Abstract
A simple method of persistent immobilization was developed for the fixing of highly efficient precrystallized (or even doped) titania (TiO2) based photocatalysts. TiO2 nanoparticles (Aeroxide P25 and VLP7000) were immobilized on the surface of Al2O3-based ceramic paper. For the immobilization, a titanium alkoxide (Ti(OEt)4) was applied as a fixing agent. This type of immobilization resulted in a photocatalytically active surface, which was used in fixed-bed flow reactors through the application of different forms of artificial or solar irradiation to activate the TiO2. To verify the stability, the decomposition of phenol was repeatedly measured on the same TiO2-covered ceramic paper; the photocatalytic performance proved to remain constant throughout five 2-h cycles. The potential for application on an industrial scale was demonstrated by a pilot-plant-scale flow reactor. The developed immobilization method is a simple technique that can be used to investigate the long-term efficiency of novel TiO2 samples, or can be applied in real air/water treatments.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Photocatalysis is an intensively investigated alternative method for the treatment of contaminated water and air, and is effective for the removal of the majority of organic pollutants [1], in contrast with the most commonly applied microbial water treatment procedure, which is unable to eliminate some industrial compounds [1, 2] (e.g. certain pharmaceuticals [3–6], dyes [2, 7–15], or toxic compounds such as pesticides [8, 16, 17] or herbicides [18] ).
Photocatalysts applied in suspensions must be separated after their use for water treatment [1, 10, 19–21]. However, in consequence, the very small grain size (5–500 nm), the filtration step demands considerable energy, which hinders their economic applicability [9, 18, 20, 22–25]. The very important challenge has therefore arisen to immobilize the photocatalyst particles in the form of photocatalytically active surfaces which can be applied in fixed-bed flow reactors [22, 25]. These methods can result in economic applications, with the utilization of solar light for the activation of the photocatalyst, without any separation costs.
The simplest method of immobilization is dip coating. The support (mainly glass) is dipped in an aqueous (or alcoholic) suspension of the photocatalyst and this is followed by a drying step. After several repetitions of the dipping/drying procedure, the final cleaning step is usually preceded by calcination [4, 11, 19, 26–29]. As described by Behnajady et al. [11], in the course of the calcination the OH groups on the catalyst surface react with the support to form oxygen bridges through water loss, and this ensures the fixing [12]. However the calcination step is difficult to carry out on large surfaces, demands the investment of considerable energy and can change the properties of the precrystallized photocatalysts.
Another possibility is to embed the photocatalytically active nanoparticles into polymers [25]. Noorjahan [30] applied a UV light-resistant acrylic polymer to make TiO2-based thin films. Polyethylene films [31] or sheets [13], polyvinyl acetate [32], polyvinyl chloride [33] and polystyrene beads [14] have also been investigated. However, organic polymers can be damaged by activated photocatalysts during utilization, which may result in mobilization of the nanoparticles.
Electrophoretic deposition is a further means of immobilization, which allows the production of thin films with controlled thickness [34]. Dunlop et al. [34] immobilized Degussa P25 TiO2 on conducting supports with the utilization of voltage, but also applied a calcination step.
Sol–gel processes, the most widely investigated methods of in situ photocatalyst generation [15, 35, 36], apply Ti compounds (mainly alkoxides or TiCl4) as precursors: an amorphous titanium hydroxide layer is produced on a support by hydrolysis and finally the photocatalytically active TiO2 surface is formed via crystallization in a calcination step. Since the photocatalytically active particles are formed during the fixing procedure, the properties of the resulting TiO2 are limited.
Special, complicated methods of synthesis to obtain highly efficient (or visible light- active) crystalline TiO2 with desired properties have been discussed in thousands of papers. The main goal of the present study was to develop a simple, stable immobilization method for the fixing of highly efficient precrystallized (or even doped) TiO2 in a UV-resistant layer which is not sensitive to the degrading action of activated TiO2 nanoparticles.
Experimental
Materials
The applied ceramic paper, produced by COTRONIC Corporation, is a highly pure Al2O3-based, non-woven, 1.6-mm-thick ceramic paper (Catalog No.: 300-040-1).
For the immobilization procedure, Ti(OEt)4 (ABCR Gmbh. & Co. Kg), which contains 10–15 % Ti(OiPr)4 and ethanol (Spektrum 3D, absolute ethanol) was applied.
The immobilized commercial photocatalysts were Aeroxide P25 (pure TiO2: ~90 m/m% anatase, ~10 m/m% rutile, Danatase ~25 nm, Drutile ~40 nm, aS BET~49 m2 g−1) produced by Evonik Industries, and VLP7000 (doped TiO2: ~100 m/m% anatase, Danatase ~7.8 nm, aS BET~297 m2 g−1) produced by Kronos Titan Gmbh. More details about the investigated photocatalysts are published elsewhere [37].
High-purity nitrogen gas (Messer, >99.995 %) was used to spray the materials onto the surfaces.
Water treatment experiments were carried out by applying phenol (Spektrum 3D; analytical grade), oxalic acid (GyKV, Hungary; analytical grade) and a pesticide (monuron; Sigma-Aldrich; analytical grade) as model contaminants, which were dissolved in MilliQ water. The pH values were ~ 6 in case of phenol and monuron solutions, while in the case of oxalic acid, the initial pH value was ~ 2.8, which increased during the decomposition.
Methods and instrumentation
Scanning electron microscopy (SEM) was used on a Hitachi S-4700 Type II FE-SEM instrument equipped with a cold field emission gun operating in the range 5–15 kV.
Changes in phenol, monuron and oxalic acid concentrations were followed with an Agilent 1100 series HPLC system. For phenol and monuron determination, it was equipped with a Lichrospher RP 18 column and a methanol/water mixture was used as eluent (detection at 210 nm). For oxalic acid measurements, a GROM-RESIN ZH column was used, with 19.3 mM aqueous sulfuric acid as eluent (λD = 206 nm).
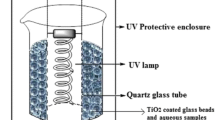
The produced TiO2-coated ceramic sheets were used in a home-made, continuous-flow recirculating reactor, illustrated in Fig. 1. The phenol solution (500 mL, 0.1 mM) was placed into a thermostated (25 °C) reservoir, while the liquid flow was maintained by a peristaltic pump (200 mL min−1) on the upper part of a tray in which a photocatalytically active impregnated ceramic sheet (10 cm × 30 cm) was placed. To activate the TiO2, UV fluorescent lamps (LIGHTECH; UV-A; 4 × 40 W), or in other cases, halogen floodlight reflectors (JEN CE-82, 2 × 500 W) were placed above the flow reactor. Fig. 2 depicts the spectra of the applied modes of irradiation.
In the case of solar photocatalytic experiments, the UV dose was measured by a hand-held UV radiometer (Optix Tech, Inc; UVTEX a + b idm).
Results and discussion
Immobilization of TiO2 on ceramic paper
A sheet of ceramic paper was perfused with ethanol to wet the surface, and was then impregnated with Ti(OEt)4 by dragging it between two plastic rollers, the lower of which was partially immersed in pure Ti(OEt)4 as shown in Fig. 3. After this step, precrystallized photocatalysts were immediately sprayed with a handheld airbrush onto the surface in suspension (ethanol) form. The concentration of the suspension was 20 g L−1, and the sprayed amount was 0.1 mL cm−2. The impregnated ceramic sheets were dried in air at room temperature for 24 h. During this step, the Ti(OEt)4 was hydrolyzed by the humidity in air, and the forming amorphous Ti(IV) oxide-hydroxide fixed the photocatalytically active particles onto the surface (Fig. 4). After the drying step, the impregnated ceramic sheets were washed with distilled water to eliminate non-immobilized nanoparticles, and were finally cleaned from possible organic surface contaminants by irradiation with UV-A light for 24 h. This immobilization method results in a titania layer which contains UV and VIS-resistant inorganic fixing material. The layer components therefore remain inert during the photocatalytic processes.
Reproducibility of the decomposition on impregnated ceramic paper
Three different ceramic papers were produced by the described impregnation method to investigate the reproducibility. Photocatalytic activities were estimated for these three ceramic papers through phenol degradation, followed during 80 min under UV irradiation. The immobilized Aeroxide P25 photocatalyst coatings displayed very similar catalytic activities (Fig. 5). The measured differences in the amounts of degraded phenol for the three different immobilized TiO2-coated ceramic papers were <1 %, i.e. the reproducibility of the preparation process was very good.
Persistence of the catalyst on impregnated ceramic paper
To demonstrate the persistence of the immobilized layer, the photocatalytic decomposition of phenol during a 2-h period was repeated five times on the same immobilized TiO2 (Aeroxide P25)-coated ceramic paper. Between the experiments, the sheet was irradiated for 24 h with UV light for the complete decomposition of the adsorbed phenol and oxidation by-products. For the five cycles, the calculated initial degradation rates were identical within the experimental error (Fig. 6). These results confirmed the stability of the coating, which was also observed visually. No detached particles were found in the phenol solutions during these experiments.
Phenol decomposition by-products
An 8-h photocatalytic experiment was carried out under UV irradiation, during which phenol was totally decomposed (its final concentration was below the detection limit). During the photodegradation of phenol, three main oxidation by-products were formed: hydroquinone, pyrocatechol and resorcinol (dihydroxybenzenes). These compounds were also degraded by this treatment during the 8-h irradiation (Fig. 7).
Experiments with different photocatalysts under visible light irradiation
Phenol decomposition experiments were carried out on immobilized Aeroxide P25 and Kronos VLP7000 TiO2 under visible light irradiation. Because of the slow degradation, the phenol concentrations were followed for 5 h (Fig. 8). The decay curves indicated a much higher activity in the case of the Kronos VLP7000-coated ceramic paper. This result confirms the possibility of the creation of photocatalytically active surfaces with higher performance through the demonstrated immobilization technique, with the application of doped, precrystallized TiO2.
Waste decomposition by solar irradiation on a semi-pilot scale
A large ceramic sheet (28 cm × 120 cm), coated with Aeroxide P25 by the described immobilization method was applied in a semi-pilot-scale flow photoreactor. Fig. 9 demonstrates that this apparatus has a very similar structure to that of the laboratory-scale flow reactor. In this system 10–50 L of model waste water could be purified. This reactor was applied to confirm that the scaling-up of the developed immobilization technique can easily be accomplished.
Outdoor solar experiments were carried out in the above-described flow reactor to demonstrate that organic contaminants can be decomposed by solar light with immobilized TiO2. The photocatalytic decontamination of 10 L of model waste water containing phenol, oxalic acid or a pesticide (monuron) was investigated on sunny days in the summer (in Szeged, Hungary). The presented results (Fig. 10) revealed that the model compounds (0.1–1 mM) were decomposed to an extent of 60–95 % after solar irradiation for 4 h.
Conclusions
In this study, a simple immobilization technique was developed to fix highly efficient precrystallized (or even doped) semiconductor nanoparticles on surfaces via an amorphous Ti(IV) oxide-hydroxide layer, which is not sensitive to the reactive radicals produced by activated TiO2 nanoparticles. The persistence was confirmed in photocatalytic experiments in which the rate of phenol decomposition remained constant throughout five 2-h cycles on the same TiO2-coated ceramic paper. The potential for application on an industrial scale was demonstrated by a pilot-plant-scale flow reactor (containing a 28 cm × 120 cm impregnated ceramic sheet), in which environmental contaminants were decomposed by solar irradiation. This immobilization method is a simple technique that can be used to investigate the long-term efficiency of novel TiO2 samples, or can even be applied in real air/water treatment processes. It seems that this method is also suitable to fix a photocatalyst on other surfaces (e.g. on the walls).
References
Černigoj U, Štangar UL, Trebše P (2007) Evaluation of a novel Carberry type photoreactor for the degradation of organic pollutants in water. J Photochem Photobiol A 188(2–3):169–176
Kwon JM, Kim YH, Song BK, Yeom SH, Kim BS, Im JB (2006) Novel immobilization of titanium dioxide (TiO2) on the fluidizing carrier and its application to the degradation of azo-dye. J Hazard Mater 134(1–3):230–236
Méndez-Arriaga F, Maldonado MI, Gimenez J, Esplugas S, Malato S (2009) Abatement of ibuprofen by solar photocatalysis process: enhancement and scale up. Catal Today 144(1–2):112–116
Khataee AR, Fathinia M, Joo SW (2013) Simultaneous monitoring of photocatalysis of three pharmaceuticals by immobilized TiO2 nanoparticles: chemometric assessment, intermediates identification and ecotoxicological evaluation. Spectrochim Acta A 112:33–45
Martínez C, Vilariño S, Fernández MI, Faria JLMC, Santaballa JA (2013) Mechanism of degradation of ketoprofen by heterogeneous photocatalysis in aqueous solution. Appl Catal B 142–143:633–646
Giraldo AL, Peñuela GA, Torres-Palma RA, Pino NJ, Palominos RA, Mansilla HD (2010) Degradation of the antibiotic oxolinic acid by photocatalysis with TiO2 in suspension. Water Res 44(18):5158–5167
Kominami H, Kumamoto H, Kera Y, Ohtani B (2001) Immobilization of highly active titanium(IV) oxide particles A novel strategy of preparation of transparent photocatalytic coatings. Appl Catal B 30:329–335
Guillard C, Disdier J, Monnet C, Dussaud J, Malato S, Blanco J, Maldonado MI, Herrmann J-M (2003) Solar efficiency of a new deposited titania photocatalyst: chlorophenol, pesticide and dye removal applications. Appl Catal B 46(2):319–332
Rao KVS, Rachel A, Subrahmanyam M, Boule P (2003) Immobilization of TiO2 on pumice stone for the photocatalytic degradation of dyes and dye industry pollutants. Appl Catal B 46(1):77–85
Rao KVS, Subrahmanyam M, Boule P (2004) Immobilized TiO2 photocatalyst during long-term use: decrease of its activity. Appl Catal B 49(4):239–249
Behnajady MA, Modirshahla N, Mirzamohammady M, Vahid B, Behnajady B (2008) Increasing photoactivity of titanium dioxide immobilized on glass plate with optimization of heat attachment method parameters. J Hazard Mater 160(2–3):508–513
Hachem C, Bocquillon F, Zahra O, Bouchy M (2001) Decolourization of textile industry wastewater by the photocatalytic degradation process. Dyes Pigment 49:117–125
Naskar S, Pillay SA, Chanda M (1998) Photocatalytic degradation of organic dyes in aqueous solution with TiO2 nanoparticles immobilized on foamed polyethylene sheet. J Photochem Photobiol A 113(3):257–264
Fabiyi ME, Skelton RL (2000) Photocatalytic mineralisation of methylene blue using buoyant TiO2-coated polystyrene beads. J Photochem Photobiol A 132(1–2):121–128
Zainal Z, Hui LK, Hussein MZ, Taufiq-Yap YH, Abdullah AH, Ramli I (2005) Removal of dyes using immobilized titanium dioxide illuminated by fluorescent lamps. J Hazard Mater 125(1–3):113–120
Atheba P, Robert D, Trokourey A, Bamba D, Weber JV (2009) Design and study of a cost-effective solar photoreactor for pesticide removal from water. Water Sci Technol 60(8):2187–2193
Fenoll J, Flores P, Hellín P, Martínez CM, Navarro S (2012) Photodegradation of eight miscellaneous pesticides in drinking water after treatment with semiconductor materials under sunlight at pilot plant scale. Chem Eng J 204–206:54–64
Mahmoodi N, Arami M, Limaee N, Gharanjig K, Nourmohammadian F (2007) Nanophotocatalysis using immobilized titanium dioxide nanoparticle degradation and mineralization of water containing organic pollutant: case study of Butachlor. Mater Res Bull 42(5):797–806
Rachel A, Lavedrine B, Subrahmanyam M, Boule P (2002) Use of porous lavas as supports of photocatalysts. Catal Commun 3:165–171
Gumy D, Rincon A, Hajdu R, Pulgarin C (2006) Solar photocatalysis for detoxification and disinfection of water: different types of suspended and fixed TiO2 catalysts study. Sol Energy 80(10):1376–1381
Antoniou MG, Dionysiou DD (2007) Application of immobilized titanium dioxide photocatalysts for the degradation of creatinine and phenol, model organic contaminants found in NASA’s spacecrafts wastewater streams. Catal Today 124(3–4):215–223
Shan AY, Ghazi TIM, Rashid SA (2010) Immobilisation of titanium dioxide onto supporting materials in heterogeneous photocatalysis: a review. Appl Catal A 389(1–2):1–8
Pozzo RL, Baltanfis MA, Cassano AE (1997) Supported titanium oxide as photocatalyst in water decontamination: state of the art. Catal Today 39:219–231
Shepharda GS, Stockenström Sonja, de Villiers David, Engelbrecht WJ, Wessels GFS (2002) Degradation of microcystin toxins in a falling film photocatalytic reactor with immobilized titanium dioxide catalyst. Water Res 36:140–146
Singh S, Mahalingam H, Singh PK (2013) Polymer-supported titanium dioxide photocatalysts for environmental remediation: a review. Appl Catal A 462–463:178–195
Hosseini SN, Borghei SM, Vossoughi M, Taghavinia N (2007) Immobilization of TiO2 on perlite granules for photocatalytic degradation of phenol. Appl Catal B 74(1–2):53–62
Scotti R, D’Arienzo M, Morazzoni F, Bellobono IR (2009) Immobilization of hydrothermally produced TiO2 with different phase composition for photocatalytic degradation of phenol. Appl Catal B 88(3–4):323–330
Lee J-M, Kim M-S, Kim B-W (2004) Photodegradation of bisphenol-A with TiO2 immobilized on the glass tubes including the UV light lamps. Water Res 38(16):3605–3613
Alrousan DMA, Polo-López MI, Dunlop PSM, Fernández-Ibáñez P, Byrne JA (2012) Solar photocatalytic disinfection of water with immobilised titanium dioxide in re-circulating flow CPC reactors. Appl Catal B 128:126–134
Noorjahan M (2004) A novel and efficient photocatalyst: TiO2-HZSM-5 combinate thin film. Appl Catal B 47(3):209–213
Tennakone K, Tilakaratne CTK, Kottegoda IRM (1995) Photocatalytic degradation of organic contaminants in water with TiO2 supported on polythene films. J Photochem Photobiol A 87:177–179
Brezova V, Jankovicova M, Soldan M, Blazkova A, Rehakova M, Surina I, Ceppan M, Havlinova B (1994) Photocatalytic degradation of p-toluenesulphonic acid in aqueous systems containing powdered an immobilized titanium-dioxide. J Photochem Photobiol A 83(1):69–75
Cho SM, Choi WY (2001) Solid-phase photocatalytic degradation of PVC-TiO2 polymer composites. J Photochem Photobiol A 143(2–3):221–228
Dunlop PSM, McMurray TA, Hamilton JWJ, Byrne JA (2008) Photocatalytic inactivation of Clostridium perfringens spores on TiO2 electrodes. J Photochem Photobiol A 196(1):113–119
Gelover S, Mondragón P, Jiménez A (2004) Titanium dioxide sol–gel deposited over glass and its application as a photocatalyst for water decontamination. J Photochem Photobiol A 165(1–3):241–246
López-Muñoz M-J, Rv Grieken, Aguado J, Marugán J (2005) Role of the support on the activity of silica-supported TiO2 photocatalysts: structure of the TiO2/SBA-15 photocatalysts. Catal Today 101(3–4):307–314
Veréb G, Manczinger L, Bozsó G, Sienkiewicz A, Forró L, Mogyorósi K, Hernádi K, Dombi A (2013) Comparison of the photocatalytic efficiencies of bare and doped rutile and anatase TiO2 photocatalysts under visible light for phenol degradation and E. coli inactivation. Appl Catal B 129:566–574
Acknowledgments
This research was supported by the European Union and the State of Hungary, co-financed by the European Social Fund in the framework of TÁMOP 4.2.4. A/2-11-1-2012-0001 ‘National Excellence Program’. This work was partially co-financed by the Swiss Contribution (SH/7/2/20). The authors are indebted to Evonik Industries and to Kronos Titan Gmbh. for supporting our work by supplying TiO2 for these studies.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Veréb, G., Ambrus, Z., Pap, Z. et al. Immobilization of crystallized photocatalysts on ceramic paper by titanium(IV) ethoxide and photocatalytic decomposition of phenol. Reac Kinet Mech Cat 113, 293–303 (2014). https://doi.org/10.1007/s11144-014-0734-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11144-014-0734-y