from 1748 to 1739 cm−1. This finding raises the possibility of n–π interactions, which explains why the dye’s aromatic ring functions as an acceptor and the oxygen in the carbonyl groups on the surface functions as a donor. Furthermore, FTIR analysis demonstrated that following MB adsorption, the peak associated with the vibration of aromatic C=C bonds slowed down and shifted (from 1537 cm−1 to 1561 cm−1). This shift suggests that MB and adsorbents interact negatively [25].
Surface area analysis
In a nitrogen atmosphere, the surface areas of acid-GL is measured using the Quantacrome NOVA 1000e Brunauer–Emmett–Teller (BET) instrument. The result, which is provided in Fig. S2, indicates that following activation change of the acid modified grape leaves produces a better surface area.
It was observed that grape leaves have some characteristics as presented in Table S1. The BET specific surface area was 6 0.5182 m2/g. Other porosity parameters such as total pore volume, and average particle size have been presented in Table S1. It was discovered that grape leaves has larger particle size of 920.502 nm compared to fig leaves 327.911 nm [14].
Parametric investigation of adsorption of MB dye
Impact of pH
The solution pH is the factor that considered one of the most important variables in adsorption procedures. Fig. S3a and Table 2 show the assessed outcomes for the adsorption of MB dye on acid-GL in the pH ranged between 2 and 11 using 60 mg/L MB and 0.06 g acid-GL. The removal % has been seen to climb when the medium’s initial pH rises for MB, peaking at pH 7, where it is most beneficial. Large H+ ions competing with MB cations for accessible spaces may be the cause of the decreased percentage of MB removal at a lower pH level. It was feasible to comprehend how the solution pH affected the disappearance of the MB. The net external charge of the surface is influenced by the pH of the surrounding environment. They can change their charge to be more negatively or positively charged by releasing or accepting H+ ions. When the initial pH is higher, the surface of acid-GL may develop a negative charge, increasing the fraction of positively charged MB that is absorbed via electrostatic attraction [29]. The majority of MB (91%) is destroyed in the pH 7 range. Thus, pH 7 was chosen for more testing.
Impact of acid-GL dose
The removal of MB using GL was studied at individually various adsorbent amounts (0.02–0.1 g), shaking velocities (200 rpm), pH (7), and times (60 min). Fig. S3b and Table 2 shows that increasing the dosage of GL results in a high proportion of MB adsorption. The doses of GL for MB that resulted in the highest percentage of dye removal were 0.1, 0.08 and 0.06 g. Initial increases in adsorbent quantity cause a rise in removal % because there are more adsorption sites available. It is well known that high amount of adsorbent resulted in high removal% but at the same time reduces the adsorption capacity. Moreover, increasing of adsorbent may affect the alkalinity of the solution then no more negative charges for methylene blue dye adsorption [30].
Influence of the initial concentration of methylene blue
Methylene blue concentration has been investigated in the range of 20–100 mg/L was examined using the adsorbent dose of 0.06 g, pH 7, and 60 min of contact period. The MB molecule is moved from a synthetic medium to an adsorbent exterior by the initial concentration of MB acting as a driving force. According to Fig. S3c and Table 2, the percentage of adsorption rises as the initial concentration of MB rises and becomes the maximum value at 20 ppm after it falls. It has been observed that the initial MB strength is crucial for the adsorption process as the concentration provides a moving force to overcome the obstacle to mass transfer that occurs between the adsorbent and adsorbate portions. The starting amount of MB overtakes the adsorption in high concentrations because there is a greater proportion of empty surfaces exposed to it. Whatever the case, the unoccupied sites are occupied at extremely high concentrations (100 mg/L), which causes a reduction in the adsorption of MB [31]. Additionally, it mentions that the clearance percentage increased as MB concentration decreased. The increased kinetic energy of the dye and the dye’s surface characteristics frequently enhance adsorption [31,32,33].
Influence of MB solution volume
When the methylene blue dye is adsorbing at different solutions volumes (25, 50, and 100 mL), acid-GL displays excellent removal efficiency with the exception of 100 mL. With a 25 mL volume, the highest removal rate was 77%. The removal effectiveness decreases as the MB dye solution volume rises. The highest adsorption capacity of 26.5 mg/g is attained at 50 mL of dye solution. These results are shown in Fig. S3d and Table 2.
Effect of temperature
Unquestionably, the temperature can affect the amount and removal rate of dye absorbed on the adsorbent. Three different temperatures were used to show the temperature effect: Depending on the disappearance percentage and adsorption capacity as shown in Fig. S3e and Table 2, 50 °C exhibited highest removal % and adsorption capacity of 86 and 21.5 mg/g. The motion of the molecules of the dye changed as the temperature rose, and more adsorption-active sites were also present [34]. Adsorption capacity increased continuously as the temperature rose from 20 to 50 °C. The adsorption capacity for MB dye reaches a minimum value of 17.8 mg/g at 20 °C and the highest value of 21.5 mg/g at 50 °C. According to the results, a rise in temperature increases the adsorption capacity because the adsorbent’s internal structure swells, allowing methylene blue to penetrate further [35].
Influence of time has been tested at all MB concentrations under these conditions: solution pH, 0.06 g acid-GL, the removal percentage and adsorption capacity of MB adsorption were determined in Fig. 2. On the adsorbate-adsorbent exposure, saturation was achieved in 60 min. The initial phase of the exposure to the adsorbate-adsorbent involved a rapid intake of dye for the first 20 min. After then, a protracted MB removal period lasted for a long period of time (> 30 min) before saturation occurred. The high concentration of MB dye exhibited a low removal percentage but high adsorption capacity compared to other concentrations. Even with the prolonged adsorption phenomena, 20 mg/L MB exhibited highest removal % and minimum adsorption capacity. The small size saturation when high concentrations are used may be due to the large number of MB molecules that attach to the surface. In contrast, the highest removal percentages were 92%, 77%, and 61% for 20, 60, and 100 mg/L MB. 25.4 mg/g is the highest adsorption capacity when 100 mg L MB used, while 19.25 and 7 mg/g were the adsorption capacities for 60 and 20 mg/L MB.
Process analysis and modeling of methylene blue adsorption
Forty six (46) trials produced by the Box-Behnken Design program were used to investigate the dynamic impacts of the starting MB concentration (A), acid-GL dose (B), solution volume (C), temperature (D), and contact length (E) on the MB removal %. Table 3 provides an overview of the analysis of variance (ANOVA) findings. Initial concentration, adsorbent dosage, solution volume, and contact duration all showed favourable effects on the removal efficiency of MB based on the evaluation of F and P values of the variables evaluated in this study. The likelihood is less than 0.05, which indicates that these variables have a substantial impact on MB removal. The amount of adsorbent used has the biggest impact on dye removal. The MB removal’s adjusted R2 and correlation coefficients (R2) are 0.9532 and 0.9740, respectively, indicating a strong connection between the experiment data and predictions. The Model F-value (46.87), on the other hand, shows that the model was important for MB removal. When a P-value is less than 0.05, it is assumed that the model’s terms are significant; when a P-value is greater than 0.05, it is assumed that the modelling terms are not significant. 9.49 is not statistically significant and supports the model’s suitability. This study’s model, which was obtained, might be used to explore the design space (Eq. 3).
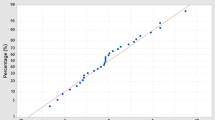
The plot of the normal probability of the residual for MB removal is shown in Fig. 3a. According to the pattern, the MB dye residue acts independently, is evenly distributed, and resembles a straight line.
The plot of the residual vs. predicted response is also displayed in Fig. 3b. Plotting the residual value versus the rising expected response value allows us to test the null hypothesis of constant variance. The residuals’ range is fixed across the entire graph, while the plot’s points are randomly distributed. The residuals of the plot depart from the middle line at random.
Fig. 4a shows the normal probability plot of the standardized effects with p 0.05 to evaluate the significance of each factor and its interactions on removal efficiency (%).It is feasible to divide a normal probability plot into its right and left half. The right sector (from B to BC) has positive coefficients, while the left region (from E to BE) contains negative coefficients. The blue balls’ parameters are not significant. The components represented by squares are thought to be significant. The Pareto chart of the standardized effects in Fig. 4b demonstrated the relative significance of the individual and interaction effects.
a Normal plot and b Pareto plot for examination of methylene blue adsorption using acid-GL under ideal circumstances at probability of 0.05 (five factors, total run 46 and one replicate). The symbols are meaning: MB concentration (A), carbon dose (B), solution volume (C), temperature (D), and shaking time (E)
The Student’s t-test was used to determine whether the estimated effects were statistically different from zero. The Pareto chart’s horizontal columns displayed the values for each effect. At a 95% confidence level, the straight line in the graph reflects the least statistically meaningful influence magnitude (2.06). For any values more than 2.06 (p 0.05) that are all taken to be significant, the right side of the solid line is broken. As the volume of the solution increased, the quantity of MB that was present in the solution was ready for adsorption on acid-GL. Every change in the dose of the adsorbent (B) led to an increase in the percent removal of MB. As a result, these variables have a big impact on the amount of MB removed. Because the interplay of two parameters BE generates a positive signal on the removal efficiency of MB, a raise in adsorbent and a decrease in volume together resulted in an increase in adsorption efficiency (%). The univariate optimization of the MB adsorption technique would be unable to distinguish whether this antagonistic influence or others.
Interaction effects on responses and process optimization
Contour plots for the ideal AB, AC, and BC interaction terms are presented in Fig. S4 with the other variables’ center levels remaining constant.
The contour plots in Fig. S4a show the MB removal efficiency at 25 mL of solution, 20 °C, and 60 min. The removal of MB molecules from a particular solution is quite effective at initial MB concentration. Contrarily, the amount of carbon determines how long the MB saturation remains on the carbon’s surface. As a result, the model is affected by the interaction effect. With an increase in carbon content, the efficiency of MB removal rose directly, reaching up to > 95%. The efficacy of adsorption normally increases with increasing the amount of cardon because more active sites are ready to bring MB molecules onto the surface. Low MB removal at low doses can be attributed to the quantity of MB molecules, which quickly saturate the active sites with enough MB. The MB removal effectiveness at 0.06 g acid-GL, 20 °C, and 60 min of agitation time is shown in the contour plot of Fig. S4b. The efficiency of MB removal was improved to > 99% when a decrease in solution volume was combined with a decrease in the initial concentration of MB. It was observed that at high concentrations of MB and large solution volumes, the adsorption of MB is too low. The reason may be attributed to the fact that a high concentration means a lot of MB molecules are free in the solution. Although large volumes of the activated carbon are distributed in the solution, there is not enough chance for it to adsorb the free methylene blue molecules. The effectiveness of MB removal at 60 mg/L dye concentration, 20 °C, and agitation time of 60 min. The combined impact of initial volume solution and acid-GL amount on the effectiveness of MB removal (response) was presented in the contour plot of Fig. S4c. Combining the two effects-reduction in solution volume and increase in carbon amount-resulted in an increase in MB removal, reaching more than 80%.
A supplementary statistical design calculation was carried out within the same conditions of experimentation to optimize and validate the projected mathematical model, and Table 4 demonstrates that the higher desirability value is identical to 0.9939 derived from the mathematical model’s results. The greatest Yield (%) (removal (%) = 98%, experimental) obtained under these circumstances corresponds to the initial concentration of 30.4 mg/L, carbon mass of 0.09 g, volume solution of 32.88 mL, temperature of 20 °C, and reaction duration of 42.97 min.
Kinetics modeling of MB adsorption process
Table 5 and Fig. 5 provided information on the determination of kinetic parameters and various kinetic model types. It was suggested by Lagergren [36] that the pseudo-first order equation, which is controlled by diffusion and is denoted by (Eq. 4)
Non-linear least squares fittings kinetics for adsorption of methylene blue dye on acid-GL adsorbent (square red color): a pseudo-first order (Qt vs t) (top) and pseudo-second order (Qt vs t) (bottom) as a function of time; b Intraparticle diffusion kinetic plot (Qt vs t0.5) as a function of square root time under the following conditions (0.06 g adsorbent, pH 7, 25 mL and 60 mg/L MB). The dashed black lines are the tangents to the plots at time = 0
Here k1 (min−1) is the adsorption rate constant and Qt and Qe (mg/g) are the capacities of adsorption at any given time t (min) and when saturated.
According to Ho and Mckay [37], chemisorptions take place on the solid’s exterior and can be expressed in terms of science by (Eq. 5),
Here k2 (g/mg.min) is the rate constant. A high correlation coefficient (> 0.99) from the pseudo-second order model indicates that the three adsorbents’ process is chemical.
Moris and Weber established the concept of intraparticle diffusion (IPD) [38], and it was acknowledged by (Eq. 6),
Here Kid (mg/g min0.5) is the rate constant and Cid (mg/g) is the IPD model constant. The greater significance of the boundary layer effect is indicated by larger Cid values. When the straight line passes through the origin, IPD will take control of the adsorption process. Sometimes, the plot indicates a number of linear portions rather than the origin, and these sections correspond to different mechanisms that predominate in adsorption. From the Qt vs. t0.5 plot, the correlation coefficients and constants are calculated and provided in Table 5.
The statistical characteristics (R2) for all kinetic models, as shown in Table 5, support the applicability of the pseudo-second order kinetic model for all three adsorbents.
Modelling of The MB adsorption process
To evaluate the various known MB (10–150 mg/L) within diverse isothermal effects, multiple isotherms have been developed. For this study, the best adsorbent dosages previously obtained were used. Experimental data and various isotherms models, including Langmuir, and Freundlich, were correlated. On the premise that the monolayer occurs with homogenous pores, Langmuir (1918) [39] was founded. It alludes to the energy of the stationary process, where there is no MB molecule emigration in the surface plane. The formula for the Langmuir isotherm is as follows (Eq. 7):
Here Qmax and KL are the model constants and Ce is the saturated medium strength (in mg/L). From the plot of the non-linear least squares fittings (Qe vs. Ce), the model constants for the adsorbent were calculated and provided in Table 6 and Fig. 6. The highest adsorption capacity was 28.85 mg/g and the Langmuir affinity constant (KL) was 0.157 L/mg. This model accurately depicts the methylene blue adsorption by acid-GL.
By successfully completing the adsorption centers of an adsorbent, the Freundlich equation shows the relationship between intake molecules and process energy in heterogeneous systems. The isotherm model (Eq. 8) looks like this:
Here the model’s constant parameters are Kf and n (Fig. 6). The capacity of adsorption and the system’s adsorbent intensity are related to the variables Kf and n [40]. The adsorbent’s Kf and n was assessed so they were 0.275 and 8.447, respectively. The values for all the model parameters from both isotherm models used for the MB ions adsorption on activated GL are listed in Table 6. The Langmuir isotherm of adsorption, which had the highest R2 values, was preferred over the Freundlich model, according to the pertinent results.
However, the present study has been compared with some other publications on different plant leaves for the adsorption of methylene blue, as reported in Table 7.
In our recent study on fig leaf, methylene blue was used as model for the adsorption process. Fig leaves were treated with phosphoric acid (20% V/V), in which 3 g of carbon were immersed in 12.5 mL of H3PO4 for two nights. Carbonization occurred at 350 °C for 2 h. The maximum capacity was 41 mg/g [14]. Pineapple leaves were soaked in a ZnCl2 aqueous solution to activate their surface for one day, then carbonized at 500 °C for 60 min. The maximum capacity was 288.34 mg MB dye per gram of activated carbon.
Grape leaves have been utilized previously to adsorb methylene blue dye. However, leaves have been directly carbonized without any reagent treatment. The carbonization spent 2 h at 500 °C [41]. The amount of activated carbon was 25 g per 2.0 L solution of dye in alkaline medium (pH 11). The maximum adsorption capacity was 0.2 mg per litter solution.
The waste leaves of Ensete ventricosum were selected to adsorb methylene blue dye from its aqueous solution. It was only dried and then sieved to 300 mesh size. However, large amount of adsorbent (50 g) was added to 250 mL of 0.1 mol/L HCl, the maximum adsorption was 35.5 mg/g [42].
Martín-González et al. created activated carbon by soaking dried banana leaves in H3PO4 then burned at different temperatures 450 °C, 550 °C and 600 °C. After that the activated carbon was applied to eliminate methylene blue dye achieving maximum adsorption capacity of 48 mg/g [43].
Thermodynamic study of MB adsorption
At different temperatures (298, 308, 318, and 323 K), the thermodynamic studies of MB adsorption were calculated for MB at fixed concentration of 60 mg/L. Equation 9 was used for each system to calculate the Kd (equilibrium constant) at various temperatures [24].
Here Ce is the dye’s equilibrium solution concentration (in mg/L), and Cs is its equilibrium solid phase concentration (in mg/L).
These formulas (Eq. 10) determine the Gibbs free energy (∆G°), enthalpy (∆H°), and entropy (∆S°).
According to Eq. 9, which suggests that adsorption nature is exergonic and that the spontaneous ness rises with rising temperature, the (−ve) value (∆G°) is determined from this equation.
However, (∆H°), and (∆S°) for acid-GL surface are provided by the plot of lnKd vs. 1/T as shown in Eq. 11.
ΔH° and ΔS° are positive, which caused the process to absorb more heat and become more disorganized.
Table 8 presents all of the data.
The values of the thermodynamic parameter are shown in Table 4. The adsorption process was shown to be viable and spontaneous by the negative values of ΔG°. The results of ΔG° decreased with increasing temperature, suggesting that sorption became more spontaneous at lower temperatures. A positive value of ΔS° described the increase in randomness at the adsorbent-solution interface during the adsorption process. A positive measurement for ΔH° confirms the general endothermic characteristic of the MB adsorption on activated carbon acid-GL.
Mechanism of adsorption
A sort of agricultural waste with a lot of organic substance is grape leaves. Grape leaves possess many active functional groups that have important effects on the eradication of MB. Investigations turned up two crucial factors that affect the adsorption process. The first is the MB’s structure, and the second is that the functional groups are found outside the leaves. The FT-IR detection revealed the presence of various functional groups (C=O, C=C, C–O, and the hydroxyl) on the adsorbents. The consumption of (+ ve) charged MB molecules may be caused by these functional groups. According to Tran et al., the (+ ve) molecules of MB and the (-ve) exterior of the leaves may interact by electrostatic interaction, Pi–Pi interaction, H-bonding, and n-Pi interaction [45]
Conclusion
The effectiveness of activated carbon acid-GL was examined using the chemical activation method. However, the ideal operating conditions, including adsorbent dosage, pH, contact time, and beginning dye concentration, were identified using the RSM method.
A SEM and FTIR spectrum characterization has been achieved on activated carbon acid-GL. From SEM, it was observed that many active sites were created to get ready for the adsorption of methylene blue dye. Furthermore, some functional groups, such as O–H, C=O, and C–H, were pointed using the FTIR spectrum.
The outcomes demonstrated the potential and effectiveness of activated carbon generated from discarded grape leaves as an adsorbent for extracting MB from an aqueous solution. The best results were obtained at 60 mg/L of MB, 30 °C of temperature, 25 mL of sample volume, 0.1 g of acid-GL, and 40 min of shaking duration, according to various replies. 92% is the greatest adsorption removal. A pseudo-second order kinetic model is also supported by the kinetic data that was acquired. The Langmuir model has explained the MB dye’s adsorption on acid-GL as an adsorbent. The adsorption process is endothermic as the enthalpy has a positive value. However, the negative value for Gibbs energy refers to the spontaneous nature of the process.
As a result, the produced adsorbent has a good capacity for adsorption and could be used to remove MB dye in a natural and economical manner, so recycling the activated carbon after adsorption could be recommended. It could also be considered for future work in terms of transferring the lab work into a large-scale pilot.
Data availability
The dataset utilized/analyzed during the current study will be available from the corresponding author upon request.
References
Boudechiche N, Fares M, Ouyahia S, Yazid H, Trari MSZ (2019) Comparative study on removal of two basic dyes in aqueous medium by adsorption using activated carbon from Ziziphus lotus stones. Microchem J 146:1010–1018
Draper B, Yee WL, Pedrana A et al (2022) Reducing liver disease-related deaths in the Asia-Pacific: the important role of decentralised and non-specialist led hepatitis C treatment for cirrhotic patients. Lancet Reg Heal Pacific. https://doi.org/10.1016/j.lanwpc.2021.100359
Zhou Y, Lu J, Zhou Y, Liu Y (2019) Recent advances for dyes removal using novel adsorbents: a review. Environ Pollut 252:352–365
Stewart GG (2016) Saccharomyces species in the production of beer. Beverages 2:34
Moghaddam SS, Moghaddam MRA, Arami M (2010) Coagulation/flocculation process for dye removal using sludge from water treatment plant: optimization through response surface methodology. J Hazard Mater 175:651–657
Chaari I, Fakhfakh E, Medhioub M, Jamoussi F (2019) Comparative study on adsorption of cationic and anionic dyes by smectite rich natural clays. J Mol Struct 1179:672–677
Bazzo A, Adebayo MA, Dias SLP et al (2016) Avocado seed powder: characterization and its application for crystal violet dye removal from aqueous solutions. Desalin water Treat 57:15873–15888
Shetti NP, Malode SJ, Malladi RS et al (2019) Electrochemical detection and degradation of textile dye congo red at graphene oxide modified electrode. Microchem J 146:387–392
Kausar A, Iqbal M, Javed A et al (2018) Dyes adsorption using clay and modified clay: a review. J Mol Liq 256:395–407
Murthy TPK, Gowrishankar BS, Prabha MNC et al (2019) Studies on batch adsorptive removal of malachite green from synthetic wastewater using acid treated coffee husk: equilibrium, kinetics and thermodynamic studies. Microchem J 146:192–201
Mohebali S, Bastani D, Shayesteh H (2019) Equilibrium, kinetic and thermodynamic studies of a low-cost biosorbent for the removal of Congo red dye: Acid and CTAB-acid modified celery (Apium graveolens). J Mol Struct 1176:181–193
Mashkoor F, Nasar A (2019) Preparation, characterization and adsorption studies of the chemically modified Luffa aegyptica peel as a potential adsorbent for the removal of malachite green from aqueous solution. J Mol Liq 274:315–327
Chakraborty S, Chowdhury S, Das SP (2011) Adsorption of crystal violet from aqueous solution onto NaOH-modified rice husk. Carbohydr Polym 86:1533–1541
Al-Asadi ST, Al-Qaim FF, Al-Saedi HFS et al (2023) Adsorption of methylene blue dye from aqueous solution using low-cost adsorbent: kinetic, isotherm adsorption, and thermodynamic studies. Environ Monit Assess 195:676
Bailey SE, Olin TJ, Bricka RM, Adrian DD (1999) A review of potentially low-cost sorbents for heavy metals. Water Res 33:2469–2479
Seidmohammadi A, Asgari G, Leili M et al (2015) Effectiveness of quercus branti activated carbon in removal of methylene blue from aqueous solutions. Arch Hyg Sci 4:217–225
Robinson T, Chandran B, Nigam P (2002) Effect of pretreatments of three waste residues, wheat straw, corncobs and barley husks on dye adsorption. Bioresour Technol 85:119–124
Hameed KS, Muthirulan P, Sundaram MM (2017) Adsorption of chromotrope dye onto activated carbons obtained from the seeds of various plants: equilibrium and kinetics studies. Arab J Chem 10:S2225–S2233
Hameed BH, Daud FBM (2008) Adsorption studies of basic dye on activated carbon derived from agricultural waste: Hevea brasiliensis seed coat. Chem Eng J 139:48–55
Kumar KV, Kumaran A (2005) Removal of methylene blue by mango seed kernel powder. Biochem Eng J 27:83–93
Ozdemir I, Şahin M, Orhan R, Erdem M (2014) Preparation and characterization of activated carbon from grape stalk by zinc chloride activation. Fuel Process Technol 125:200–206
Al-Qaim FF, Mussa ZH, Yuzir A et al (2018) Full factorial experimental design for carbamazepine removal using electrochemical process: a case study of scheming the pathway degradation. J Braz Chem Soc. https://doi.org/10.21577/0103-5053.20180047
Jawad AH, Alkarkhi AFM, Mubarak NSA (2015) Photocatalytic decolorization of methylene blue by an immobilized TiO2 film under visible light irradiation: optimization using response surface methodology (RSM). Desalin Water Treat 56:161–172
Prola LDT, Acayanka E, Lima EC et al (2013) Comparison of Jatropha curcas shells in natural form and treated by non-thermal plasma as biosorbents for removal of reactive red 120 textile dye from aqueous solution. Ind Crops Prod 46:328–340
Tran HN, You S-J, Nguyen TV, Chao H-P (2017) Insight into the adsorption mechanism of cationic dye onto biosorbents derived from agricultural wastes. Chem Eng Commun 204:1020–1036
Guo D, Li Y, Cui B et al (2020) Natural adsorption of methylene blue by waste fallen leaves of Magnoliaceae and its repeated thermal regeneration for reuse. J Clean Prod 267:121903
Jawad AH, Ramlah AR, Khudzir I, Sabar S (2017) High surface area mesoporous activated carbon developed from coconut leaf by chemical activation with H3PO4 for adsorption of methylene blue. Desalin Water Treat 74:326–335
Kushwaha AK, Gupta N, Chattopadhyaya MC (2014) Removal of cationic methylene blue and malachite green dyes from aqueous solution by waste materials of Daucus carota. J Saudi Chem Soc 18:200–207
De Marco C, Mauler RS, Daitx TS et al (2020) Removal of malachite green dye from aqueous solutions by a magnetic adsorbent. Sep Sci Technol 55:1089–1101
Izan NR, Zainol MM, Nordin AH et al (2022) Removal of methylene blue via adsorption using magnetic char derived from food waste. Malaysian J Chem 24:283–292
Shoukat S, Bhatti HN, Iqbal M, Noreen S (2017) Mango stone biocomposite preparation and application for crystal violet adsorption: a mechanistic study. Microporous Mesoporous Mater 239:180–189
Konicki W, Cendrowski K, Bazarko G, Mijowska E (2015) Study on efficient removal of anionic, cationic and nonionic dyes from aqueous solutions by means of mesoporous carbon nanospheres with empty cavity. Chem Eng Res Des 94:242–253
Ahmad R, Kumar R (2010) Adsorption studies of hazardous malachite green onto treated ginger waste. J Environ Manage 91:1032–1038
Bharathi KS, Ramesh ST (2013) Removal of dyes using agricultural waste as low-cost adsorbents: a review. Appl Water Sci 3:773–790
Hu X-S, Liang R, Sun G (2018) Super-adsorbent hydrogel for removal of methylene blue dye from aqueous solution. J Mater Chem A 6:17612–17624
Ho Y-S, McKay G (1998) Sorption of dye from aqueous solution by peat. Chem Eng J 70:115–124
Ho Y-S, McKay G (1999) Pseudo-second order model for sorption processes. Process Biochem 34:451–465
Weber WJ Jr, Morris JC (1963) Kinetics of adsorption on carbon from solution. J Sanit Eng Div 89:31–59
Langmuir I (1918) The adsorption of gases on plane surfaces of glass, mica and platinum. J Am Chem Soc 40:1361–1403
Khaled A, El Nemr A, El-Sikaily A, Abdelwahab O (2009) Treatment of artificial textile dye effluent containing direct yellow 12 by orange peel carbon. Desalination 238:210–232
Mousavi SA, Mahmoudi A, Amiri S et al (2022) Methylene blue removal using grape leaves waste: optimization and modeling. Appl Water Sci 12:1–11
Mekuria D, Diro A, Melak F, Asere TG (2022) Adsorptive removal of methylene blue dye using biowaste materials: barley Bran and Enset midrib leaf. J Chem. https://doi.org/10.1155/2022/4849758
Martín-González MA, Susial P, Pérez-Peña J, Doña-Rodríguez JM (2013) Preparation of activated carbons from banana leaves by chemical activation with phosphoric acid. Adsorption of methylene blue. Rev Mex Ing química 12:595–608
Mahamad MN, Zaini MAA, Zakaria ZA (2015) Preparation and characterization of activated carbon from pineapple waste biomass for dye removal. Int Biodeterior Biodegrad 102:274–280
Tran HN, You S-J, Chao H-P (2017) Fast and efficient adsorption of methylene green 5 on activated carbon prepared from new chemical activation method. J Environ Manage 188:322–336
Acknowledgements
Many thanks to university of Babylon for the support.
Funding
No funding was obtained for this study.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interest
The authors declare that they have no conflict of interest.
Ethical approval
All authors have read, understood, and have complied as applicable with the statement on “Ethical responsibilities of Authors” as found in the Instructions for Authors.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Al-Qaim, F.F., Al-Saedi, H.F.S., Mussa, Z.H. et al. Application of the response surface approach to the adsorption of methylene blue from water using acid-modified grape leaves. Reac Kinet Mech Cat 137, 399–422 (2024). https://doi.org/10.1007/s11144-023-02542-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11144-023-02542-5