Abstract
Purpose
Both metabolic syndrome (MetS) and cognitive dysfunction impair health-related quality of life (HRQOL). This study aims to determine whether individuals experiencing both MetS and cognitive dysfunction have lower HRQOL.
Methods
This cross-sectional study enrolled 567 participants who attended outpatient clinics at a medical center in northern Taiwan. MetS was diagnosed according to the modified criteria for the Asian population. Cognitive function was categorized as normal, mild cognitive dysfunction, and advanced cognitive dysfunction according to the score of the Montreal Cognitive Assessment, Taiwanese version. HRQOL was assessed using the SF-36v2® Health Survey (SF-36v2). The associations of the comorbidity status of MetS and cognitive dysfunction with HRQOL were analyzed using linear regression models, adjusting for age, sex, marital status, education level, income groups, and activities of daily living.
Results
Out of 567 participants, 33 (5.8%) had MetS with mild cognitive dysfunction, and 34 (6.0%) had MetS with advanced cognitive dysfunction. Participants with both MetS and advanced cognitive dysfunction exhibited the lowest scores in the physical component summary and almost all scales of HRQOL. MetS exacerbated the inverse association between mild cognitive dysfunction and the mental component summary. For those with MetS, the scores on scales of role physical, bodily pain, vitality, and social functioning worsened as cognitive function deteriorated (all Ptrend<0.05).
Conclusion
As the severity of comorbidity between MetS and cognitive dysfunction varies, patients exhibited poorer performance in different aspects of HRQOL. Future research is needed to find solutions to improve HRQOL for patients with both MetS and cognitive dysfunction.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Metabolic syndrome (MetS), characterized by a constellation of metabolic disorders, is currently a formidable global health concern. Its association with increased risks of type 2 diabetes mellitus, chronic renal diseases, cardiovascular diseases, and all-cause mortality underscores its significant impact on overall health [1,2,3]. The burden of metabolic diseases is substantial, with obesity contributing to the highest number of deaths in 2019 (5.0 million), followed by hyperlipidemia (4.3 million), type 2 diabetes mellitus (1.4 million), and hypertension (1.1 million) [4]. Moreover, MetS has been linked to an increased incidence of mild cognitive impairment and the development of dementia [5].
MetS and cognitive dysfunction are closely related. Many studies have found that MetS accelerates the rate of cognitive decline, and the affected domains include global cognition, memory, executive functions, and attention [6]. Patients with MetS are more likely to experience cognitive impairment due to systemic inflammation and neurodegeneration [7,8,9]. MetS component, such as hyperglycemia, can lead to neurological damage in the brain [10], was found to be associated with amnestic mild cognitive impairment [11]. Additionally, MetS patients are prone to atherosclerosis, leading to cerebral hypoperfusion, which further contributes to cognitive dysfunction [8]. On the flip side, cognitive dysfunction can lead to overeating due to altered neural responses to food, impaired memory, and executive function [12]. This overeating can prompt weight gain and worsen MetS. Therefore, experiencing both MetS and cognitive dysfunction can exacerbate each condition individually (Fig. 1) and potentially worsen other health indicators associated with each disease.
Conceptual framework of the study hypotheses. Metabolic syndrome and cognitive dysfunction are closely related and affect each other. Thus, the coexistence of metabolic syndrome and cognitive dysfunction is possibly associated with a poorer health-related quality of life through impaired physical and mental health
Cognitive dysfunction is a broad term referring to abnormalities in cognitive function, ranging from mild cognitive impairment to dementia. It includes different domains of cognitive impairment and various etiologies. Cognitive impairment in learning, memory, attention, language, motor speed, executive functions, and visuospatial processing can significantly impact an individual’s social functioning and roles [13]. Cognitive dysfunction also causes limitations in physical function, affecting activities of daily living (ADL) and instrumental activities of daily living (IADL) [14, 15]. The correlation between cognitive dysfunction and lower health-related quality of life (HRQOL) is evident. Older adults experiencing cognitive dysfunction more likely to report pain, discomfort, anxiety, and depression [16]. Studies by Stites et al. also highlight a connection between cognitive dysfunction and lower quality of life, including lower satisfaction in physical well-being, family, marital status, living, and financial situation [17].
In addition to cognitive dysfunction, MetS is correlated with compromised HRQOL in both physical and mental domains [18]. A persistent MetS status has been found to adversely affect mental HRQOL, particularly in vitality and mental health [19]. Individuals with MetS are also more susceptible to limited mobility and depressive symptoms [20, 21]. Furthermore, those with mild cognitive impairment exhibit limited physical performance compared to those with normal cognition [14]. Depression is common in cognitive dysfunction [22]. Together with the close association between MetS and cognitive dysfunction, we hypothesize that the convergence of MetS and cognitive dysfunction poses a dual threat to HRQOL through impaired physical function and mental health (Fig. 1). However, research investigating the association of the coexistence of MetS and cognitive dysfunction with HRQOL remains scarce.
This cross-sectional study aims to fill this research gap by examining the association of the comorbidity status of MetS and cognitive dysfunction with HRQOL. We infer that individuals with both MetS and cognitive dysfunction will experience the poorer HRQOL than those with MetS alone or cognitive dysfunction alone. To the best of our knowledge, no study has explored the association of MetS and cognitive dysfunction with HRQOL. Understanding this crucial issue can empower healthcare providers to adopt a holistic perspective in caring for these patients, enabling them to deliver more compassionate and comprehensive care.
Methods
Study design and study population
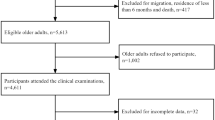
This study was a cross-sectional study. Participants were recruited from the health examinations and outpatient clinics of the Taipei Veterans General Hospital, Taipei, Taiwan during 2020–2023. Community-dwelling adults aged 50 and above, with the capacity for independent behavior, were invited to participate in this study. Exclusion criteria included severe cognitive dysfunction (inability to comprehend the contents of the informed consent form or diagnosed with moderate to severe dementia) and residency in institutions (Fig. 2). Eligible individuals who agreed to participate in this study needed to sign a written informed consent. The study received approval from the Research Ethics Committee of Taipei Veterans General Hospital, Taipei, Taiwan, on May 9, 2022 (Protocol Code: 2020-06-001 A). The design and execution of this study fully comply with relevant regulations. For estimating the sample size, we set the effect size to 0.02 (indicating a small effect size), with α = 0.05 and power = 0.90, resulting in a minimum sample size of 528.
During the interview, research assistants guided participants in completing a questionnaire to collect the necessary information for the study. This information includes details such as age, sex, marital status, education level, income level, religion, smoking, alcohol consumption, past medical history, medication use, ADL, IADL, as well as assessments of cognitive function and HRQOL. The basic physical function was evaluated using the Barthel Index [23]. The Barthel Index comprises 10 items, including feeding, bathing, grooming, dressing, bowel and bladder function, toilet use, transfers, mobility, and stair climbing. The total ADL score ranges from 0 to 100. The Lawton IADL scale is the most commonly used instrument for assessing IADL [24]. In this study, a slightly adapted Chinese version was used to evaluate 8 domains [25]. These domains include ability to use telephone, shopping, food preparation, housekeeping, laundry, mode of transportation, responsibility for own medications, and ability to handle finances. The total IADL score ranges from 0 to 24, allowing for a more detailed differentiation of the ability to live independently. Details of the questionnaire are provided in the supplements.
Definition of metabolic syndrome
The diagnostic criteria used to identify MetS adhered to the guidelines outlined in the National Cholesterol Education Program Adult Treatment Panel III (ATP III) [26]. MetS was diagnosed based on the presence of three or more than three of the following components: (1) abdominal obesity, defined as a waist circumference of ≥ 90 cm in men and ≥ 80 cm in women for the Asian population [26]; (2) hypertriglyceridemia, indicated by a serum triglyceride level ≥ 150 mg/dl or undergoing drug treatment; (3) low serum high-density lipoprotein cholesterol (HDL-C), with HDL-C < 40 mg/dL for men or < 50 mg/dL for women, or undergoing drug treatment; (4) hypertension, characterized by blood pressure ≥ 130/85 mmHg or undergoing drug treatment; and (5) hyperglycemia, indicated by a serum fasting glucose level ≥ 100 mg/dl or undergoing drug treatment.
The waist circumference was measured by experienced nurses at the midpoint between the inferior margin of the ribs and the superior border of the iliac crest at the end of expiration. The biochemical analyses, including serum glucose, triglyceride, and HDL-C, were conducted using the automatic chemistry analyzer in the central laboratory of Taipei Veterans General Hospital.
Global cognition assessment
The assessment of global cognitive function was conducted using the Montreal Cognitive Assessment, Taiwanese version (MoCA-T), version 7.0. The MoCA-T evaluates the following domains: visuospatial/executive functions, naming, verbal memory, attention, language, abstraction, delayed verbal memory, and orientation. The form and administration instructions of the MoCA-T test are available from the official MoCA website at http://www.mocatest.org.
The cut-off points for mild cognitive dysfunction and advanced cognitive dysfunction in this study were determined based on Tsai et al.‘s validated research of the MoCA-T in Taiwanese populations [27]. The cut-off values were set at 23/24 and 21/22 for mild cognitive dysfunction and advanced cognitive dysfunction, respectively. According to Tsai’s report, these cut-off values yield a sensitivity and specificity of 92% and 78% for mild cognitive dysfunction, and 98% and 95% for advanced cognitive dysfunction.
Evaluation of health-related quality of life
The Short Form Health Survey (SF-36) is a widely used instrument for quality of life assessment [28]. In this study, we used Taiwanese version of SF-36v2® [29]. The SF-36 questionnaires consist of eleven major sections to investigate the physical and mental health status of the participants. The responses from these sections can be transformed with different degrees of weighting and direction, constituting scores for eight scales, including physical functioning, role participation with physical health problems (role-physical), bodily pain, general health, vitality, social functioning, role participation with emotional health problems (role-emotional), and mental health domains. The eight scales then go through a standardization process, where they’ll be multiplied by specific coefficients and then computed to produce scores for both physical and mental components. Detailed information regarding the scoring procedures can be found in the user’s manual for the SF-36 [30].
In a previous study involving 1,180 Chinese participants, the reliability and validity of the SF-36 were analyzed. The study found that the practice factor loadings for the eight scales of the SF-36 performed well on a single factor, with loadings ranging from 0.66 to 0.80 for the physical component summary (PCS) and from 0.71 to 0.86 for the mental component summary (MCS). However, the scale of social functioning had factor loadings of 0.61 for PCS and 0.60 for MCS, indicating a moderate correlation with both factors. Reliability analysis showed that the overall Cronbach’s alpha coefficient of the SF-36 questionnaire was 0.82, and the Cronbach’s alpha coefficients for all eight domains were greater than 0.70 [31].
Grouping
Through the combinations of MetS status and cognitive function level, we created a variable with the following six groups: no MetS and normal cognitive function, no MetS with mild cognitive dysfunction, no MetS with advanced cognitive dysfunction, MetS and normal cognitive function, MetS with mild cognitive dysfunction, and MetS with advanced cognitive dysfunction. The main analysis used individuals without MetS and with normal cognitive function as the reference group, comparing the HRQOL with the other five groups.
We further conducted trend tests to determine if the eight scales of the SF-36 changed with the progression of cognitive dysfunction in patients with and without MetS [32]. In the subgroup analyses, participants with MetS were compared across normal cognitive function, mild cognitive dysfunction, and advanced cognitive dysfunction. Similarly, participants without MetS were compared across these three levels of cognitive function. In both analyses, participants with normal cognitive function were used as the control group.
Statistical analyses
For the univariate analysis, categorical variables were assessed using Chi-square tests and Fisher’s exact tests, while continuous variables were examined through the Mann-Whitney U tests and Kruskal-Wallis tests. We calculated Cronbach’s α for reliability measures of scales used in this study, including ADL, IADL, and SF-36. Multivariable linear regression models were used to investigate the association of the comorbidity status of MetS and cognitive dysfunction with HRQOL, adjusting for age, sex, marital status, educational level, income level, and ADL. The analysis of outcomes includes PCS, MCS, and the eight scales of the SF-36. All analyses were performed using SAS version 9.4 (SAS Institute Inc., Cary, NC, USA). Statistical significance was defined as a two-tailed p-value < 0.05.
Sensitivity analyses
Comorbidity is a possible confounder that may affect HRQOL. We further adjusted for a history of ischemic heart disease, stroke, and malignancy for sensitivity analysis.
Results
There were 567 adults eligible for the study. Out of 567 eligible adults, 8 participants had incomplete blood data, but these items were not included as analytic variables in this study. Therefore, 567 eligible participants entered the analysis (Fig. 2). The reliability measures showed high internal reliability for ADL, IADL, and most dimensions of SF-36, (data not showing in table; Cronbach’s α = 0.89, 0.93, 0.99, 0.99, 0.86, 0.85, 0.83, and 0.79 for ADL, IADL, role physical, role emotional, physical functioning, bodily pain, vitality, and mental health of SF-36, respectively), and poorer results for social functioning and general health on SF-36 (Cronbach’s α = 0.65 and 0.56, respectively). Additionally, the Pearson correlation coefficient between ADL and PCS is 0.22 (p < 0.001), and between ADL and MCS is 0.09 (p = 0.02).
Table 1 shows the demographic characteristics of the study population. There were 267 participants (47.1%) who did not have MetS and had normal cognitive function. Following this, 127 participants (22.4%) had only MetS. Additionally, 60 participants (10.6%) had only mild cognitive dysfunction, while 46 (8.1%) had only advanced cognitive dysfunction. Participants with both MetS and mild cognitive dysfunction numbered 33 (5.8%), and those with both MetS and advanced cognitive dysfunction accounted for 34 (6.0%). The median age of participants was 66.3 years. Males accounted for 39.3% of the participants. Participants with MetS and advanced cognitive dysfunction were likely to be older (median age 75.7 vs. 64.7–70.5 in other groups) and had lower proportions of being married (61.8% vs. 65.2–78.7% in other groups). They were also more likely to have lower scores in ADL, IADL, and SF-36. Participants with mild and advanced cognitive dysfunction had higher proportions of depression compared to those with normal cognitive function (15.0% and 17.4% in participants without MetS but with mild and advanced cognitive dysfunction, and 12.1% and 17.7% in participants with MetS and mild and advanced cognitive dysfunction vs. 3.8% and 5.6% in participants with normal cognitive function with and without MetS). There were no significant differences among the six groups in religion, smoking, alcohol consumption, and history of malignancy.
Table 2 displays the association of the comorbidity status of MetS and cognitive dysfunction with the PCS and MCS of HRQOL. Compared to participants with no MetS and normal cognitive function, those with MetS and normal cognitive function had a lower PCS score [mean score (95% CI) = -1.12 (-2.14, -0.09)]. However, when MetS was combined with advanced cognitive dysfunction, the PCS score was even worse [mean score (95% CI) = -4.84 (-6.73, -2.94)].
Similar convergence effects were observed in the MCS. Participants with mild cognitive dysfunction and no MetS had a lower MCS score compared to those with no MetS and normal cognitive function [mean score (95% CI) = -2.07 (-3.51, -0.64)]. However, when mild cognitive dysfunction coexisted with MetS, the MCS score was even worse [mean score (95% CI) = -2.58 (-4.43, -0.74)].
In Fig. 3, the group with both MetS and advanced cognitive dysfunction represents the most severe comorbid condition. Compared to participants with no MetS and normal cognitive function, this group had the poorest scores across most scales (except for role emotional and mental health) and also fared worse than those with only MetS or only mild cognitive dysfunction in the graphical distributions. The graphical representation reveals that among patients with MetS, cognitive function deterioration exacerbates trends in certain domains. The stratified analysis for MetS showed that worsening cognitive dysfunction is associated with declining scores in role physical, bodily pain, vitality, and social functioning among participants with MetS (Table 3, Ptrend <0.05). However, these four scales did not show a worsening trend with the progression of cognitive dysfunction in individuals without MetS. The full regression results of Fig. 3 and Table 3 are shown in Supplementary Tables 1 to 6.
The associations of the comorbidity status of metabolic syndrome and cognitive function with eight scales of SF-36 (β coefficient and 95% confidence interval). The statistics were analyzed by multivariable linear regression models adjusting for age, sex, marital status, education level, income groups, and activities of daily living. The details of the regression results are shown in Supplementary Tables 1 and 2
In sensitivity analyses (Table 4), when incorporating past history of ischemic heart disease, stroke, and malignancy into the statistical models, the associations between different statuses of MetS and cognitive dysfunction and HRQOL were similar to those in the main analysis. Compared to those without MetS and with normal cognitive function, individuals with MetS and normal cognitive function and those with MetS and advanced cognitive dysfunction had lower PCS scores [β (95% CI) = -1.10 (-2.12, -0.08) and − 5.03 (-6.91, -3.14), respectively]. Participants with mild cognitive dysfunction exhibited poorer performance on MCS, and the presence of MetS exacerbated the association [β (95% CI) = -2.14 (-3.58, -0.71) and − 2.67 (-4.52, -0.83) for mild cognitive dysfunction without and with MetS, respectively]. Ischemic heart disease and malignancy showed no significant association with PCS or MCS. Stroke was slightly inversely associated with PCS [β (95% CI) = -3.86 (-7.71, -0.01)] but had no significant association with MCS.
Discussions
This study discovered the association of the comorbidity status of MetS and cognitive dysfunction with HRQOL. We found that individuals with MetS and advanced cognitive dysfunction had the worst HRQOL, including PCS and almost all scales except role emotional and mental health. MetS exacerbates the inverse association of mild cognitive dysfunction on MCS. Moreover, in individuals with MetS, as the severity of cognitive dysfunction increases, deteriorating trends are observed in HRQOL scales such as role physical, bodily pain, vitality, and social functioning.
The study focused on middle-aged and older adults, revealing a MetS prevalence of 34.2%. A prior epidemiological study on MetS in Taiwan showed that in the 60–69 age group, the prevalence was 25.2% for males and 38.3% for females [33], aligning with the findings of our study. The prevalence of cognitive dysfunction in our research was 30.5%, exceeding rates reported in other Asian studies. For instance, Wu et al. identified a 22.2% prevalence of cognitive impairment in the elderly population in Taiwan [34], while Kitamura et al. reported a 21.5% prevalence of cognitive impairment in the Japanese elder population [35]. In these studies, cognitive impairment was diagnosed using the Mini-Mental State Examination (MMSE). In contrast, our study employed the MoCA-T for diagnosis, a more sensitive tool than the MMSE, and resulting in a higher observed prevalence of cognitive impairment [36]. The prevalence rate of cognitive dysfunction varies when using different diagnostic tools.
In this study, participants with MetS and advanced cognitive dysfunction exhibited the poorest HRQOL, except in the MCS and scales measuring role emotional and mental health. Previous literature has reported that individuals with advanced cognitive impairment often demonstrate limited abilities in emotional perception and comprehension [37, 38]. Therefore, participants with advanced cognitive dysfunction in this study may struggle to accurately identify various emotions, potentially affecting the scoring in the domains of role emotional and mental health. This inference is supported by the lower performance in MCS and the scales of role emotional and mental health among participants with mild cognitive dysfunction compared to those with normal cognition.
This study found that mild cognitive dysfunction was inversely correlated with MCS in HRQOL, and MetS further exacerbates the inverse correlation. Older adults with mild cognitive impairment tend to report greater depression, increased subjective stress, and more limitations in IADL [17]. MetS also lead to depressive symptoms through inflammatory cytokines and impaired circulation in the brain [39, 40]. There is a bidirectional relationship between MetS and depression [41]. Furthermore, MetS increased the incidences of limitations in mobility, ADL, and IADL [20]. Depression and functional impairment are key factors impacting quality of life among individuals with cognitive impairment [42]. Therefore, MetS may have an additive effect on the impaired HRQOL of MCS caused by mild cognitive dysfunction.
This study identified trends in how cognitive dysfunction affects certain HRQOL scales among individuals with MetS, such as role physical, bodily pain, vitality, and social functioning. A study from Greece that utilized SF-36 to assess HRQOL found that individuals with MetS scored lower on various scales, such as role physical, vitality, and social functioning [43]. As cognitive dysfunction worsens, physical function is likely to be affected [44], potentially involving related aspects of HRQOL, such as role physical and vitality. Kotwal et al. reported that as cognitive function transitions from normal to mild cognitive impairment and dementia, individuals’ social resources and social engagement tend to decrease accordingly [45]. Additionally, cognitive impairment is linked to bodily pain, particularly in areas such as low back pain, waist pain, sciatica, and pain experienced in more than two locations [46]. This study found that the comorbidity status of MetS and cognitive dysfunction was inversely associated with these scales of HRQOL. It may be because MetS-related inflammatory cytokines and impaired microcirculation mediate painful perception [47, 48]. Furthermore, MetS increases the likelihood of physical limitation and depression [20, 42], thereby exacerbating the inverse association between cognitive dysfunction and scales such as role physical, vitality, and social functioning.
In our conceptual framework of the study hypotheses (Fig. 1), MetS and cognitive dysfunction may affect HRQOL through impaired physical function. Logically, this physical function impairment should first affect IADL, which requires a higher level of executive functioning. Therefore, we adjusted ADL rather than IADL. Some questions in the SF-36 partially overlapped with the ADL scale. However, ADL primarily assesses physical function, whereas the SF-36 was designed to assess the impact of both physical and mental health on HRQOL. Therefore, they are not entirely equivalent, and the correlation coefficient between ADL and SF-36 show a very low correlation. Our results showed that even after controlling for ADL, poorer HRQOL was still observed for individuals with MetS and cognitive dysfunction. This suggests a more nuanced association beyond basic physical function.
The choice of confounders adjusted in the statistical models included important demographic factors (age, sex) and factors that showed differences among the groups classified by the comorbidity status of MetS and cognitive dysfunction (ADL, marital status, education level, and income level). We further conducted sensitivity analyses adjusting for comorbidities including ischemic heart disease, stroke, and malignancy. Hypertension, diabetes mellitus, and dyslipidemia were the diagnostic criteria for MetS, so we did not adjust for them. Depression, which may be an important mediator of the association between MetS, cognitive dysfunction, and HRQOL, was also not included in the sensitivity analyses. The results of the sensitivity analyses were similar to our main analyses. For establishing a parsimonious model, these comorbidities were not adjusted in the final models.
This study had some limitations. First, the cross-sectional design does not allow us to interpret the causal inference between the comorbidity status of MetS and cognitive dysfunction and impaired HRQOL. Further longitudinal studies are warranted to elucidate the causal relationship. Second, the role of physical activity and dietary patterns were not analyzed in this study. However, physical activity and dietary patterns were highly associated with socioeconomic status, and we adjusted for important demographic and socioeconomic confounding factors [49]. Third, the study population was mainly recruited from adults who attended health examinations at a medical center in the capital of Taiwan. This study population may be healthier and may have a higher socioeconomic status than the general population, which may lead to a participation bias. However, a higher socioeconomic level and better health status tend to be related to a good HRQOL [50], thus the participation bias is more likely to underestimate the effect. Additionally, the internal reliability of social functioning and general health in this study showed poor levels, which may lead to results that do not accurately reflect the participants’ true states, potentially resulting in misleading conclusions. Although the SF-36 has been previously validated in the literature, further research is needed to validate it specifically in the Taiwanese population. Finally, the study was conducted in a single institution, thus the representativeness of the study population and the generalizability of our results may be limited. Future research should include different populations to validate the findings in this study.
Despite these limitations, the association between the comorbidity status of MetS and cognitive dysfunction and HRQOL have seldom been discussed before. In addition to the inverse association, we further observed that as cognitive dysfunction becomes more severe, several HRQOL scales in MetS patients deteriorate accordingly. This serves as a reminder to healthcare providers that when dealing with patients having both MetS and cognitive dysfunction, as the condition worsens, patients may experience increased suffering. Identifying possible solutions is a crucial issue. Beyond treating the diseases, can a more comprehensive long-term care system, increased active social engagement, or other social welfare resolutions mitigate the inverse associations between MetS and cognitive dysfunction and HRQOL? More relevant research is warranted in the future to address this question.
Conclusion
We found that the comorbidity status of MetS and cognitive dysfunction was inversely associated with HRQOL in both PCS and MCS. Worsening trends related to the comorbidity status were also observed in scales such as role physical, bodily pain, vitality, and social functioning. Individuals with both MetS and cognitive dysfunction face greater challenges in their HRQOL. Future research needs to discover solutions to alleviate the inverse association.
Data availability
The raw data is available on the Open Science Framework (OSF): https://osf.io/fhbe5/?view_only=b1b3a182642d44aea54bfe9ca677f83b.
References
Malik, S., Wong, N., Franklin, S., Kamath, T., L’Italien, G., Pio, J., & Williams, G. (2004). Impact of the metabolic syndrome on mortality from coronary heart disease, cardiovascular disease and all cause in United States adults. Circulation, 110(10), 1245–1250. https://doi.org/10.1161/01.CIR.0000140677.20606.0E
Chen, J. (2004). The metabolic syndrome and chronic kidney disease in U.S. adults. Annals of Internal Medicine, 140(3), 167–174. https://doi.org/10.7326/0003-4819-140-3-200402030-00007
Shin, J. A., Lee, J. H., Lim, S. Y., Ha, H. S., Kwon, H. S., Park, Y. M., Lee, W. C., Kang, M. I., Yim, H. W., Yoon, K. H., & Son, H. Y. (2013). Metabolic syndrome as a predictor of type 2 diabetes, and its clinical interpretations and usefulness. Journal of Diabetes Investigation, 4(4), 334–343. https://doi.org/10.1111/jdi.12075
Chew, N. W. S., Ng, C. H., Tan, D. J. H., Kong, G., Lin, C., Chin, Y. H., Lim, W. H., Huang, D. Q., Quek, J., Fu, C. E., Xiao, J., Syn, N., Foo, R., Khoo, C. M., Wang, J. W., Dimitriadis, G. K., Young, D. Y., Siddiqui, M. S., Lam, C. S. P., et al. (2023). The global burden of metabolic disease: Data from 2000 to 2019. Cell Metabolism, 35(3), 414–428. https://doi.org/10.1016/j.cmet.2023.02.003
Ng, T. P., Feng, L., Nyunt, M. S. Z., Feng, L., Gao, Q., Lim, M. L., Collinson, S. L., Chong, M. S., Lim, W. S., Lee, T. S., Yap, P., & Yap, K. B. (2016). Metabolic syndrome and the risk of mild cognitive impairment and progression to dementia: Follow-up of the Singapore Longitudinal Ageing Study Cohort. JAMA Neurology, 73(4), 456–463. https://doi.org/10.1001/jamaneurol.2015.4899
Koutsonida, M., Markozannes, G., Bouras, E., Aretouli, E., & Tsilidis, K. K. (2022). Metabolic syndrome and cognition: A systematic review across cognitive domains and a bibliometric analysis. Frontiers in Psychology, 13, 981379. https://doi.org/10.3389/fpsyg.2022.981379
Yaffe, K., Kanaya, A., Lindquist, K., Simonsick, E. M., Harris, T., Shorr, R. I., Tylavsky, F. A., & Newman, A. B. (2004). The metabolic syndrome, inflammation, and risk of cognitive decline. Journal of the American Medical Association, 292(18), 2237–2242. https://doi.org/10.1001/jama.292.18.2237
Yates, K. F., Sweat, V., Yau, P. L., Turchiano, M. M., & Convit, A. (2012). Impact of metabolic syndrome on cognition and brain: A selected review of the literature. Arteriosclerosis Thrombosis and Vascular Biology, 32(9), 2060–2067. https://doi.org/10.1161/atvbaha.112.252759
Capucho, A. M., Chegão, A., Martins, F. O., Miranda, V., H., & Conde, S. V. (2022). Dysmetabolism and neurodegeneration: Trick or treat? Nutrients, 14(7), 1425. https://doi.org/10.3390/nu14071425
Srikanth, V., Sinclair, A. J., Hill-Briggs, F., Moran, C., & Biessels, G. J. (2020). Type 2 diabetes and cognitive dysfunction-towards effective management of both comorbidities. The Lancet Diabetes & Endocrinology, 8(6), 535–545. https://doi.org/10.1016/s2213-8587(20)30118-2
Bae, S., Shimada, H., Lee, S., Makizako, H., Lee, S., Harada, K., Doi, T., Tsutsumimoto, K., Hotta, R., Nakakubo, S., Park, H., & Suzuki, T. (2017). The relationships between components of metabolic syndrome and mild cognitive impairment subtypes: A cross-sectional study of Japanese older adults. Journal of Alzheimer’s Disease, 60(3), 913–921. https://doi.org/10.3233/jad-161230
Gunstad, J., Sanborn, V., & Hawkins, M. (2020). Cognitive dysfunction is a risk factor for overeating and obesity. The American Psychologist, 75(2), 219–234. https://doi.org/10.1037/amp0000585
Carrión, R. E., Goldberg, T. E., McLaughlin, D., Auther, A. M., Correll, C. U., & Cornblatt, B. A. (2011). Impact of neurocognition on social and role functioning in individuals at clinical high risk for psychosis. The American Journal of Psychiatry, 168(8), 806–813. https://doi.org/10.1176/appi.ajp.2011.10081209
Jefferson, A. L., Byerly, L. K., Vanderhill, S., Lambe, S., Wong, S., Ozonoff, A., & Karlawish, J. H. (2008). Characterization of activities of daily living in individuals with mild cognitive impairment. The American Journal of Geriatric Psychiatry, 16(5), 375–383. https://doi.org/10.1097/JGP.0b013e318162f197
Jekel, K., Damian, M., Wattmo, C., Hausner, L., Bullock, R., Connelly, P. J., Dubois, B., Eriksdotter, M., Ewers, M., Graessel, E., Kramberger, M. G., Law, E., Mecocci, P., Molinuevo, J. L., Nygård, L., Olde-Rikkert, M. G., Orgogozo, J. M., Pasquier, F., Peres, K., et al. (2015). Mild cognitive impairment and deficits in instrumental activities of daily living: A systematic review. Alzheimer’s Research & Therapy, 7(1), 17. https://doi.org/10.1186/s13195-015-0099-0
Pan, C. W., Wang, X., Ma, Q., Sun, H. P., Xu, Y., & Wang, P. (2015). Cognitive dysfunction and health-related quality of life among older Chinese. Scientific Reports, 5(1), 17301. https://doi.org/10.1038/srep17301
Stites, S. D., Harkins, K., Rubright, J. D., & Karlawish, J. (2018). Relationships between cognitive complaints and quality of life in older adults with mild cognitive impairment, mild Alzheimer disease dementia, and normal cognition. Alzheimer Disease and Associated Disorders, 32(4), 276–283. https://doi.org/10.1097/wad.0000000000000262
Saboya, P. P., Bodanese, L. C., Zimmermann, P. R., Gustavo, A. D., Assumpção, C. M., & Londero, F. (2016). Metabolic syndrome and quality of life: A systematic review. Revista Latino-americana de Enfermagem, 24, e2848. https://doi.org/10.1590/1518-8345.1573.2848
Lin, Y. H., Chang, H. T., Tseng, Y. H., Chen, H. S., Chiang, S. C., Chen, T. J., & Hwang, S. J. (2021). Changes in metabolic syndrome affect the health-related quality of life of community-dwelling adults. Scientific Reports, 11(1), 20267. https://doi.org/10.1038/s41598-021-99767-y
Carriere, I., Pérès, K., Ancelin, M. L., Gourlet, V., Berr, C., Barberger-Gateau, P., Bouillon, K., Kivimaki, M., Ritchie, K., & Akbaraly, T. (2014). Metabolic syndrome and disability: Findings from the prospective three-city study. The Journals of Gerontology Series A Biological Sciences and Medical Sciences, 69(1), 79–86. https://doi.org/10.1093/gerona/glt101
Kim, H. B., Wolf, B. J., & Kim, J. H. (2023). Association of metabolic syndrome and its components with the risk of depressive symptoms: A systematic review and meta-analysis of cohort studies. Journal of Affective Disorders, 323, 46–54. https://doi.org/10.1016/j.jad.2022.11.049
Pellegrino, L. D., Peters, M. E., Lyketsos, C. G., & Marano, C. M. (2013). Depression in cognitive impairment. Current Psychiatry Reports, 15(9), 384. https://doi.org/10.1007/s11920-013-0384-1
Mahoney, F. I., & Barthel, D. W. (1965). Functional evaluation: The Barthel Index. Maryland State Medical Journal, 14, 61–65.
Lawton, M. P., & Brody, E. M. (1969). Assessment of older people: Self-maintaining and instrumental activities of daily living. The Gerontologist, 9(3), 179–186. https://doi.org/10.1093/geront/9.3_Part_1.179
Chang, H. T. (2009, April). National digital library of theses and dissertations in Taiwan. Determinants in older veterans’ quality of life: The case of four veterens’ homes in southern Taiwan. Retrieved August 19, 2024, from https://hdl.handle.net/11296/v4frwj
Grundy, S. M., Cleeman, J. I., Daniels, S. R., Donato, K. A., Eckel, R. H., Franklin, B. A., Gordon, D. J., Krauss, R. M., Savage, P. J., Smith, S. C. Jr., Spertus, J. A., & Costa, F. (2005). Diagnosis and management of the metabolic syndrome: An American Heart Association/National Heart, Lung, and Blood Institute Scientific Statement. Circulation, 112(17), 2735–2752. https://doi.org/10.1161/circulationaha.105.169404
Tsai, C. F., Lee, W. J., Wang, S. J., Shia, B. C., Nasreddine, Z., & Fuh, J. L. (2012). Psychometrics of the Montreal Cognitive Assessment (MoCA) and its subscales: Validation of the Taiwanese version of the MoCA and an item response theory analysis. International Psychogeriatrics, 24(4), 651–658. https://doi.org/10.1017/s1041610211002298
Pequeno, N. P. F., Cabral, N. L. A., Marchioni, D. M., Lima, S. C. V. C., & Lyra, C. O. (2020). Quality of life assessment instruments for adults: A systematic review of population-based studies. Health and Quality of Life Outcomes, 18(1), 208. https://doi.org/10.1186/s12955-020-01347-7
Lu, J. F. R., Tseng, H. M., & Tsai, Y. J. (2003). Assessment of health-related quality of life in Taiwan (I): Development and psychometric testing of SF-36 Taiwan Version. Taiwan Journal of Public Health, 22(6), 501–511.
Maruish, M. E. (2011). User’s manual for the SF-36v2 health survey third edition. QualityMetric.
Qu, B., Guo, H. Q., Liu, J., Zhang, Y., & Sun, G. (2009). Reliability and validity testing of the SF-36 questionnaire for the evaluation of the quality of life of Chinese urban construction workers. The Journal of International Medical Research, 37(4), 1184–1190. https://doi.org/10.1177/147323000903700425
Patino, C. M., & Ferreira, J. C. (2016). Test for trend: Evaluating dose-response effects in association studies. Jornal Brasileiro De Pneumologia, 42(4), 240. https://doi.org/10.1590/s1806-37562016000000225
Hwang, L. C., Bai, C. H., & Chen, C. J. (2006). Prevalence of obesity and metabolic syndrome in Taiwan. Journal of the Formosan Medical Association, 105(8), 626–635. https://doi.org/10.1016/s0929-6646(09)60161-3
Wu, M. S., Lan, T. H., Chen, C. M., Chiu, H. C., & Lan, T. Y. (2011). Socio-demographic and health-related factors associated with cognitive impairment in the elderly in Taiwan. BMC Public Health, 11(1), 22. https://doi.org/10.1186/1471-2458-11-22
Kitamura, K., Watanabe, Y., Nakamura, K., Sanpei, K., Wakasugi, M., Yokoseki, A., Onodera, O., Ikeuchi, T., Kuwano, R., Momotsu, T., Narita, I., & Endo, N. (2016). Modifiable factors associated with cognitive impairment in 1,143 Japanese outpatients: The Project in Sado for Total Health (PROST). Dementia and Geriatric Cognitive Disorders Extra, 6(2), 341–349. https://doi.org/10.1159/000447963
Jia, X., Wang, Z., Huang, F., Su, C., Du, W., Jiang, H., Wang, H., Wang, J., Wang, F., Su, W., Xiao, H., Wang, Y., & Zhang, B. (2021). A comparison of the Mini-mental State Examination (MMSE) with the Montreal Cognitive Assessment (MoCA) for mild cognitive impairment screening in Chinese middle-aged and older population: A cross-sectional study. Bmc Psychiatry, 21(1), 485. https://doi.org/10.1186/s12888-021-03495-6
Shimokawa, A., Yatomi, N., Anamizu, S., Torii, S., Isono, H., Sugai, Y., & Kohno, M. (2001). Influence of deteriorating ability of emotional comprehension on interpersonal behavior in Alzheimer-type dementia. Brain and Cognition, 47(3), 423–433. https://doi.org/10.1006/brcg.2001.1318
Elferink, M. W., van Tilborg, I., & Kessels, R. P. (2015). Perception of emotions in mild cognitive impairment and Alzheimer’s dementia: Does intensity matter? Translational Neuroscience, 6(1), 139–149. https://doi.org/10.1515/tnsci-2015-0013
Schiepers, O. J., Wichers, M. C., & Maes, M. (2005). Cytokines and major depression. Progress in Neuro-psychopharmacology & Biological Psychiatry, 29(2), 201–217. https://doi.org/10.1016/j.pnpbp.2004.11.003
Taylor, W. D., Aizenstein, H. J., & Alexopoulos, G. S. (2013). The vascular depression hypothesis: Mechanisms linking vascular disease with depression. Molecular Psychiatry, 18(9), 963–974. https://doi.org/10.1038/mp.2013.20
Pan, A., Keum, N., Okereke, O. I., Sun, Q., Kivimaki, M., Rubin, R. R., & Hu, F. B. (2012). Bidirectional association between depression and metabolic syndrome: A systematic review and meta-analysis of epidemiological studies. Diabetes Care, 35(5), 1171–1180. https://doi.org/10.2337/dc11-2055
Burks, H. B., des Bordes, J. K. A., Chadha, R., Holmes, H. M., & Rianon, N. J. (2021). Quality of life assessment in older adults with dementia: A systematic review. Dementia and Geriatric Cognitive Disorders, 50(2), 103–110. https://doi.org/10.1159/000515317
Tziallas, D., Kastanioti, C., Kostapanos, M. S., Skapinakis, P., Elisaf, M. S., & Mavreas, V. (2012). The impact of the metabolic syndrome on health-related quality of life: A cross-sectional study in Greece. European Journal of Cardiovascular Nursing, 11(3), 297–303. https://doi.org/10.1016/j.ejcnurse.2011.02.004
Gray, M., Gills, J. L., Glenn, J. M., Vincenzo, J. L., Walter, C. S., Madero, E. N., Hall, A., Fuseya, N., & Bott, N. T. (2021). Cognitive decline negatively impacts physical function. Experimental Gerontology, 143, 111164. https://doi.org/10.1016/j.exger.2020.111164
Kotwal, A. A., Kim, J., Waite, L., & Dale, W. (2016). Social function and cognitive status: Results from a US nationally representative survey of older adults. Journal of General Internal Medicine, 31(8), 854–862. https://doi.org/10.1007/s11606-016-3696-0
Huang, C. C., Lee, L. H., Lin, W. S., Hsiao, T. H., Chen, I. C., & Lin, C. H. (2022). The association between bodily pain and cognitive impairment in community-dwelling older adults. Journal of Personalized Medicine, 12(3), 350. https://doi.org/10.3390/jpm12030350
Zhang, C., Ward, J., Dauch, J. R., Tanzi, R. E., & Cheng, H. T. (2018). Cytokine-mediated inflammation mediates painful neuropathy from metabolic syndrome. PloS One, 13(2), e0192333. https://doi.org/10.1371/journal.pone.0192333
Coderre, T. J. (2023). Contribution of microvascular dysfunction to chronic pain. Frontiers in Pain Research, 4, 1111559. https://doi.org/10.3389/fpain.2023.1111559
Stringhini, S., Dugravot, A., Shipley, M., Goldberg, M., Zins, M., Kivimäki, M., Marmot, M., Sabia, S., & Singh-Manoux, A. (2011). Health behaviours, socioeconomic status, and mortality: Further analyses of the British Whitehall II and the French GAZEL prospective cohorts. PLoS Medicine, 8(2), e1000419. https://doi.org/10.1371/journal.pmed.1000419
Ma, X., & McGhee, S. M. (2013). A cross-sectional study on socioeconomic status and health-related quality of life among elderly Chinese. British Medical Journal Open, 3(2), e002418. https://doi.org/10.1136/bmjopen-2012-002418
Funding
This work was supported by grants from Taipei Veterans General Hospital. [Grant number: V109E-005-4, V110E-005-4, V111E-006-4, V112E-004-4]
Author information
Authors and Affiliations
Contributions
Yi-Hsuan Lin designed the study, conducted statistical analysis, interpreted the results, and wrote the paper. Hsiao-Ting Chang designed the study, organized the original data, coordinated the research, and editing. Yen-Feng Wang, Jong-Ling Fuh, and Shuu-Jiun Wang planned the research direction and assisted in collecting the original data. Harn-Shen Chen assisted in formulating the research question and study design. Sih-Rong Li assisted in the enrollment of participants and maintaining the original data. Ming-Hwai Lin, Tzeng-Ji Chen, and Shinn-Jang Hwang contributed to conceptualization, review, and editing.
Corresponding author
Ethics declarations
Ethical approval
This study was performed in line with the principles of the Declaration of Helsinki. Approval was granted by the Research Ethics Committee of Taipei Veterans General Hospital, Taipei, Taiwan, on May 9, 2022 (Protocol Code: 2020-06-001 A).
Consent to participate
All participants in this study provided written informed consent before participating in the research.
Competing interests
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Lin, YH., Chang, HT., Wang, YF. et al. The association of the comorbidity status of metabolic syndrome and cognitive dysfunction with health-related quality of life. Qual Life Res (2024). https://doi.org/10.1007/s11136-024-03784-z
Accepted:
Published:
DOI: https://doi.org/10.1007/s11136-024-03784-z