Abstract
Purpose
To fill the gap in knowledge about associations of health-related quality of life (HRQoL) with comorbid diabetes mellitus (DM), hypertension (HTN), and/or mild cognitive impairment (MCI) in the elderly, we explored associations of comorbid DM, HTN, and/or MCI with HRQoL.
Methods
Data for this study were from a population-based cross-sectional survey of elderly Taiwanese (≥ 65 years old). Participants (N = 4,634; 47.9% male) were categorized into eight chronic-illness groups: DM only (n = 224); HTN only (n = 1226); DM and HTN (n = 365); MCI only (n = 497); DM and MCI (n = 58); HTN and MCI (n = 303); DM, HTN, and MCI (n = 101); and none (healthy; n = 1860). Associations were examined between the eight chronic-illness groups and HRQoL (measured by EQ-5D scores) using binary logistic regression analyses and generalized linear models adjusted for covariates. Index scores were calculated from EQ-5D scores using Taiwan’s general population-preference weights.
Results
Compared to the healthy group, after adjusting covariates, MCI alone or with other comorbidities was significantly, negatively associated with HRQoL. Among all chronic-illness groups, comorbid DM, HTN, and MCI exhibited the lowest HRQoL. After adjusting covariates, between-group odds ratios for index scores were significant when comparing comorbid DM and MCI to DM only, comparing comorbid HTN and MCI to HTN only and comorbid DM, comparing HTN and MCI to comorbid DM and HTN, suggesting that MCI additively affects HRQoL.
Conclusions
HRQoL of older Taiwanese adults was negatively associated with having MCI. Thus, clinicians managing older persons with chronic illnesses should assess their cognitive function to identify high-risk groups needing HRQoL assistance.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The aging population is increasing rapidly worldwide and in Taiwan. In 2018, Taiwan became an aged society in which the proportion of older adults became 14.5%, exceeding 14% of the total population [1]. This proportion is projected to become 20.5% in 2030, creating a super-aged society, and will be at 38.6% in 2060 [2]. One of the most common clinical problems among the elderly is mild cognitive impairment (MCI) [3]. In a 2014 national survey, the prevalence of MCI in elderly Taiwanese (≥ 65 years old) was 18.76%, with prevalence increasing as age advanced [4]. Therefore, as the aging population increases, the number of people with MCI will increase dramatically and become an important health issue in Taiwan.
MCI, a syndrome defined by cognitive decline that is more severe than expected for an individual’s age and education level, does not notably interfere with affected individuals’ activities of daily living (ADL) [3]. MCI often represents a degenerative process from normal cognition to early dementia [3]. Indeed, 41% (n = 18/44) of patients with amnesic MCI in a prospective cohort study progressed to dementia by the end of a 4-year follow-up period [5]. Comorbid diabetes mellitus (DM) and hypertension (HTN) had a negative impact on cognitive function [6, 7] and increased the risk of cognitive impairment [8]. Specifically, DM and HTN were identified as risk factors for predicting progression from MCI to Alzheimer’s disease [9]. In Taiwan, DM and HTN are also highly prevalent among older persons [10]. A thorough understanding of outcomes associated with these diseases is vital in primary care settings. Therefore, it is essential to explore the impact of MCI in combination with comorbid DM and/or HTN among older persons in Taiwan.
An important outcome for evaluating the influence of disease, particularly chronic illnesses [11], and the effectiveness of medical interventions among the elderly is health-related quality of life (HRQoL). The most common chronic illnesses among older adults are DM and HTN, which often have serious complications that impact health outcomes [12]. Individuals in the general population with DM or HTN have poorer quality of life than those without [13, 14], and their quality of life decreases under comorbid conditions [15, 16]. More specifically, DM and HTN in the general population were each found to have comparable adverse effects on HRQoL [13], whereas comorbid DM and HTN had additive effects on HRQoL [17]. In older persons, DM and HTN are commonly comorbid, with a high prevalence rate [7]. However, few studies to date have examined correlates of HRQoL among older persons with comorbid DM and HTN.
Quality of life has been shown to be affected in the early stages of cognitive decline, i.e., with the onset of MCI [18]. Specifically, MCI is accompanied by increasing neuropsychiatric symptoms [19] and decreasing functional abilities [20], thus contributing significantly to poor quality of life among the elderly. For example, individuals with MCI had significantly lower quality of life (measured by the Quality of Life-Alzheimer’s Disease scale) than those with normal cognition, and their poor quality of life was associated with neuropsychiatric symptoms and functional decline [21]. Nevertheless, the relationship of MCI in combination with comorbid DM and/or HTN with HRQoL in the elderly has yet to be studied extensively, and the magnitude and profile of differences among persons with different combinations of DM, HTN, and MCI have yet to be determined.
To address these gaps in knowledge, the main purpose of this study was to explore the relationships of MCI in combination with comorbid DM and/or HTN with HRQoL in a representative sample of older persons in Taiwan. We compared HRQoL among older people with DM only, HTN only, MCI only, comorbid DM and HTN, DM and MCI, HTN and MCI, DM, HTN and MCI, and no chronic disease (i.e., healthy). We hypothesized that older individuals with DM or HTN in conjunction with MCI would have poorer HRQoL than those without MCI, that those with comorbid DM, HTN, and MCI would have the worst HRQoL of all chronic-illness groups, and that those with no chronic illness would have the best HRQoL.
Methods
Design and sample
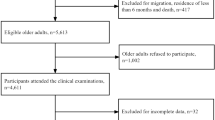
Data for this descriptive, secondary analysis study were from a subset of data used in a nationwide, population-based, cross-sectional survey of the prevalence of MCI and dementia [4]. In the original survey, a national representative sample of Taiwanese persons aged 65 and older was generated by computerized multistage random sampling from December 2011 to March 2013 [4]. Participants were surveyed in face-to-face interviews conducted by trained home-care nurses during door-to-door visits. The most frequent chronic illnesses self-reported by participants were prompted by asking, “Have you ever been told by a doctor that you have …?” We defined participants as having a chronic illness if they reported having been diagnosed with it. MCI was diagnosed in the original study based on core clinical criteria recommended by the National Institute on Aging-Alzheimer’s Association [22]. MCI was also assessed by the ADL scale, instrumental activities of daily living scale (IADL), Clinical Dementia Rating scale (CDR), and the Taiwanese Mental State Evaluation. Participants with MCI and impaired in > 1 cognitive domain but without impaired social or occupational functioning were evaluated as such by the ADL, IADL, and CDR. The original study was approved by the authors’ Institutional Review Boards, and participants in the original study provided written informed consent. The sample for the original survey comprised 10,432 older adults, including 176 proxies (1.68%) who responded instead. Of the 4634 original participants whose data were analyzed for the current study, 224 (2 proxies) had DM only, 1226 (12 proxies) had HTN only, 365 (4 proxies) had comorbid DM and HTN, 497 (7 proxies) had MCI only, 58 had comorbid DM and MCI, 303 had comorbid HTN and MCI, 101 (3 proxies) had comorbid DM, HTN, and MCI, and 1,860 (15 proxies) were healthy (without any chronic illness).
Measures
The outcome variable, HRQoL, was measured using the EuroQol Group EQ-5D-3L, comprising the EQ-5D descriptive system. This system is a standardized self-classifier of health status in the dimensions of mobility, self-care, usual activities, pain/discomfort, and anxiety/depression on three levels (no problems, some problems, and extreme problems). EQ-5D descriptive-system scores were converted to index scores (also known as preference or value weights) using Taiwan’s general population-preference weights [23]. EQ-5D scores indicate 243 possible self-rated health states, from − 0.674 (most severe impairment across all five dimensions) to 1 (no problems in any dimension).
Independence level was assessed by ADL performance (dependencies in eating, transferring, grooming, toileting, bathing, walking, climbing stairs, and dressing, along with bowel and bladder control) and IADL performance (dependencies in shopping, using transportation, handling finances, telephoning, preparing meals, housekeeping, and doing laundry). ADL and IADL scores were calculated by summing all individual item scores. ADL scores ranged from 0 to 20; higher scores indicate higher levels of independence. IADL scores ranged from 0 to 28; lower scores indicate higher levels of independence.
Statistical analyses
Participants were categorized into eight groups: no chronic disease (i.e., healthy), DM only, HTN only, MCI only, comorbid DM and HTN, DM and MCI, HTN and MCI, and DM, HTN, and MCI. Of all participants, 91 (2%) reported having extreme problems (level 3) in one or more dimensions (i.e., at least one EQ-5D score was 3). Given this relatively low number, participants’ data from EQ-5D levels 2 (some problems) and 3 (extreme problems) were combined to create the category “with problems.” All EQ-5D scores were then dichotomized into “no problems” (level 1 EQ-5D scores) or “with problems.” Binary variables (with problems = 1, no problems = 0) were generated to identify any health problems across all EQ-5D dimensions.
Participants’ characteristics included socio-demographic variables, lifestyle habits, and health status. Socio-demographics included age, gender, illiteracy status (uneducated/educated), marital status (married/single), and living status (with/without family). Lifestyle habits included smoking status (smoker/non-smoker), alcohol drinking status (drinker/non-drinker), regular exercise (at least 20 min of physical activity each week, intense enough to make one sweat), sleep quality (insomnia/no insomnia), and regular social activities (social activities at least once/week, e.g., attending clubs or social groups, engaging in religious activities, meeting friends, family, or others). Health status included independence level and body mass index (BMI). Differences in mean scores for characteristics among chronic-illness groups were determined by Chi-square tests for categorical variables and F-tests (or Welch tests when homogeneity was rejected by the variance test) for continuous variables. Older adults who were included and excluded did not differ significantly in terms of gender, illiteracy status, marital status, living status, smoking status, and BMI. Participants had a slightly lower mean age (M = 75.5, SD = 6.3) than older adults who were excluded (M = 76.8, SD = 7.0).
Associations were examined between the eight chronic-illness groups and EQ-5D health status (self-classifier and summary index) using binary logistic regression analyses (for binary variables) and generalized linear models (for continuous variables), with covariates of age, gender, illiteracy status (illiterate = 1), marital status (married = 1), living status (living with family = 1), smoking status (non-smoker = 1), alcohol drinking status (non-drinker = 1), regular exercise (yes = 1), sleep quality (good = 1), regular social activities (yes = 1), independence level (i.e., ADL and IADL scores), and BMI. The strength of associations was expressed by odds ratios (OR) and 95% confidence intervals (CI). OR measures what might be reported as equality of HRQoL among chronic-illness groups and, if the OR significantly deviated from 1, whether participants within each chronic-illness group were more likely to report different levels of HRQoL than a reference group. An effect was additive if the comorbid effect of two or more chronic illnesses on HRQoL approximated the sum of each chronic-illness’ effect on HRQoL. To provide additional insight into the additive effects of DM, HTN, and MCI on HRQoL, we tested the associations of HRQoL with comorbid DM, HTN, and/or MCI. For example, if DM had an additive effect on HRQoL, the OR would deviate significantly from 1 when comorbid DM and HTN are compared to HTN only, comorbid DM and MCI are compared to MCI only, and comorbid DM, HTN, and MCI are compared to comorbid HTN and MCI. Statistical analyses were performed using SPSS 22 for Windows (SPSS Inc., Chicago, IL).
Results
Participant characteristics
Socio-demographic characteristics, lifestyle habits, and health status of each chronic-illness group are shown in Table 1. In terms of socio-demographic characteristics, participants in all groups were on average 74.3 to 78.3 years old. Those in groups with MCI tended to be older than those without MCI. Among all groups, the proportion of males was 29.7 to 54.9%, with lower percentages of males in chronic-illness groups having MCI (29.7 and 41.4%) than in those without (45.5 and 54.9%). A similar pattern was found for the proportion of illiterate participants (24.1 to 60.4%). Similarly, lower proportions of participants in chronic-illness groups with MCI had regular exercise, good sleep quality, and regular social activities than those in groups without MCI. In terms of independence level, groups with MCI had mean ADL scores of 19.3 to 19.6 and IADL scores of 10.5 to 12.5, indicating greater dependence than groups without MCI, whose ADL scores were 19.8 to 19.9 and IADL scores were 7.8 to 7.9. The average BMI of all participants ranged from 23.3 to 25.2. Among all chronic-illness groups, participants with comorbid DM and HTN had the highest BMI (25.2).
Comparison of HRQoL among chronic-illness groups
The percentages of participants reporting problems in each EQ-5D dimension are summarized in Table 2. Of the five EQ-5D dimensions of HRQoL, pain/discomfort was the most frequently reported problem. More specifically, the mean percentages of individuals with MCI reporting pain/discomfort problems ranged from 34.1 to 52.5%, whereas the averages for participants without MCI ranged from 15.7 to 24.0%. Similar results were found for other EQ-5D dimensions. More participants with MCI (5.2 to 12.9%) reported self-care problems than did those without MCI (0.5 to 1.1%), despite self-care being the dimension least reported as a problem. The group with comorbid DM, HTN, and MCI reported the most problems, with 26.7% reporting problems in mobility, 12.9% in self-care, 33.7% in usual activities, 52.5% in pain/discomfort, and 24.8% in anxiety/depression.
In terms of mean index scores (Table 2), the healthy group had the highest score (M = 0.88, SD = 0.25), followed by the group with HTN only (M = 0.84, SD = 0.27). The group with comorbid DM, HTN, and MCI had the lowest mean index score (M = 0.54, SD = 0.37), and all groups with comorbid MCI had lower mean index scores than those without MCI.
Associations of comorbid DM, HTN, and/or MCI with HRQoL
The odds ratios for HRQoL of each chronic-illness group, after adjusting for covariates, are compared to those of the healthy group given in Table 3. In terms of EQ-5D index scores, each chronic-illness group was associated with significantly poorer scores than the healthy group (coefficient estimate [b] = − 0.05, p = 0.01 for DM, b = − 0.03, p = 0.006 for HTN, b = − 0.04, p = 0.016 for comorbid DM and HTN, b = − 0.10, p < 0.001 for MCI, b = − 0.15, p < 0.001 for comorbid DM and MCI, b = − 0.11, p < 0.001 for comorbid HTN and MCI, b = − 0.21, p < 0.001 for comorbid DM, HTN, and MCI). Moreover, all groups with MCI were more negatively associated with index scores than groups without MCI. In terms of EQ-5D dimensions, problems in each dimension were more likely to be experienced by individuals in groups with MCI than those in the healthy group. All groups with MCI had higher likelihoods of reporting HRQoL problems than the groups without MCI. Participants with comorbid DM, HTN, and MCI were the most likely among all chronic-illness groups to have problems across all EQ-5D dimensions. Compared to the healthy group, individuals in the DM, HTN, and MCI group were 4.68 times more likely to report problems in mobility (95% CI 2.31, 9.49, p < 0.001), 10.71 times more likely to report problems in self-care (95% CI 2.95, 38.89, p < 0.001), 5.27 times more likely to report problems in usual activities (95% CI 2.61, 10.67, p < 0.001), 3.51 times more likely to report problems in pain/discomfort (95% CI 2.14, 5.77, p < 0.001), and 2.58 times more likely to report problems in anxiety/depression (95% CI 1.45, 4.58, p = 0.001).
To clarify whether the effects of DM, HTN, or MCI on HRQoL were additive, we tested the ORs for HRQoL by comparing chronic-illness groups on each additive effect (Table 4). After controlling for covariates, ORs for EQ-5D health status (self-classifier and index) did not deviate significantly from 1 when comorbid DM and HTN were compared to HTN only and comorbid DM and MCI were compared to MCI only, indicating that DM did not have an additive effect on reducing HRQoL. Similar results were found for the effect of HTN. In contrast, ORs for index scores were significant between groups when comorbid DM and MCI were compared to DM only, comorbid HTN and MCI were compared to HTN only, and comorbid DM, HTN, and MCI were compared to comorbid DM and HTN. These results suggest that MCI has an additive effect on HRQoL. However, in terms of EQ-5D dimensions, not all comparisons for testing the additive effect of MCI had significant results. Notably, compared to participants with HTN, participants with comorbid HTN and MCI had the greatest OR of reporting problems in anxiety/depression (OR = 1.94, 95% CI 1.33, 2.83, p = 0.001). Similarly, compared to participants with comorbid DM and HTN, participants with comorbid DM, HTN, and MCI had the greatest OR of reporting problems in usual activities (OR = 3.59, 95% CI 1.56, 8.26, p = 0.003).
Discussion
The main finding of this study is that older Taiwanese diagnosed with MCI had a significantly greater OR for poor HRQoL than those diagnosed with various combinations of DM and/or HTN [18, 24]. Similarly, cognitive impairment was reported to be the strongest predictor of HRQoL for older persons with chronic illness in Taiwan, and it alone explained 27% of the variance in HRQoL [25]. Furthermore, better cognitive function was associated with higher EQ-5D scores in older community-dwelling persons in Taiwan [26]. Expanding on these findings, our study demonstrates that MCI alone or with other chronic illnesses (DM or HTN) is negatively associated with HRQoL when compared to healthy older Taiwanese adults. These associations with HRQoL were stronger than those of chronic illnesses without MCI. Among the different chronic-illness groups with comorbid MCI, the lowest HRQoL was among participants with comorbid DM, HTN, and MCI.
Our findings confirm the negative associations of MCI with HRQoL among the elderly in Taiwan [25, 26] and are noteworthy in two regards. First, individuals with MCI were reported to have significantly lower HRQoL than those with normal cognition, and this lower HRQoL was associated with neuropsychiatric symptoms and functional decline [21]. Neuropsychiatric symptoms such as depression, apathy, and anxiety are commonly present with MCI [19]. Indeed, our participants with MCI, either alone or with other comorbidities, had 2.28 to 2.88 times the likelihood of reporting a problem in the anxiety/depression dimension than individuals with normal cognition. Moreover, the OR of reporting problems in anxiety/depression was greatest when comorbid HTN and MCI was compared to HTN only. Depression and cognitive impairment have been found to severely and negatively impact the health and quality of life of older persons with chronic diseases [25], patients with early Parkinson’s disease [27], and people with dementia in long-term care facilities [28]. Accordingly, treating cognitive impairment and alleviating depressive symptoms may play important roles in improving HRQoL [29].
Second, we found that participants with MCI had a greater OR than those without MCI of reporting problems in the EQ-5D dimension of usual activities (e.g., work, study, housework, family, or leisure activities). Additionally, participants with comorbid DM, HTN, and MCI had the greatest OR of reporting problems in usual activities than participants with comorbid DM and HTN. These results confirm that MCI is associated with significant difficulties in performing IADL [20, 30], which require more complex neuropsychological functioning and are therefore more likely to deteriorate with cognitive decline [31]. Diminished IADL performance was also found to be negatively associated with quality of life in older persons with chronic diseases in Taiwan [25, 26]. Accordingly, the likelihood of improving HRQoL in older persons with MCI may be increased by interventions focusing on neuropsychiatric symptoms and/or IADL impairment [25, 26, 29].
We found that, across chronic-illness groups, the most frequently reported HRQoL problem was pain/discomfort, indicating that pain/discomfort was a critical factor in decreasing HRQoL. Our findings are consistent with a report that pain/discomfort was a common problem among a sample of Canadians (≥ 18 years) with chronic illnesses [32]. In contrast, we found the lowest percentages of HRQoL problems across each chronic-illness group in self-care, as was reported for community-dwelling older adults (≥ 65 years) in Britain [33] and the Netherlands [34].
Our study likewise provides evidence that older Taiwanese with DM only, HTN only, or comorbid DM and HTN have significantly greater ORs of poorer HRQoL in index scores than older people without chronic illness. Similarly, DM and HTN had a significant negative impact on outpatients’ HRQoL in Hong Kong, and outpatients with one or more comorbidities had lower HRQoL scores than those without comorbidities [35]. Nevertheless, we found that comorbid DM and HTN did not have an additive effect in reducing HRQoL. On the other hand, our results are inconsistent with previous findings that comorbid DM and HTN had comparable adverse effects in Croatian adults [13] or an additive effect in ethnic Chinese, Malay, and Indian adults [17] on HRQoL when compared to those with DM or HTN only. These differing results might be due to different sampling criteria regarding age; our participants were ≥ 65 years old, whereas the other samples were ≥ 18 years old [13] and 21 to 65 years old [17]. Nonetheless, more than 50% of patients with DM was also diagnosed with HTN, making it difficult to differentiate between the associations of HRQoL with DM, HTN, and/or comorbid DM and HTN, as they shared a common risk for cardiovascular disease or mortality [36]. Since understanding the negative associations of comorbid DM and HTN with HRQoL is critical for older persons with DM to effectively self-manage their disease each day, these associations warrant further investigation.
The results of this study have several implications. First, given that cognitive function is not routinely assessed in current practice, clinicians managing older persons with DM and HTN should assess their cognitive function to identify high-risk groups needing assistance with HRQoL. Second, when these high-risk older persons with DM and HTN also have MCI, their needs must be considered, especially in terms of managing anxiety/depression. Routine screening for depression among older persons with DM, HTN, and MCI is suggested for timely diagnoses and interventions. Third, specific attention needs to be paid to individuals with comorbid DM, HTN, and MCI because they are most vulnerable to poor HRQoL across all dimensions, but particularly in mobility, self-care, and usual activities. Impairment in mobility often leads to falls [37] and impairment in self-care ability suggests the need for home-care visits and caregivers, requiring the attention of geriatric health care providers [25]. Finally, our results can serve as a basis for developing health care policies for older persons with chronic illnesses and MCI. For example, policies can be established to provide routine screening of cognitive function for persons with DM and HTN in national chronic-illness prevention and management programs. Also, mechanisms can be developed to regularly assess HRQoL in persons with DM and/or HTN with MCI to provide timely interventions or referrals for related services.
This study had several limitations. First, diagnoses of DM and HTN were self-reported, and participants were taking medications without medical prescriptions, which may have limited the validity of the diagnoses. Strict adherence to prescribed antihypertensive medication has been positively correlated with HRQoL in older adults [38], suggesting the need for further study. Second, the study design was cross-sectional, preventing confirmation of causal relationships between chronic illnesses and HRQoL. Third, participants’ cognitive function was assessed primarily by clinical history, the Mini-Mental State Examination, and CDR, without a detailed psychiatric evaluation to exclude other major disorders that may have been misdiagnosed as MCI. Finally, other factors besides those examined in this study might have affected HRQoL. For example, depression was negatively associated with HRQoL in older Taiwanese persons with chronic diseases [25], suggesting the need for further study with more confounders.
Conclusion
This secondary analysis of a nationwide epidemiological study found that MCI, alone or in conjunction with other chronic illnesses, was significantly associated with the HRQoL of older persons living in Taiwan. These associations with HRQoL were stronger than the associations of chronic illnesses without MCI. Among the chronic-illness groups with comorbid MCI, the lowest HRQoL was found among participants with comorbid DM, HTN, and MCI. We also found that DM or HTN only and comorbid DM and HTN were negatively associated with HRQoL, but the effects of comorbid DM and HTN on HRQoL were not additive. In contrast, MCI might have a negative additive effect on HRQoL. Our findings indicate that clinicians managing older persons with chronic illnesses should assess cognitive function to identify high-risk patients needing assistance across all HRQoL dimensions, particularly in anxiety/depression and usual activities.
References
Department of Statistics, Ministry of Interior, Taiwan. (2018). Monthly bulletin of interior statistics: 1.5 Population by age of 0–14, 15–64, 65+ and by 6-year age group [Data file]. Retrieved from https://www.moi.gov.tw/files/site_stuff/321/1/month/m1-05.xls.
National Development Council, Taiwan. (2016). Population projections for R.O.C. (Taiwan): 2016–2060. Retrieved from https://www.ndc.gov.tw/en/cp.aspx?n=2E5DCB04C64512CC.
Eshkoor, S. A., Hamid, T. A., Mun, C. Y., & Ng, C. K. (2015). Mild cognitive impairment and its management in older people. Clinical Interventions in Aging, 10, 687–693. https://doi.org/10.2147/CIA.S73922.
Sun, Y., Lee, H. J., Yang, S. C., Chen, T. F., Lin, K. N., Lin, C. C., et al. (2014). A nationwide survey of mild cognitive impairment and dementia, including very mild dementia, in Taiwan. PLoS ONE, 9(6), e100303. https://doi.org/10.1371/journal.pone.0100303.
Lonie, J. A., Parra-Rodriguez, M. A., Tierney, K. M., Herrmann, L. L., Donaghey, C., O’Carroll, R. E., et al. (2010). Predicting outcome in mild cognitive impairment: 4-year follow-up study. The British Journal of Psychiatry, 197(2), 135–140. https://doi.org/10.1192/bjp.bp.110.077958.
Ryuno, H., Kamide, K., Gondo, Y., Kabayama, M., Oguro, R., Nakama, C., et al. (2017). Longitudinal association of hypertension and diabetes mellitus with cognitive functioning in a general 70-year-old population: The SONIC study. Hypertension Research, 40(7), 665–670. https://doi.org/10.1038/hr.2017.15.
Hassing, L. B., Hofer, S. M., Nilsson, S. E., Berg, S., Pedersen, N. L., McClearn, G., et al. (2004). Comorbid type 2 diabetes mellitus and hypertension exacerbates cognitive decline: Evidence from a longitudinal study. Age and Ageing, 33(4), 355–361.
Hazari, M. A. H., Reddy, B. R., Uzma, N., & Kumar, B. S. (2015). Cognitive impairment in type 2 diabetes mellitus. International Journal of Diabetes Mellitus, 3(1), 19–24. https://doi.org/10.1016/j.ijdm.2011.01.001.
Li, J. Q., Tan, L., Wang, H. F., Tan, M. S., Tan, L., Xu, W., et al. (2015). Risk factors for predicting progression from mild cognitive impairment to Alzheimer’s disease: A systematic review and meta-analysis of cohort studies. Journal of Neurology, Neurosurgery, and Psychiatry, 87(5), 476–484. https://doi.org/10.1136/jnnp-2014-310095.
Chi, M. J., Lee, C. Y., & Wu, S. C. (2011). The prevalence of chronic conditions and medical expenditures of the elderly by chronic condition indicator (CCI). Archives of Gerontology and Geriatrics, 52(3), 284–289. https://doi.org/10.1016/j.archger.2010.04.017.
Musekamp, G., Bengel, J., Schuler, M., & Faller, H. (2016). Improved self-management skills predict improvements in quality of life and depression in patients with chronic disorders. Patient Education and Counseling, 99(8), 1355–1361. https://doi.org/10.1016/j.pec.2016.03.022.
Kirkman, M. S., Briscoe, V. J., Clark, N., Florez, H., Haas, L. B., Halter, J. B., et al. (2012). Diabetes in older adults. Diabetes Care, 35(12), 2650–2664. https://doi.org/10.2337/dc12-1801.
Poljičanin, T., Ajduković, D., Šekerija, M., Pibernik-Okanović, M., Metelko, Ž, & Mavrinac, G. V. (2010). Diabetes mellitus and hypertension have comparable adverse effects on health-related quality of life. BMC Public Health, 10(1), 12. https://doi.org/10.1186/1471-2458-10-12.
Trevisol, D. J., Moreira, L. B., Kerkhoff, A., Fuchs, S. C., & Fuchs, F. D. (2011). Health-related quality of life and hypertension: A systematic review and meta-analysis of observational studies. Journal of Hypertension, 29(2), 179–188. https://doi.org/10.1097/HJH.0b013e328340d76f.
Solli, O., Stavem, K., & Kristiansen, I. S. (2010). Health-related quality of life in diabetes: The associations of complications with EQ-5D scores. Health and Quality of Life Outcomes, 8(1), 18. https://doi.org/10.1186/1477-7525-8-18.
Green, A. J., Bazata, D. D., Fox, K. M., & Grandy, S. (2012). Quality of life, depression, and healthcare resource utilization among adults with type 2 diabetes mellitus and concomitant hypertension and obesity: A prospective survey. Cardiology Research and Practice. 2012. https://doi.org/10.1155/2012/404107.
Wee, H. L., Cheung, Y. B., Li, S. C., Fong, K. Y., & Thumboo, J. (2005). The impact of diabetes mellitus and other chronic medical conditions on health-related Quality of Life: Is the whole greater than the sum of its parts? Health and Quality of Life Outcomes, 3(1), 2.
Bárrios, H., Narciso, S., Guerreiro, M., Maroco, J., Logsdon, R., & De Mendonça, A. (2013). Quality of life in patients with mild cognitive impairment. Aging & Mental Health, 17(3), 287–292. https://doi.org/10.1080/13607863.2012.747083.
Apostolova, L. G., & Cummings, J. L. (2008). Neuropsychiatric manifestations in mild cognitive impairment: A systematic review of the literature. Dementia and Geriatric Cognitive Disorders, 25(2), 115–126. https://doi.org/10.1159/000112509.
Jekel, K., Damian, M., Wattmo, C., Hausner, L., Bullock, R., Connelly, P. J., et al. (2015). Mild cognitive impairment and deficits in instrumental activities of daily living: A systematic review. Alzheimer’s Research & Therapy, 7(1), 17. https://doi.org/10.1186/s13195-015-0099-0.
Teng, E., Tassniyom, K., & Lu, P. H. (2012). Reduced quality-of-life ratings in mild cognitive impairment: Analyses of subject and informant responses. The American Journal of Geriatric Psychiatry, 20(12), 1016–1025. https://doi.org/10.1097/JGP.0b013e31826ce640.
McKhann, G. M., Knopman, D. S., Chertkow, H., Hyman, B. T., Jack, C. R., Kawas, C. H., et al. (2011). The diagnosis of dementia due to Alzheimer’s disease: Recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimer’s & Dementia, 7(3), 263–269. https://doi.org/10.1016/j.jalz.2011.03.005.
Lee, H. Y., Hung, M. C., Hu, F. C., Chang, Y. Y., Hsieh, C. L., & Wang, J. D. (2013). Estimating quality weights for EQ-5D (EuroQol-5 dimensions) health states with the time trade-off method in Taiwan. Journal of the Formosan Medical Association, 112(11), 699–706. https://doi.org/10.1016/j.jfma.2012.12.015.
Johansson, M. M., Marcusson, J., & Wressle, E. (2012). Cognition, daily living, and health-related quality of life in 85-year-olds in Sweden. Aging, Neuropsychology, and Cognition, 19(3), 421–432. https://doi.org/10.1080/13825585.2011.629290.
Chen, H. M., & Chen, C. M. (2017). Factors associated with quality of life among older adults with chronic disease in Taiwan. International Journal of Gerontology, 11(1), 12–15. doi: 0.1016/j.ijge.2016.07.002.
Yang, K.-F., Hsu, C.-H., Tang, Y.-J., & Kung, C.-C. (2012). Correlation among activities of daily living, quality of life, and sense of well-being in elderly community dwellers. Taiwan Geriatrics & Gerontology, 7(4), 217–232.
Lawson, R. A., Yarnall, A. J., Duncan, G. W., Khoo, T. K., Breen, D. P., Barker, R. A., et al. (2014). Severity of mild cognitive impairment in early Parkinson’s disease contributes to poorer quality of life. Parkinsonism & Related Disorders, 20(10), 1071–1075. https://doi.org/10.1016/j.parkreldis.2014.07.004.
Beerens, H. C., Zwakhalen, S. M., Verbeek, H., Ruwaard, D., & Hamers, J. P. (2013). Factors associated with quality of life of people with dementia in long-term care facilities: A systematic review. International Journal of Nursing Studies, 50(9), 1259–1270. https://doi.org/10.1016/j.ijnurstu.2013.02.005.
Bourassa, K. J., Memel, M., Woolverton, C., & Sbarra, D. A. (2017). Social participation predicts cognitive functioning in aging adults over time: comparisons with physical health, depression, and physical activity. Aging & Mental Health, 21(2), 133–146. https://doi.org/10.1080/13607863.2015.1081152.
Lindbergh, C. A., Dishman, R. K., & Miller, L. S. (2016). Functional disability in mild cognitive impairment: A systematic review and meta-analysis. Neuropsychology Review, 26(2), 129–159. https://doi.org/10.1007/s11065-016-9321-5.
Reppermund, S., Sachdev, P. S., Crawford, J., Kochan, N. A., Slavin, M. J., Kang, K., et al. (2011). The relationship of neuropsychological function to instrumental activities of daily living in mild cognitive impairment. International Journal of Geriatric Psychiatry, 26(8), 843–852. https://doi.org/10.1002/gps.2612.
Agborsangaya, C. B., Lau, D., Lahtinen, M., Cooke, T., & Johnson, J. A. (2013). Health-related quality of life and healthcare utilization in multimorbidity: Results of a cross-sectional survey. Quality of Life Research, 22(4), 791–799. https://doi.org/10.1007/s11136-012-0214-7.
Parker, L., Moran, G. M., Roberts, L. M., Calvert, M., & McCahon, D. (2014). The burden of common chronic disease on health-related quality of life in an elderly community-dwelling population in the UK. Family Practice, 31(5), 557–563. https://doi.org/10.1093/fampra/cmu035.
Mangen, M. J. J., Bolkenbaas, M., Huijts, S. M., van Werkhoven, C. H., Bonten, M. J., & de Wit, G. A. (2017). Quality of life in community-dwelling Dutch elderly measured by EQ-5D-3L. Health and Quality of Life Outcomes, 15(1), 3. https://doi.org/10.1186/s12955-016-0577-5.
Xu, R. H., Cheung, A. W. L., & Wong, E. L. Y. (2017). Examining the health-related quality of life using EQ-5D-5L in patients with four kinds of chronic diseases from specialist outpatient clinics in Hong Kong SAR, China. Patient Preference and Adherence, 11, 1565–1572. https://doi.org/10.2147/PPA.S143944.
Lastra, G., Syed, S., Kurukulasuriya, L. R., Manrique, C., & Sowers, J. R. (2014). Type 2 diabetes mellitus and hypertension: An update. Endocrinology and Metabolism Clinics, 43(1), 103–122. https://doi.org/10.1016/j.ecl.2013.09.005.
Xu, T., Clemson, L., O’Loughlin, K., Lannin, N. A., Dean, C., & Koh, G. (2018). Risk factors for falls in community stroke survivors: A systematic review and meta-analysis. Archives of Physical Medicine and Rehabilitation, 99(3), 563–573.e5. https://doi.org/10.1016/j.apmr.2017.06.032.
Holt, E. W., Muntner, P., Joyce, C. J., Webber, L., & Krousel-Wood, M. A. (2010). Health-related quality of life and antihypertensive medication adherence among older adults. Age and Ageing, 39(4), 481–487. https://doi.org/10.1093/ageing/afq040.
Acknowledgements
The authors would like to express their gratitude to the Taiwan Alzheimer’s Disease Association for generously allowing us to use an invaluable database that they partly developed and supporting us under the commission of the Ministry of Health and Welfare of Taiwan (DOH100-TD-M113-100001). The views expressed throughout this study are exclusively those of the authors and do not represent the views or opinions of the Ministry of Health and Welfare, Taiwan.
Funding
This work was supported by the Chang Gung Medical Foundation [grant numbers BMRP297, CMRPD1E0162] and Healthy Aging Research Center, Chang Gung University, Taiwan [grant number EMRPD1H0361, EMRPD1H0551].
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
The data were approved by the National Taiwan University Hospital’s Institutional Review Board. Informed consent was obtained from the participants or their proxy.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Liu, HY., Tsai, WC., Chiu, MJ. et al. Mild cognitive impairment in combination with comorbid diabetes mellitus and hypertension is negatively associated with health-related quality of life among older persons in Taiwan. Qual Life Res 28, 1281–1291 (2019). https://doi.org/10.1007/s11136-019-02101-3
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11136-019-02101-3