Abstract
A green one-step catalytic process of obtaining microcrystalline cellulose (MCC) from wood in the medium “acetic acid–hydrogen peroxide–water” in the presence 2% wt. sulfuric acid catalyst is described. The influence of wood nature and conditions of the process on the yield, composition and structure of obtained samples of MCC was investigated. For hardwood (aspen wood and birch wood) the minimal content of residual lignin (<1% wt.) was achieved under the following conditions: temperature 130 °C, the concentration of H2O2 4% wt., the concentration of CH3COOH ~ 26% wt., liquor ratio of 10 and the process time of 3 h. At these conditions, the degree of delignification of softwood (spruce wood and larch wood) is lower than for hardwood. According to X-ray and FTIR data, the structure of MCC samples obtained from wood is close to that of MCC Avicel and MCC from cotton linter.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Advanced methods of oxidative catalytic delignification with “green” reagents (molecular oxygen, hydrogen peroxide, ozone) make it possible to produce cellulose in environmentally friendly ways [1, 2].
Previously, it was shown that a significant intensification of oxidative delignification of wood by hydrogen peroxide in acetic acid–water medium at 120–130 °C takes place in the presence of sulfuric acid catalyst or suspended TiO2 [3, 4]. Under these conditions, along with the oxidative destruction of lignin and hemicelluloses, hydrolysis of the amorphous parts of cellulose was observed. The hydrolysis of amorphous cellulose is intensified with an increase of liquor ratio and the time of wood treatment. In our early study, the one-step method of obtaining microcrystalline cellulose (MCC) from aspen wood was suggested based on the combined action of TiO2 catalyst and of UV-pretreatment of [5].
MCC is widely used as a biologically active additive, filler and stabilizer of medicines, for the manufacture of health food and confectionery, water–latex emulsions and paints, and in many other areas. Traditional technologies of MCC production from wood include stages of wood delignification, pulp bleaching and mild hydrolysis to remove easily hydrolyzable polysaccharides [6]. The use of sulfur and chlorine-containing reagents in the stages of delignification and bleaching makes traditional multi-stage technology of MCC production dangerous for the environment.
The present paper describes a “green” one-step catalytic process of obtaining MCC in the medium “acetic acid–hydrogen peroxide–water” in the presence of sulfuric acid catalyst. The influence of wood nature and the conditions of the combined process, which includes reactions of oxidative degradation of lignin, hydrolysis of hemicelluloses and some part of amorphous cellulose on the yield, composition and structure of obtained MCC was established.
Experimental technique
Air dry sawdust (fraction 2–5 mm) of Siberian origin wood (Populus tremula L., Betula pendula Roth., Abies sibirica Ledeb., Larix sibirica Ledeb.) was used as initial raw material. The contents of wood components were determined by standard methods of chemical analysis [7]. The chemical composition (% wt. on abs. dry wood): of aspen wood: 46.3 cellulose, 21.8 lignin, 24.5 hemicelluloses, 7.8 extractive substances; of birch wood: 41.3 cellulose, 19.9 lignin, 30.3 hemicelluloses, 8.4 extractive substances; of abies wood: 50.3 cellulose, 27.7 lignin, 15.4 hemicelluloses, 6.8 extractive substances; of larch wood: 34.5 cellulose, 26.1 lignin, 27.2 hemicelluloses, 13.0 extractive substances.
The delignification medium was composed of acetic acid of “chemically pure” grade (GOST 61-75), hydrogen peroxide of medical grade (GOST 177-88), sulfuric acid of “chemically pure” grade (GOST 4204-77) and distilled water (GOST 6709-72).
Delignification of wood sawdust was carried out in a shaking metal reactor of 200 cm3 volume according to procedure described in [4]. The parameter indicating the residual lignin content in the cellulosic products of wood delignification was used to evaluate the delignification activity of sulfuric acid catalyst.
The yield of cellulosic product was estimated by weighing. The structural characteristics of cellulosic products were studied by XRD, FTIR and scanning electron microscope (SEM) methods.
X-ray diffraction scattering analysis of MCC was carried out on PANalytical X’Pert Pro diffractometer using a Cu–Kα source (λ = 0.154 nm) in the 2θ range 5–70° and scanning step width of 0.01°/scan. The samples of MCC were analyzed by the powder method in a cuvette with 2.5 cm diameter. Each analysis was repeated twice. The phone scattering was subtracted from the cellulose diffraction diagram. The crystalline reflections and amorphous halo were defined according to [8].
Crystallinity index (Kp) was calculated as [9]:
where Sk and Sa are area of peaks the scattering intensities from crystalline and amorphous regions of cellulose, respectively. Ik and Io are the scattering intensities from crystalline and general regions of cellulose, respectively.
The average width of crystallites in the (002) lattice plane was determined from the Scherrer equation [8]:
where β002 is a width on the middle height of (002) reflection, rad; θ002—is the maximum of (002) reflection, rad; λ—wavelength of X-ray source (0.154 nm); δL and dL are parameters related to the lattice distortion perpendicular to the (002) plane (direction 90.05) and the distance between (002) lattice planes (0.395 nm), respectively.
Infrared spectroscopy analysis (FTIR) of the MCC was carried out in transmission mode (solid pattern MSS was prepared in pellet with matrix KBr). The mass of MCC and matrix was regulated 3 mg for each. The spectra were recorded with Vector-22 spectrometer in the 400–4,000 cm−1 wavelength range. Spectral data were processed by program OPUS/YR (version 2.2).
The crystallinity index was calculated from the relative intensities of the infrared bands [10]:
where I1,370 represents the intensity of the band at 1,370 cm−1 belonging to the CH bending vibration, and I2,900 is the intensity of the band at 2,900 cm−1 belonging to the CH stretching vibration.
The electron microphotographs were obtained by a SEM TM-1000 HITACHI (Japan) with accelerating potential 15 kV. Samples were coated on carbon support.
Results and discussion
The influence of delignification process conditions (temperature, treatment time, liquid/wood ratio, concentration of CH3COOH and H2O2) on the yield and composition of the cellulosic product was studied. The concentration of 2% wt. H2SO4 catalyst was taken according to [11]. For hardwood (aspen wood and birch wood) the minimal content of residual lignin (<1% wt.) was achieved under the following conditions: temperature 130 °C, the concentration of H2O2 4% wt., of CH3COOH ~ 26% wt., liquor ratio of 10 and the process time of 3 h (Table 1). However, under these conditions, the degree of delignification of softwood (spruce wood and larch wood) is lower than for hardwood. A possible reason for the observed effect is the difference in the chemical compositions of hardwood and softwood lignins. Hardwood lignin consists mainly of syringylic phenylpropane units, and softwood lignin contains guacylic units [12].
As was shown earlier [3], the most effective catalyst for the oxidative delignification of abies wood is TiO2 and larch wood—NaMoO4. These catalysts allow the production of the cellulosic product with the residual lignin content of less than 1% wt.
For obtaining the microcrystalline cellulose from cellulosic product, the easily hydrolyzable polysaccharides have to be removed. For this purpose, the mild acid hydrolysis of cellulose with dilute mineral acids is applied in industry. In the system CH3COOH–H2O2–H2O–H2SO4, the hydrolytic reactions can be significantly intensified by increasing the concentration of sulfuric and acetic acids, as well as by growth of liquor ratio and time of the treatment.
The influence of these factors on the yield and composition of cellulosic products obtained by oxidative delignification of aspen wood and birch wood was investigated. It was found that the increase of process temperature above 130 °C accelerates not only the decomposition of easily hydrolyzable polysaccharides but also intensifies the polycondensation reactions, resulting into the formation of humic substances (so-called, pseudolignin).
The growth of sulfuric acid catalyst concentrations higher than 2% wt. intensifies the hydrolysis reactions, which results in a significant decrease of cellulosic product yield.
The increase of acetic acid concentration in the reaction medium from 10 to 50% reduces the yield of cellulosic product significantly and the content of residual lignin. The optimum concentration of acetic acid in solution, which provides a high yield of MCC with the lignin content less 1% wt., is about 30%. Independently of the wood nature, the growth of process temperature and time result in a decrease of MCC yield and degree of MCC polymerization (Table 2).
Fig. 1 shows electron microscope images of MCC samples obtained by oxidative catalytic delignification of birch wood and aspen wood. Cellulose fibers are collected in bunches after their dispersion in the water.
IR spectra of MCC samples obtained from aspen wood and birch wood correspond to the spectrum of industrial MCC Avicel from cotton raw material (Fig. 2).
The crystallinity of MCC samples obtained from aspen wood and birch wood, calculated from the infrared spectra in respect of the absorption bands (D1370/D2900) varies between 70 and 80%. These values correspond to the crystallinity values calculated from X-ray diffraction patterns (Table 3).
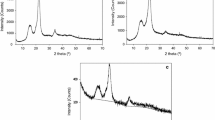
A well crystallized and homogeneous on the lattice parameters sample of MCC gives narrow and high diffraction peaks. X-ray data (Fig. 3) suggests that the crystal structure of MCC samples obtained from the birch wood and aspen wood, is monoclinic cellulose I [13].
As calculated from the X-ray data, the crystallinity of MCC samples obtained from wood (67–74%) are close to the crystallinity of industrial MCC Avicel PH 102 (64%) [6] and MCC from cotton linter (80%) (Table 3).
References
Weinstock LA, Atalla RH, Reiner RS (1997) J Mol Catal A 116:59
Kuznetsov BN, Kuznetsova SA, Danilov VG et al (2002) Catal Today 75:211
Kuznetsov BN, Tarabanko VE, Kuznetsova SA (2008) Kinet Catal 49(4):517
Kuznetsov BN, Kuznetsova SA, Danilov VG, Yatsenkova OV (2008) React Kinet Catal Lett 94:311
Kuznetsov BN, Kuznetsova SA, Danilov VG, Yatsenkova OV (2009) React Kinet Catal Lett 97:295
Vasiliu-opera C, Nicoleanu J (1990) Polym Plast Technol Eng 32(3):181
Obolenskaya AV, El’nitskaya ZP, Leonovich AA (1991) Laboratory work on chemistry of wood and cellulose, Ecology, Moscow
Rebuzzi F, Evtuguin DV (2006) Macromol Symp 232:121
Ioelovitch MYa, Veveris GP (1983) Khimiya Drevesiny 2:10
Chen Hua, Chen Kefu, Yang Rendang, Yang Fei, Gao Wenhua (2011) BioResources 6(3):2399
Kuznetsova SA, Danilov VG, Yatsenkova OV, Ivanchenko NM (2008) J. Sib Fed Univ Chem 1:80
Fengel D, Wegener G Wood (1984) Chemistry, ultrastructure, reactions. Walter de Gruyter, Berlin/New York
Nishiyama Y, Langan P, Chanzy H (2002) J Am Chem Soc 124(31):9074
Ardizzone S, Dioguardi F, Mussini T et al (1999) Cellulose 6:57
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kuznetsov, B.N., Kuznetsova, S.A., Danilov, V.G. et al. A green one-step process of obtaining microcrystalline cellulose by catalytic oxidation of wood. Reac Kinet Mech Cat 104, 337–343 (2011). https://doi.org/10.1007/s11144-011-0354-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11144-011-0354-8