Abstract
Fig leaf, an environmentally friendly byproduct of fruit plants, has been used for the first time to treat of methylene blue dye. The fig leaf-activated carbon (FLAC-3) was prepared successfully and used for the adsorption of methylene blue dye (MB). The adsorbent was characterized by Fourier transform infrared spectroscopy (FTIR), X-ray diffraction (XRD), scanning electron microscopy (SEM), and the Brunauer–Emmett–Teller (BET). In the present study, initial concentrations, contact time, temperatures, pH solution, FLAC-3 dose, volume solution, and activation agent were investigated. However, the initial concentration of MB was investigated at different concentrations of 20, 40, 80, 120, and 200 mg/L. pH solution was examined at these values: pH3, pH7, pH8, and pH11. Moreover, adsorption temperatures of 20, 30, 40, and 50 °C were considered to investigate how the FLAC-3 works on MB dye removal. The adsorption capacity of FLAC-3 was determined to be 24.75 mg/g for 0.08 g and 41 mg/g for 0.02 g. The adsorption process has followed the Langmuir isotherm model (R2 = 0.9841), where the adsorption created a monolayer covering the surface of the adsorbent. Additionally, it was discovered that the maximum adsorption capacity (Qm) was 41.7 mg/g and the Langmuir affinity constant (KL) was 0.37 L/mg. The FLAC-3, as low-cost adsorbents for methylene blue dye, has shown good cationic dye adsorption performance.
Graphical Abstract

Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Water is the most essential element for life on earth and has a significant impact on the production of food and energy, industrial output, and the quality of our environment (A. Sharma et al., 2022). Waterborne illnesses cause a considerable economic burden since they not only reduce worker productivity but also drive-up national healthcare expenses (Shannon et al., 2008; A. Sharma et al., 2022). The production of textiles, plastic, paper, pharmaceuticals, and cosmetics all use methylene blue (Fig. 1), a cationic water-soluble dye (Stewart, 2016).
However, due to its complex structure, natural degradation is challenging (it is non-biodegradable), and above a certain concentration, it is toxic and carcinogenic. The removal of methylene blue from wastewater necessitates the employment of effective, environmentally friendly technology due to its presence in effluents (very apparent at modest levels of 1 ppm), resulting from dying procedures or the production of pharmaceuticals or cosmetics (Omer et al., 2018; Patra et al., 2021a, 2021b, 2021c). The direct or indirect release of several sophisticated and powerful contaminants into water bodies results from domestic and industrial operations. One of these pollutants that is widely used in the textile, fabric, pharmaceutical, plastics, pulp and paper, printing, food, and printing industries is dye. The poisonous substances, acids, organics, bases, and other contaminants present in the discharged dyes have a negative impact on the natural habitats and metabolism of aquatic and soil life (Patra et al., 2021a, 2021b, 2021c; Patra et al., 2021a). Biological methods, such as anaerobic and aerobic digestion, physical methods, such as sedimentation, coagulation, filtration, ion exchange, adsorption, and reverse osmosis, and chemical methods, such as oxidation, ozonation, photochemical, and electrochemical degradation, are all used in conventional wastewater treatment to remove dyes (Patra et al., 2021b). Consequently, it is important to safeguard the current water resources. Insoluble chemical compounds, heavy metals, pharmaceutical micropollutants, and dyes are just a few of the contaminants that can be removed from water thanks to ongoing research efforts that have led to certain modified technologies and effective adsorbents (Huang & Shi, 2014; Khomri et al., 2022). The most effective method among them is adsorption because of its high efficiency and accessibility (Mangla et al., 2022). The collection of solid wastes is addressed and prevented from contaminating the air and water during the natural decomposition process by converting biomass into beneficial activated carbon that is an environmentally friendly adsorbent (El Messaoudi et al., 2021; Singh et al., 2021). Additionally, the biomass adsorbents exhibit exceptional high surface area, substantially greater effectiveness, and a faster adsorption rate in water treatment when compared to traditional materials (L. Liu et al., 2019; Singh et al., 2021). The removal of azo dyes and other harmful compounds from wastewater can be done with biochar nanoparticles since they are affordable, effective, and environmentally acceptable (Zhu et al., 2021). There are two different types of modification processes for biomass carbon materials now available: direct carbonization and chemical activation. Under the protection of an inert gas, high-temperature carbonization of biomass carbon materials is carried out in order to break down large molecules in organic matter into smaller ones, such as carbon, water vapor, and carbon dioxide (Wu et al., 2020; Yağmur & Kaya, 2021). Low specific surface area and ineffective adsorption effectiveness are drawbacks, though. When using the chemical activation method, the carbonization and activation processes can be completed in one step activation method, and the carbonization and activation processes can be completed in one step. The use of activators such as NaOH, KOH, K2CO3, H3PO4, H2SO4, and ZnCl2 allows for the synthesis of carbon compounds with a high specific surface area and large empty volume (El Messaoudi et al., 2022; Jawad et al., 2016; Kılıç et al., 2012; Rashid et al., 2016; Wu et al., 2020; Yağmur & Kaya, 2021). Adsorption techniques are advantageous since they are economical and environmentally friendly (Gutub et al., 2013). Rice husk was altered by Liu et al. (Z. Liu et al., 2020) by chemical activation with KOH at 400 °C for 30 min and 850 °C for 1 h. Due to its favorable effects on the environment and resource efficiency, modified rice husk was used to remediate wastewater that included mercury ions. Using date palm bark as the raw material, Haghbin et al. (Haghbin & Shahrak, 2021) created a highly porous activated carbon using a thermal decomposition process at 400 °C for 3 h followed by chemical activation with H3PO4. The materials as-prepared shown high porosity and efficient adsorption of diverse contaminants including heavy metals, dyes, and quercetin due to their large surface area and acidic functional groups on their surface (Haghbin & Shahrak, 2021). Sunflower seed hulls, walnut shells, coconut shells, and corncob waste plant biomass might all be used using this straightforward process. In order to create extremely porous carbon material for the removal of phenolic compounds from aqueous medium, Prashanthakumar et al. (2018) pyrolyzed coconut spathe at 800 °C for 90 min while using KOH as an activating agent in a nitrogen atmosphere. By hydrothermally carbonizing coconut shell at 200 °C for 2 h and impregnating it in NaOH for 4 h, the mesoporous coconut shell-activated carbon was created (Islam et al., 2017). The activated hydrochar was then heated for 1 h at 600 °C in a N2 environment, acting as a more effective adsorbent to remove cationic dyes. KOH has been extensively utilized in comparison to other chemical activating agents to produce activated carbon that has a greater specific surface area (up to 3000 m2/g) and is effective at removing both organic and inorganic pollutants from wastewater (Choma et al., 2015; Li et al., 2017). Fallen leaves are a fantastic and promising option for making activated carbon. They are regarded as inexpensive adsorbents since they are abundant in nature, affordable, need little preparation, and serve as working materials as well (Choudhry et al., 2021). Plant leaves are a promising choice for producing activated carbon because of their high carbon contents, which will have a significant ecological impact (Choudhry et al., 2021;A. Sharma et al., 2023). Chemical or gas activation can be used to create activated carbon from materials that contain carbon. They are the most widely utilized adsorbents because of their enormous surface area and exceptional adsorption capacity (Jawad et al., 2016).
There has not been any research on the use of fig leaves for the adsorption of the dye methylene blue, despite the fact that many papers on the preparation of activated carbon from various less expensive and substitute agricultural wastes and byproducts with chemical activation have been published recently. Regarding the chemical synthesis of activated carbon from fig leaves with H3PO4 or any other chemical agent, no investigations have been recorded previously. As a result, the goal of this research is to identify the ideal circumstances for producing activated carbon from fig leaf using H3PO4 activation. Investigations have been done into the effects of the activation temperature, pH, carbonization agents, and other variables. To characterize the activated carbon before and after adsorption, Fourier transform infrared spectroscopy (FTIR), X-ray diffraction (XRD), a scanning electron microscope (SEM), and the Brunauer–Emmett–Teller (BET) instruments were used. Moreover, the obtained activated carbon was compared with other fruit leaf activated carbon.
Materials and methods
Materials collection and pretreatment
The fig leaves used in the current project were collected from waste at an Iraqi fig farm in Babylon, Iraq. After being cleaned with distilled water, fig leaves were allowed to air dry. The crushed, undersized particles from the sieving of the dried fig leaves through a 75-mesh screen were used for the subsequent stages. H3PO4, H2SO4, and NaOH as activation agents were provided by Merck. Methylene blue (MB) (C16H18ClN3S), a basic (cationic) dye, is the adsorbate model utilized in adsorption studies. Methylene blue dye was purchased from Dyestuffs and Chemicals Co. (China). Analytical grade chemicals were utilized exclusively and no further action was required.
Activated carbon preparation
The fig leaves were collected as crispy (i.e., dry), washed strongly with water (3–5 times) to remove the dust and other solid matters, and then dried. Afterwards, the fig leaves were crushed using an electric grinder and sieved for 75 mesh. A 10 mL deionized water was added in beaker contained 3 g fig leaves powder. And then, 2.5 mL of concentrated H3PO4, H2SO4, and NaOH was added separately and stirred continuously until all the powder was well immersed. It was left for 2 h and then washed with water several time (> 10 times). After washing, the pH was measured using a pH meter (Lovibond@Water Testing) to record a pH value of 6.6. After that, sample was dried in normal conditions for 1–2 nights. Dried powder was then carbonized in a furnace at 350 °C for 2 h. The full experimental methodology has been presented in Fig. 2.
Fig leaves activated carbon (FLAC-3) surface characterization
Fourier transform infrared spectroscopy (Perkin Elmer Spectrum) was used to determine the presence of surface functional groups and chemical interactions for the treated fig leaf-activated carbon using potassium bromide pellets in the range of 400–4000 cm−1. The crystalline and non-crystalline powders have been tested using X-ray powder diffraction (Bruker D2 Phaser). The micrographs of the adsorbent were investigated before and after adsorption using a field emission scanning electron microscope. The specific surface area of FLAC-3 adsorbent has been calculated by Brunauer–Emmett–Teller.
Adsorption experiments
A total of 25 mL of different concentrations of methylene blue dye solution (20, 40, 80, 120, and 200 mg/L) were mixed with 0.08 g of adsorbents and subjected to the shaker for 60 min at room temperature. The samples were centrifuged at 3000 rpm after adsorption, and the filtrates were examined. Using a UV–vis spectrophotometer (UH4150, Hitachi) and the calibration curve, the residual MB dye concentrations in the solution were measured at their highest absorbance wavelengths (665 nm). The following Eqs. (1, 2) are used to calculate the removal% and adsorption capacity of MB dye:
where the initial dye concentration (C0) (measured in mg/L for MB) is given, the residual concentration (Ct) was measured for the MB dye in mg/L, is given.
R% is the percentage of MB dye solution removed after adsorption; Q is the adsorption capacity (mg/g) at various times; and m is the amount of FLAC-3 (g).
In order to create the necessary dye solutions, stock MB solution (1000 mg/L) was dissolved in double distilled water. Adsorbent was added to 25 mL of a wide spectrum of MB concentrations (20, 40, 80, 120, and 200 mg/L) together with 0.08 g to test the effects of contact time and the initial concentration of dye on adsorption removal and adsorption capacity. At a pH of 3, 7, 8, and 11, with a 80 mg/L concentration of MB dye and 0.08 g of FLAC-3, the impact of the pH was tested. Temperature has been investigated on adsorption of methylene blue dye in four different temperature values which are 20, 30, 40, and 50 °C at 60 min. The concentration of MB dye was 80 mg/L, and the adsorbent was 0.08 g. Different amounts of FLAC-3 (0.02, 0.04, 0.06, 0.08, and 0.1 g) have been investigated on 80 mg/L of MB dye at 60 min. The effect of volume solution has been investigated at different values of 25, 50, and 100 mL at fixed conditions 80 mg/L MB and 0.08 g FLAC-3. Activation agent: three activating agents (H3PO4, H2SO4 and NaOH) have been applied to select the best agent for further experiments under the same conditions. All samples of methylene blue dye were centrifuged after adsorption and examined using a UV–visible spectrophotometer.
Results and discussion
Fourier transform infrared spectroscopy
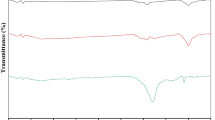
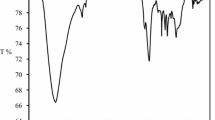
Fourier transform infrared spectroscopy is the method that can be used to qualitatively assess the presence of the principal functional groups on the exterior surface of the biosorbent. Leaves are thought to be a biomass-rich lignocellulosic source because they display a lot of oxygen functional groups on their surface (Tran et al., 2017a, 2017b). However, from the searching on the analysis and characterization of the functional groups on the surface of waste leaf plants, it was observed that the most common functional groups present in leaf-activated carbon are the O–H group, the C = O group, the C-O group, the C-H group, and the C = C group as shown in the previous studies (Guo et al., 2020; Jawad et al., 2017; Kushwaha et al., 2014). The FTIR characterization of FLAC-3 is further performed in Fig. 3a to study the surface functional groups before and after methylene blue adsorption. It is possible to attribute the signal at 3487 cm−1 to vibrations of hydroxyl functional groups, which is too close to the band of the same group that was detected at 3750 and 3500 cm−1 (Abdulhameed et al., 2021). The bands at 1626 cm−1 represent the vibrational stretching of the carbonyl group C = O, which is too close to the band of the same group that was detected at 1750 and 1500 cm−1 (Abdulhameed et al., 2021; Jawad et al., 2020). A significant band at 1317 cm−1 that was C-O band had a drop in intensity. Out of plane vibration of the C-H band had been observed at 783 cm−1. After MB adsorption, the findings of the FTIR study showed that the O–H peak shifted toward a lower wavenumber (3464 cm−1), indicating the presence of both hydrogen bonding and dipole–dipole interactions. After MB adsorption, the C = O group peaks significantly diminished in intensity and somewhat shifted from 1626 to 1616 cm−1 in wavenumber. This result suggests the existence of n–π interactions. Moreover, FTIR research revealed that following MB adsorption, the peak corresponding to the C-O bond reduced in intensity and downshifted (from 1317 to 1311 cm−1). This downshift shows that adsorbents and MB have interactions that are negative (Tran et al., 2017a, 2017b).
X-ray diffraction
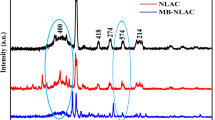
The XRD spectrum of a FLAC-3 is shown in Fig. 3b. A largely amorphous structure is revealed by the appearance of a broad diffraction background and the lack of a sharp peak. The outcome demonstrates that the FLAC-3 sample has an amorphous structure, indicating that the organic components of fig leaf waste were mostly affected by the H3PO4 alteration.
Scanning electron microscopy
A FLAC-3 adsorbent has been evaluated using scanning electronic microscopy, and it was found that a more uniform surface and high porosity were present, which led to a high adsorption capacity (Fig. 3c). The exterior surfaces of the activated carbons feature various-sized voids. The presence of these holes may facilitate the facile diffusion and trapping of large numbers of MB molecules in the pore structure of activated carbon. As a result, the use of chemical treatment helps to thoroughly clean and remove the natural colors that are present in fig leaves, freeing up the pores that they occupy and improving the fig leaves’ capacity to absorb things. The pores on the surfaces of activated carbon that are created after chemical treatment are caused by the evaporation of the activating agent during carbonization, which leaves behind the ruptured surface of activated carbon with pores formerly occupied by the activating agent (Bencheikh et al., 2020).
Nitrogen adsorption–desorption isotherms
Nitrogen adsorption–desorption tests were carried out to describe the porous FLAC-3 architectures (Fig. 3d). The FLAC-3 was estimated to have a BET-specific surface area of 18.3 m2/g. Other porosity parameters such as total pore volume, and average particle size have been presented in Table 1. The high level of surface activity and vast surface area of porous carbons frequently lead to effective dye adsorption (Khangwichian et al., 2022). It was discovered that larger particles (327.9 nm) helped boost adsorption capacity. Since heteroatoms can produce a redistribution of the surface charge of carbon materials, it has been demonstrated that the inclusion of heteroatoms in carbon materials greatly improves their performance.
Effect of initial MB concentration
UV–vis spectroscopy measurement is used to assess the removal% and adsorption capabilities of prepared fig leaf-activated carbon. In order to study the removal efficiencies and adsorption capacities of the MB dye, the initial MB concentrations are tested at these concentrations: 20, 40, 80, 120, and 200 mg/L at room temperature, as shown in Fig. 4 and Table 2.
Research on how different adsorbents respond to the initial concentration of adsorbate has shown consistent results. In one reported study regarding the adsorption of methylene blue using oil palm trunk nanocrystalline cellulose, it was observed that an increase in the initial concentration of methylene blue is related to an increase in the removal effectiveness of methylene blue. They explained that a high concentration of dye can overcome the liquid-mass transfer resistance so the low concentration of MB led to creating large number of empty active site on the surface, thus decreasing the adsorption capacity (Mustikaningrum et al., n.d.). Figure 4 shows the graph of the fluctuation in methylene blue concentration on adsorption capacity (mg/g). In general, it may be said that the adsorption capacity increases with increasing starting concentration. The mass transfer resistance between the liquid (methylene blue) and the solid adsorbent is significantly overcome by the higher starting concentration (Al-Ghouti & Al-Absi, 2020). The number of active site on FLAC-3 might not be enough to adsorb enough methylene blue molecules at high concentrations, thus resulting in lower color removal.
Effect of contact time
It is well known that the mass of dye adsorbed on the adsorbent and the cost of the adsorption process in wastewater treatment can both be affected by the contact time without a doubt. However, the experiments have been provided as follows: a 0.08 mg of FLAC-3 was added to 25 mL of different concentrations 20, 40, 80, 120, and 200 mg/L MB solution. The mixture was then agitated strongly for a variety of times at room temperature. From Fig. 5a, the adsorption of MB by FLAC-3 is<77% when the concentration is 40, 80, or 120 mg/L at a contact time of 10 min. On the other side, the removal efficiency rises to > 94% when the concentration was 20, 40, 80, and 120 mg/L at a contact time of 60 min which is the equilibrium time.
Adsorption capability is also taken into account and assessed, as seen in Fig. 5b. It is clear that removal percentage and adsorption capacity have opposite relationships. However, the lowest adsorption capacity was 6.25 mg/g at equilibrium time with a MB dye concentration of 20 mg/L, while the maximum adsorption capacity was 38.9 mg/g at the MB concentration of 200 mg/L. This pattern aligns with previously published findings. The percentage of MB removed decreased significantly as the initial concentration of methylene blue dye was raised on magnetic char (Izan et al., n.d.). The MB intake, however, went from 42.6 to 63.7 mg/g. The leftover biomass from the brewing sector has been used to examine how the initial concentration of methylene blue affected adsorption capability (Blaga et al., 2022). He discovered that raising the MB dye concentration from 5 to 70 mg/L caused the adsorption capacity to rise from 50 to 230 mg/g. This suggests that at higher initial MB concentrations, MB cation-adsorbent surface collisions take place more frequently, increasing MB adsorption capacity (Jiang et al., 2021).
Effect of temperature
Figure 6 and Table 2 show the change in MB adsorption removal and capacity by fig leaf-activated carbon at various temperatures. The elimination efficiency of FLAC-3 is 93.3% at 20 °C. At 50 °C, the removal efficiency increases to up to 98.9%. The mobility of the dye molecules was dynamic as the temperature rose, and there were more active sites for adsorption as well (Bharathi & Ramesh, 2013). The adsorption capacity increased steadily with increasing temperature from 20 to 50 °C. For MB dye adsorption, the adsorption capacity reaches 30.9 mg/g at 50 °C, while it is at its lowest value of 29.2 mg/g at 20 °C. Based on the data presented, the temperature increase causes an increase in the adsorption capacity due to the swelling of the internal structure of the adsorbent, which allows methylene blue to penetrate further(Hu et al., 2018).
Effect of initial solution pH
Figure 7 and Table 2 show how the fig leaf-activated carbon performs in terms of MB adsorption at various initial solution pH ranges (pH3, pH7, pH8, and pH11). With acidic conditions, the MB absorption by FLAC-3 is comparatively low, whereas the high adsorption property is realized under basic concentration. The cationic MB dye molecules experienced electrostatic mutual repulsion with more H+ ions on the FLAC-3 surface at lower pH levels. As we know, the OH group’s active site was beneficial for the adsorption of adsorbed material on activated carbon surfaces (Islam et al., 2017). Therefore, at pH 3, the elimination effectiveness is just 82.5%. When the pH is increased from 3 to 7, the adsorption removal rises to 99.3%. The elimination effectiveness of FLAC-3 is 95% when pH reaches 8 and 11. This finding was in agreement with other previous studies. According to a publication, decreased MB adsorption at pH 3 may be caused by the adsorbent’s predominantly protonated amino and carboxylic acid functional groups, which increase the cationic MB dye’s electrostatic repulsion. When the initial pH rose to an alkaline medium, high MB adsorption onto the adsorbents was observed (Shelke et al., 2022).
Additionally, it was found that the proton generation competes with the MB cation for adsorption on the active FLAC-3 sites under acidic conditions, resulting in a reduction in adsorption capacity (Nordin et al., 2021). The high cation exchange capacity of FLAC-3 is probably to blame for the high MB adsorption that becomes apparent as the pH of the solution rises to a high 11. The alkaline state of the solution suggests that the adsorbent surface has more negative charges than positive ones (Murthy et al., 2020). The high adsorption capacity of FLAC-3 roughly 24.5 mg/g is caused by electrostatic attraction to cationic MB due to the negative charge on its surface. Thus, it is evident that FLAC-3 requires a neutral or basic environment in order to achieve high adsorption efficiency.
Effect of FLAC-3 amount
Figure 8 and Table 2 display the results of the evaluation of the dosage of fig leaf activated carbon for the adsorption of 80 mg/L of MB dye solution at pH 7. As can be seen, the plot demonstrates that at FLAC-3 dosages of 0.02 and 0.1 g, respectively, the adsorption efficiency of MB rose from 41 to 100%. This is brought on by the rise in the number of available empty adsorption sites and the adsorbent's surface area for adsorption (Abuzerr et al., 2018). High removal efficiency may result from a high adsorbent dose.
But as the dosage is increased, the adsorption ability decreases. It might not be enough to give negative charges for MB adsorption if the adsorbent dosage is increased further since it could change the nature of the solution (Izan et al. n.d.). As can be seen, the adsorption capacity was decreased from 41 to 20 mg/g when the FLAC-3 increased from 0.02 to 0.1 g.
Effect of solution volume
With the exception of 100 mL, FLAC-3 exhibits great removal efficiency in the adsorption of the methylene blue dye at different volumes of solution (25, 50, and 100 mL). The highest removal rate was 99.5% with a 25 mL dosage. As the volume of the MB dye solution increases, the removal efficiency declines. At 50 mL of dye solution, the greatest adsorption capacity of 41.3 mg/g is obtained. These findings are depicted in Fig. 9 and Table 2.
Effect of activation agent
The activation process can undoubtedly influence the removal% and mass of adsorbed dye on the adsorbent. The effect of the activation process was presented in five aspects: AC1: only burn; AC2: pristine powder; AC3 (FLAC-3): impregnated in H3PO4; AC4: impregnated in NaOH; and AC5: impregnated in H2SO4 on the removal% and adsorption capacity as presented in Fig. 10 and Table 2. A 80 mg of adsorbents was added individually to 25 mL of an 80 mg/L MB solution in conical flasks and shaken for 60 min at room temperature. The elimination percent of MB by AC3 is 99.6%, and the adsorption capacity is 24.9 mg/g. By testing the others, the lowest removal efficiency is 75% when pristine powder is used without any further chemical treatment. The impregnation of fig leaf powder in NaOH solution has not improved the efficiency of adsorption as much as acid did.
Adsorption isotherms
The Langmuir and Freundlich models’ equation method was used to determine the value of the adsorption equilibrium constant (Fig. 11 and Table 3). The Freundlich model has the lowest R2 of the two models (0.9130), but the Langmuir model has a high R2 of 0.9841. Adsorption kinetics models like the Langmuir isotherm model are frequently used to explain intricate adsorption dynamics. The maximal adsorption capacity in the current investigation was 41.7 mg/g, and the Langmuir affinity constant (KL) was 0.37 L/mg. This model accurately depicts the methylene blue adsorption by FLAC-3. In addition to the adsorbent’s pores, the adsorption mechanism also depends on hydrogen bonds and Van der Waals interactions. The FLAC-3 hydroxyl group, which binds the nitrogen element of methylene blue, contains hydrogen, which is what distinguishes the hydrogen bond in this adsorption method. Dipole-ion interactions and electrostatic interactions are features of the Van der Waals force. An appropriate model to explain the chemical adsorption mechanism is the Langmuir isotherm. The observable adsorption process, specifically the monolayer (Wang & Guo, 2020), indicates this. This monolayer surface demonstrates that an active site, which can only be occupied by one molecule at a time, is responsible for carrying out the adsorption (Hasan et al., 2020). Other earlier investigations also supported the Langmuir isotherm model for the methylene blue adsorption procedure utilizing activated carbon from leaf waste plants (Guo et al., 2020; Jawad et al., 2017).
According to the previous studies, utilization of fig leaves in terms of adsorption dyes has not reported yet. However, the present study has compared with some other publications on different plant leaves for adsorption of methylene blue as reported in Table 4.
Commonly used as a reference dye in adsorption investigations is methylene blue. It has been claimed that using too much methylene blue can directly oxidize hemoglobin, leading to methemoglobinemia. Additionally, it can lead to hemolysis-related issues, particularly in newborns. Continual exposure may cause noticeable anemia (Kadhom et al., 2020). After being dried, the residual pineapple leaves were put in a 1:1 ratio to a 500-mL beaker with 136.28 g/mol of ZnCl2 as an activator agent. This mixture was then permitted to soak for 24 h at room temperature. Throughout this time, a glass rod was occasionally used for stirring. After that, it was carbonized for an hour at 500 °C and dried for 24 h at 110 °C. The ability of the activated carbon to efficiently remove dye was evaluated using methylene blue (MB). The maximum adsorption capacity was 288.34 mg/g.
In other study, the grape leaf has been used to create the activated carbon. The grape leaves are thoroughly cleansed with distilled water before being dried in an oven for 3 h at 150 °C. It was then thermally activated for 2 h at 500 °C in a nitrogen-filled electric furnace (Mousavi et al., 2022). With a starting concentration of 100 mg/L, an adsorbent concentration of 12.5 g/L, a pH solution of 11, and a time period of 90 min, the maximum adsorption capacity was 0.2 mg/L, and the removal efficiency was 97.4%.
The Ensete ventricosum midrib leaf (EVML) was used as the optimal adsorbent in a study to remove methylene blue from synthetic water (Mekuria et al., 2022). The adsorbents were crushed with a mortar and pestle and then sieved to a 300-m mesh size. Then, 50 g of the adsorbent was added to a 250-mL flask containing 0.1 M HCl and stirred for 6 h to remove the colored components. Over a broad pH range, it was discovered that EVML adsorbents have noticeably high MB adsorption capacity of 35.5 mg/g. Instead of using activated carbon, the necessary adsorbent was generated as ash from banana leaves (Alam et al., 2022). Under the following conditions, methylene blue dye was subjected to adsorption: 23.9 mg/100 mL of adsorbent dose, 3 h of shaking time, and 356 rpm shaking speed. To get the greatest reduction of MB (93.75%), all these variables were statistically analyzed (Alam et al., 2022). The same study found that when banana leaf ash was compared to other carbon sources, it had the maximum adsorption capacity, up to 128.5 mg/g, while the range for other materials was between 73.8 and 93.6 mg/g (Alam et al., 2022).
According to the findings of the other study, banana leaves were chemically activated to create activated carbon, in which they were then impregnated with H3PO4 and burned at three different temperatures of 450, 550, and 600 °C (Martín-González et al., 2013). The end product, activated carbon, was utilized to batch-process methylene blue absorption. The dynamic experimental data were modeled using pseudo-second-order kinetic models utilizing non-linear regression, while the equilibrium experimental data were fitted to be correlated to the Langmuir model (Martín-González et al., 2013). The highest adsorption capacity was found to be between 19.08 and 48.01 mg/g, while the removal percentage ranged between 40 and 90% in less than 20 min (Martín-González et al., 2013).
Agave salmiana leaves have been reacted with phosphoric acid to produce microwave-activated carbon (Canales-Flores & Prieto-García, 2020). The samples were cleaned in distilled water, allowed to dry in the sun for 72 h and then crushed and sieved to create particles of 0.3 to 1 mm diameters. Prior to activation, the precursors were pyrolyzed with nitrogen gas at 500 °C in a muffle furnace. The materials that resulted were then H3PO4 impregnated (Canales-Flores & Prieto-García, 2020). Activated carbon showed significant MB removal efficiency of 72% and an adsorption capacity of 89.3 mg/g as a result of the activation agent and microwaves (Canales-Flores & Prieto-García, 2020).
A low-cost biosorbent known as Saccharum arundinaceum leaf powder (PSAL), which is derived from agricultural waste, can be used to remove MB from wastewater in the textile, printing, and industrial industries. The dried powder is first soaked in acetic acid and hydrogen peroxide before being washed with water to create activated carbon. The samples were then dried at room temperature after that (Halysh et al., 2020). Methylene blue’s equilibrium adsorption and kinetics were examined using batch studies (MB). The largest amount of MB dye that could be adsorbed was 25.4 mg/g in alkaline media (pH 10), where it was more easily absorbed.
Peels from the cucumber plant, Cucumis sativus, were utilized as an adsorbent for the MB (Shakoor & Nasar, 2017). By increasing the adsorbent dosage, the removal efficiency rose until it optimally reached 85% at 6 g/L. Subsequent dosage increases only slightly improved the efficiency. However, as the dosage of adsorbent was increased, the adsorption capacity dropped. After 1 h of contact, the adsorption capacity achieved its equilibrium value of 21.45 mg/g.
Thermodynamic study
At 293, 303, 313, and 323 K, the impact of temperature on the adsorption of MB on FLAC-3 adsorbent was examined. As the temperature rose from 293 to 323 K, it was found that the adsorption capacity increased from 29.2 to 30.9 mg/g. These results suggested that the MB dye may be pushed from the solution phase to the solid surface due to the increased feasibility of adsorption at higher temperatures caused by the rise in kinetic energy of dye molecules (Dural et al., 2011). In the study on the adsorption of MB onto FLAC-3, a related finding was also made. Using Eq. (3) and Eq. (4), the thermodynamic parameters change in enthalpy (∆H°), entropy (∆S°), and Gibbs free energy (∆G°) are calculated for the adsorption of MB on FLAC-3.
where Ce is the dye’s equilibrium concentration in solution (mg/L) and Cs is its equilibrium concentration in the solid phase (mg/L). Temperature is T, and gas constant R is 8.314 J/mol/K. Changes in enthalpy (kJ/mol), entropy (J/mol/K), and Gibb’s free energy (kJ/mol) are denoted by the symbols ∆H°, ∆S°, and ∆G°, respectively. The slope (∆H°/R) and intercept (∆S°/R) of the plots of ln (Cs/Ce) vs. 1/T were used to get the values of ∆H° and ∆S°.
The coefficient of distribution is calculated using Eq. (5) which is named Kd.
Table 5 displays the thermodynamic parameter values. Negative values of ∆G° demonstrated the viability and spontaneity of the adsorption process. As the temperature rose, the values of ∆G° fell, indicating that the adsorption was more spontaneous at low temperatures. The increase in randomness at the adsorbent-solution interface during the adsorption was described by a positive value of ∆S°. The overall endothermic nature of the MB adsorption on FLAC-3 is confirmed by a positive value for ∆H°.
Kinetic study
The kinetic data are investigated using the pseudo-first-order and pseudo-second-order linear models shown in Eqs. (6) and (7), respectively (Mousavi et al., 2022).
where Qe and Qt are the adsorption capacities (measured in mg/g of MB adsorbed on the material) at equilibrium and any time t (min), respectively. The rate constants for the pseudo-first-order (min−1) and pseudo-second-order (g/mg.min) adsorption processes are Kad1 and Kad2, respectively. The pseudo-first-order and pseudo-second-order models, respectively, for the kinetic processes of adsorption are shown in Fig. 12a and b and Table 6. Each model’s parameter values are displayed. When compared to the correlation coefficients obtained for the pseudo-first-order model, the data are demonstrated to suit the pseudo-second-order model well (R2 = 0.9972) (Fig. 12b). Chemisorption is therefore shown to occur because the process is dependent on the adsorbent and the concentration of the adsorbate.
Methylene blue adsorption mechanism
Fig leaves are agricultural waste and contain higher quantities of compounds that are rich in organic composition. However, after activation and carbonization the fig leaves, FLAC-3 is the promising adsorbent to adsorb methylene blue dye from its aqueous solution. In MB adsorption, various active functional groups on the surface show the main role in adsorption of dye. Studies revealed two key elements that have an impact on the adsorption process. The methylene blue dye’s structure is the first, and the presence of functional groups on the outside of the leaves is the second. The FTIR detection shows that different functional groups, including C = O, C-H, C-O, and O–H, were reachable from the adsorbents. These functional groups may be the reason why positively charged MB molecules are taken in. The positive charge molecules of MB and the negative charge exterior of the leaves may interact via electrostatic interaction, -interaction, H-bonding, π-π-interaction, and n-π-interaction (Tran et al., 2017a, 2017b).
Selectivity dye adsorption
In the field of selective dye adsorption and separation, earlier studies have shown that leaf waste-activated carbon displayed excellent affinity for organic dyes. The heterocyclic aromatic organic compound known as the cationic MB dye is frequently utilized as a target molecule for wastewater treatment and water purification. Figure 13 describes the chemical composition of the methylene blue organic dye in the study of selective dye adsorption. After 60 min of using 0.06 g FLAC-3 as the MB adsorbent, the color of the 25 mL 40 mg/L MB solution is almost vanished to the human sight. The cationic MB is electrostatically bound to the anionic hydroxyl groups on the FLAC-3 surface. To demonstrate the selective dye adsorption property (Fig. 13), UV–vis spectroscopy was used to record the solution’s absorbance spectra. The MB peak is hardly discernible after adsorption.
Conclusion
Fig leaves have been used as an activated carbon, and it was applied in treatment of dyes for the first time. However, the viability of using fig leaves as a novel, inexpensive precursor in the production of activated carbon is examined in this present work. The outcomes show that FLAC-3 is a powerful adsorbent for the adsorption of the methylene blue dye. This study has shown that H3PO4 treatment could improve fig leaf-activated carbon’s ability to adsorb MB and from its aqueous solutions compared to other activating agents such as NaOH and H2SO4.
SEM scans showed that after adsorption, MB molecules had filled the active sites of FLAC-3. The surface of FLAC-3 has numerous significant functional groups, including hydroxyl O–H, alkane C-H, and C = O, according to FTIR spectra. The FLAC-3 possesses non-crystalline characteristics, according to the XRD instrument. When the initial MB dye concentration, contact time, temperature, and FLAC-3 dose rose, so did the dye uptake clearly and explicitly. The FLAC-3’s adsorption capacity was found to be 24.75 mg/g for 0.08 g and 41 mg/g for 0.02 g.
The results of the adsorption experiments showed that the pseudo-second-order model best described the kinetic uptake characteristics. The overall endothermic nature of the MB adsorption on FLAC-3 is confirmed by a positive value for ∆H°. The negative values of ∆G° indicate that the adsorption was more spontaneous at low temperatures. The Langmuir model, on the other hand, does a good job of describing the adsorption isotherms. It was discovered that the maximum adsorption capacity was 41.7 mg/g and the Langmuir affinity constant was 0.37 L/mg. The FLAC-3 as low-cost adsorbents for methylene blue dye has shown good cationic dye adsorption performance.
Data availability
The dataset utilized/analyzed during the current study will be available from the corresponding author upon request.
References
Abdulhameed, A. S., Hum, N. N. M. F., Rangabhashiyam, S., Jawad, A. H., Wilson, L. D., Yaseen, Z. M., et al. (2021). Statistical modeling and mechanistic pathway for methylene blue dye removal by high surface area and mesoporous grass-based activated carbon using K2CO3 activator. Journal of Environmental Chemical Engineering, 9(4), 105530.
Abuzerr, S., Darwish, M., & Mahvi, A. H. (2018). Simultaneous removal of cationic methylene blue and anionic reactive red 198 dyes using magnetic activated carbon nanoparticles: Equilibrium, and kinetics analysis. Water Science and Technology, 2017(2), 534–545.
Alam, M. Z., Bari, M. N., & Kawsari, S. (2022). Statistical optimization of methylene blue dye removal from a synthetic textile wastewater using indigenous adsorbents. Environmental and Sustainability Indicators, 14, 100176.
Al-Ghouti, M. A., & Al-Absi, R. S. (2020). Mechanistic understanding of the adsorption and thermodynamic aspects of cationic methylene blue dye onto cellulosic olive stones biomass from wastewater. Scientific Reports, 10(1), 1–18.
Bencheikh, I., Azoulay, K., Mabrouki, J., El Hajjaji, S., Dahchour, A., Moufti, A., & Dhiba, D. (2020). The adsorptive removal of MB using chemically treated artichoke leaves: Parametric, kinetic, isotherm and thermodynamic study. Scientific African, 9, e00509.
Bharathi, K. S., & Ramesh, S. T. (2013). Removal of dyes using agricultural waste as low-cost adsorbents: A review. Applied Water Science, 3(4), 773–790.
Blaga, A. C., Tanasă, A. M., Cimpoesu, R., Tataru-Farmus, R.-E., & Suteu, D. (2022). Biosorbents based on biopolymers from natural sources and food waste to retain the methylene blue dye from the aqueous medium. Polymers, 14(13), 2728.
Canales-Flores, R. A., & Prieto-García, F. (2020). Taguchi optimization for production of activated carbon from phosphoric acid impregnated agricultural waste by microwave heating for the removal of methylene blue. Diamond and Related Materials, 109, 108027.
Choma, J., Osuchowski, Ł, Dziura, A., Marszewski, M., & Jaroniec, M. (2015). Benzene and methane adsorption on ultrahigh surface area carbons prepared from sulphonated styrene divinylbenzene resin by KOH activation. Adsorption Science & Technology, 33(6–8), 587–594.
Choudhry, A., Sharma, A., Khan, T. A., & Chaudhry, S. A. (2021). Flax seeds based magnetic hybrid nanocomposite: An advance and sustainable material for water cleansing. Journal of Water Process Engineering, 42, 102150.
Dural, M. U., Cavas, L., Papageorgiou, S. K., & Katsaros, F. K. (2011). Methylene blue adsorption on activated carbon prepared from Posidonia oceanica (L.) dead leaves: Kinetics and equilibrium studies. Chemical Engineering Journal, 168(1), 77–85.
El Messaoudi, N., El Mouden, A., El Khomri, M., Bouich, A., Fernine, Y., Ciğeroğlu, Z., et al. (2022). Experimental study and theoretical statistical modeling of acid blue 25 remediation using activated carbon from Citrus sinensis leaf. Fluid Phase Equilibria, 563, 113585.
Guo, D., Li, Y., Cui, B., Hu, M., Luo, S., Ji, B., & Liu, Y. (2020). Natural adsorption of methylene blue by waste fallen leaves of Magnoliaceae and its repeated thermal regeneration for reuse. Journal of Cleaner Production, 267, 121903.
Gutub, S. A., Bassyouni, M., & Abdel-Hamid, S. M. S. (2013). Dissolved solids adsorption of freshwater using synthesized bio-foam composite. Life Science Journal, 10(2), 464–471.
Haghbin, M. R., & Shahrak, M. N. (2021). Process conditions optimization for the fabrication of highly porous activated carbon from date palm bark wastes for removing pollutants from water. Powder Technology, 377, 890–899.
Halysh, V., Sevastyanova, O., Pikus, S., Dobele, G., Pasalskiy, B., Gun’ko, V. M., & Kartel, M. (2020). Sugarcane bagasse and straw as low-cost lignocellulosic sorbents for the removal of dyes and metal ions from water. Cellulose, 27(14), 8181–8197.
Hasan, R., Ying, W. J., Cheng, C. C., Jaafar, N. F., Jusoh, R., Jalil, A. A., & Setiabudi, H. D. (2020). Methylene blue adsorption onto cockle shells-treated banana pith: Optimization, isotherm, kinetic, and thermodynamic studies. Indonesian Journal of Chemistry, 20(2), 368–378.
Hu, X.-S., Liang, R., & Sun, G. (2018). Super-adsorbent hydrogel for removal of methylene blue dye from aqueous solution. Journal of Materials Chemistry A, 6(36), 17612–17624.
Huang, S., & Shi, J. (2014). Monolithic macroporous carbon materials as high-performance and ultralow-cost sorbents for efficiently solving organic pollution. Industrial & Engineering Chemistry Research, 53(12), 4888–4893.
Islam, M. A., Ahmed, M. J., Khanday, W. A., Asif, M., & Hameed, B. H. (2017). Mesoporous activated coconut shell-derived hydrochar prepared via hydrothermal carbonization-NaOH activation for methylene blue adsorption. Journal of Environmental Management, 203, 237–244.
Izan, N. R., Zainol, M. M., Nordin, A. H., Asmadi, M., Wong, S. L., Azhar, M. A. I., & Alias, N. H. (2020). Removal of methylene blue via adsorption using magnetic char derived from food waste. Malaysian Journal of Chemistry, 24(2) 283–292.
Jawad, A. H., Rashid, R. A., Ishak, M. A. M., & Wilson, L. D. (2016). Adsorption of methylene blue onto activated carbon developed from biomass waste by H2SO4 activation: Kinetic, equilibrium and thermodynamic studies. Desalination and Water Treatment, 57(52), 25194–25206.
Jawad, A. H., Ramlah, A. R., Khudzir, I., & Sabar, S. (2017). High surface area mesoporous activated carbon developed from coconut leaf by chemical activation with H3PO4 for adsorption of methylene blue. Desalination and Water Treatment, 74, 326–335.
Jawad, A. H., Bardhan, M., Islam, M. A., Islam, M. A., Syed-Hassan, S. S. A., Surip, S. N., et al. (2020). Insights into the modeling, characterization and adsorption performance of mesoporous activated carbon from corn cob residue via microwave-assisted H3PO4 activation. Surfaces and Interfaces, 21, 100688.
Jiang, W., Zhang, L., Guo, X., Yang, M., Lu, Y., Wang, Y., et al. (2021). Adsorption of cationic dye from water using an iron oxide/activated carbon magnetic composites prepared from sugarcane bagasse by microwave method. Environmental Technology, 42(3), 337–350.
Kadhom, M., Albayati, N., Alalwan, H., & Al-Furaiji, M. (2020). Removal of dyes by agricultural waste. Sustainable Chemistry and Pharmacy, 16, 100259.
Khangwichian, W., Pattamasewe, S., Leesing, R., Knijnenburg, J. T. N., & Ngernyen, Y. (2022). Adsorption of cationic dye on activated carbon from hydrolyzed Dipterocarpus alatus leaves: Waste from biodiesel production. Engineering and Applied Science Research, 49(4), 531–544.
Khomri, M. E., Messaoudi, N. E., Dbik, A., Bentahar, S., Fernine, Y., Bouich, A., et al. (2022). Modification of low-cost adsorbent prepared from agricultural solid waste for the adsorption and desorption of cationic dye. Emergent Materials, 5(6), 1679–1688.
Kılıç, M., Apaydın-Varol, E., & Pütün, A. E. (2012). Preparation and surface characterization of activated carbons from Euphorbia rigida by chemical activation with ZnCl2, K2CO3, NaOH and H3PO4. Applied Surface Science, 261, 247–254.
Kushwaha, A. K., Gupta, N., & Chattopadhyaya, M. C. (2014). Removal of cationic methylene blue and malachite green dyes from aqueous solution by waste materials of Daucus carota. Journal of Saudi Chemical Society, 18(3), 200–207.
Li, Z., Gao, X., Wu, L., Wang, K., & Kobayashi, N. (2017). Preparation of activated carbons from poplar wood by chemical activation with KOH. Journal of Porous Materials, 24(1), 193–202.
Liu, L., Li, Y., & Fan, S. (2019). Preparation of KOH and H3PO4 modified biochar and its application in methylene blue removal from aqueous solution. Processes, 7(12), 891.
Liu, Z., Sun, Y., Xu, X., Qu, J., & Qu, B. (2020). Adsorption of Hg (II) in an aqueous solution by activated carbon prepared from rice husk using KOH activation. ACS Omega, 5(45), 29231–29242.
Mahamad, M. N., Zaini, M. A. A., & Zakaria, Z. A. (2015). Preparation and characterization of activated carbon from pineapple waste biomass for dye removal. International Biodeterioration & Biodegradation, 102, 274–280.
Mangla, D., Sharma, A., & Ikram, S. (2022). Synthesis of ecological chitosan/PVP magnetic composite: Remediation of amoxicillin trihydrate from its aqueous solution, isotherm modelling, thermodynamic, and kinetic studies. Reactive and Functional Polymers, 175, 105261.
Martín-González, M. A., Susial, P., Pérez-Peña, J., & Doña-Rodríguez, J. M. (2013). Preparation of activated carbons from banana leaves by chemical activation with phosphoric acid. Adsorption of methylene blue. Revista mexicana de ingeniería química, 12(3), 595–608.
Mekuria, D., Diro, A., Melak, F., & Asere, T. G. (2022). Adsorptive removal of methylene blue dye using biowaste materials: Barley Bran and enset midrib leaf. Journal of Chemistry. https://doi.org/10.1155/2022/4849758
El Messaoudi, N., El Khomri, M., Goodarzvand Chegini, Z., Chlif, N., Dbik, A., & Bentahar, S., et al. (2021). Desorption study and reusability of raw and H2SO4 modified jujube shells (Zizyphus lotus) for the methylene blue adsorption. International Journal of Environmental Analytical Chemistry, 1–17.
Mousavi, S. A., Mahmoudi, A., Amiri, S., Darvishi, P., & Noori, E. (2022). Methylene blue removal using grape leaves waste: Optimization and modeling. Applied Water Science, 12(5), 1–11.
Murthy, T. P. K., Gowrishankar, B. S., Krishna, R. H., Chandraprabha, M. N., & Mathew, B. B. (2020). Magnetic modification of coffee husk hydrochar for adsorptive removal of methylene blue: Isotherms, kinetics and thermodynamic studies. Environmental Chemistry and Ecotoxicology, 2, 205–212.
Mustikaningrum, M., Cahyono, R. B., & Yuliansyah, A. T. (n.d.). Adsorption of methylene blue on nano-crystal cellulose of oil palm trunk: Kinetic and thermodynamic studies. Indonesian Journal of Chemistry, 22(4), 953–964.
Nordin, A. H., Wong, S., Ngadi, N., Zainol, M. M., Abd Latif, N. A. F., & Nabgan, W. (2021). Surface functionalization of cellulose with polyethyleneimine and magnetic nanoparticles for efficient removal of anionic dye in wastewater. Journal of Environmental Chemical Engineering, 9(1), 104639.
Omer, O. S., Hussein, M. A., Hussein, B. H. M., & Mgaidi, A. (2018). Adsorption thermodynamics of cationic dyes (methylene blue and crystal violet) to a natural clay mineral from aqueous solution between 293.15 and 323.15 K. Arabian Journal of Chemistry, 11(5), 615–623.
Patra, B. R., Mukherjee, A., Nanda, S., & Dalai, A. K. (2021a). Biochar production, activation and adsorptive applications: A review. Environmental Chemistry Letters, 19(3), 2237–2259.
Patra, B. R., Nanda, S., Dalai, A. K., & Meda, V. (2021b). Slow pyrolysis of agro-food wastes and physicochemical characterization of biofuel products. Chemosphere, 285, 131431.
Patra, B. R., Nanda, S., Dalai, A. K., & Meda, V. (2021c). Taguchi-based process optimization for activation of agro-food waste biochar and performance test for dye adsorption. Chemosphere, 285, 131531.
Prashanthakumar, T. K. M., Kumar, S. K. A., & Sahoo, S. K. (2018). A quick removal of toxic phenolic compounds using porous carbon prepared from renewable biomass coconut spathe and exploration of new source for porous carbon materials. Journal of Environmental Chemical Engineering, 6(1), 1434–1442.
Rashid, R. A., Jawad, A. H., Ishak, M. A. M., & Kasim, N. N. (2016). KOH-activated carbon developed from biomass waste: Adsorption equilibrium, kinetic and thermodynamic studies for Methylene blue uptake. Desalination and Water Treatment, 57(56), 27226–27236.
Shakoor, S., & Nasar, A. (2017). Adsorptive treatment of hazardous methylene blue dye from artificially contaminated water using cucumis sativus peel waste as a low-cost adsorbent. Groundwater for Sustainable Development, 5, 152–159.
Shannon, M. A., Bohn, P. W., Elimelech, M., Georgiadis, J. G., Mariñas, B. J., & Mayes, A. M. (2008). Science and technology for water purification in the coming decades. Nature, 452(7185), 301–310.
Sharma, A., Mangla, D., & Chaudhry, S. A. (2022). Recent advances in magnetic composites as adsorbents for wastewater remediation. Journal of Environmental Management, 306, 114483.
Sharma, A., Rasheed, S., Mangla, D., Choudhry, A., Shukla, S., & Chaudhry, S. A. (2023). Cobalt ferrite incorporated ocimum sanctum nanocomposite matrix as an interface for adsorption of organic dyes: A sustainable alternative. ChemistrySelect, 8(5), e202203709.
Shelke, B. N., Jopale, M. K., & Kategaonkar, A. H. (2022). Exploration of biomass waste as low cost adsorbents for removal of methylene blue dye: A review. Journal of the Indian Chemical Society, 99(7), 100530.
Singh, A., Nanda, S., Guayaquil-Sosa, J. F., & Berruti, F. (2021). Pyrolysis of Miscanthus and characterization of value-added bio-oil and biochar products. The Canadian Journal of Chemical Engineering, 99, S55–S68.
Stewart, G. G. (2016). Saccharomyces species in the Production of Beer. Beverages, 2(4), 34.
Tran, H. N., You, S.-J., & Chao, H.-P. (2017a). Fast and efficient adsorption of methylene green 5 on activated carbon prepared from new chemical activation method. Journal of Environmental Management, 188, 322–336.
Tran, H. N., You, S.-J., Nguyen, T. V., & Chao, H.-P. (2017b). Insight into the adsorption mechanism of cationic dye onto biosorbents derived from agricultural wastes. Chemical Engineering Communications, 204(9), 1020–1036.
Wang, J., & Guo, X. (2020). Adsorption kinetic models: Physical meanings, applications, and solving methods. Journal of Hazardous Materials, 390, 122156.
Wu, H.-Y., Chen, S. S., Liao, W., Wang, W., Jang, M.-F., Chen, W.-H., et al. (2020). Assessment of agricultural waste-derived activated carbon in multiple applications. Environmental Research, 191, 110176.
Yağmur, H. K., & Kaya, İ. (2021). Synthesis and characterization of magnetic ZnCl2-activated carbon produced from coconut shell for the adsorption of methylene blue. Journal of Molecular Structure, 1232, 130071.
Zhu, R., Yu, Q., Li, M., Zhao, H., Jin, S., Huang, Y., et al. (2021). Analysis of factors influencing pore structure development of agricultural and forestry waste-derived activated carbon for adsorption application in gas and liquid phases: A review. Journal of Environmental Chemical Engineering, 9(5), 105905.
Acknowledgements
The authors thank the College of Sciences for Women, University of Babylon, for facilitating this work.
Author information
Authors and Affiliations
Contributions
Safaa Talib Al-Asadi: wrote the first draft of the manuscript, did the experiments and organized the structure of the manuscript.
Fouad Fadhil Al-Qaim: analyzed the data and edited the final draft of the manuscript.
Haider Falih SHamikh Al-Saedi: collected the references and improved the final revised draft.
Issa Farhan Deyab: revised the whole manuscript and improved the structure of the final draft.
Hesam Kamyab: revised the whole manuscript and improvement the English language.
Shreeshivadasan Chelliapan: revised the whole manuscript and improvement the English language.
All authors read and approved the final manuscript.
All authors have read, understood, and have complied as applicable with the statement on “Ethical responsibilities of Authors” as found in the Instructions for Authors.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Al-Asadi, S.T., Al-Qaim, F.F., Al-Saedi, H.F.S. et al. Adsorption of methylene blue dye from aqueous solution using low-cost adsorbent: kinetic, isotherm adsorption, and thermodynamic studies. Environ Monit Assess 195, 676 (2023). https://doi.org/10.1007/s10661-023-11334-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10661-023-11334-2