Abstract
Parkinson’s disease (PD) is an incurable neurodegenerative disease characterized by motor and non-motor disabilities resulting from neuronal cell death in the substantia nigra and striatum. Microglial activation and oxidative stress are two of the primary mechanisms driving that neuronal death. Here, we evaluated the effects of geranium oil on 1-methyl-4-phenyl-1,2,3,6-tetra-hydropyridine (MPTP) mouse model for PD, on microglial activation, and oxidative stress. We demonstrate that oral treatment with geranium oil improved motor performance in this model. The therapeutic effects of geranium oil were observed as a significant increase in rotarod latency and distance among the mice treated with geranium oil, as compared to vehicle-treated MPTP mice. Geranium oil also prevented dopaminergic neuron death in the substantia nigra of the treated mice. These therapeutic effects can be partially attributed to the antioxidant and anti-inflammatory properties of geranium oil, which were observed as attenuated accumulation of reactive oxygen species and inhibition of the secretion of proinflammatory cytokines from geranium oil-treated activated microglial cells. A repeated-dose oral toxicity study showed that geranium oil is not toxic to mice. In light of that finding and since geranium oil is defined by the FDA as generally recognized as safe (GRAS), we do not foresee any toxicity problems in the future and suggest that geranium oil may be a safe and effective oral treatment for PD. Since the MPTP model is only one of the preclinical models for PD, further studies are needed to confirm that geranium oil can be used to prevent or treat PD.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Parkinson’s disease (PD) is one of the most common neurodegenerative disorders, affecting approximately 2% of adults over the age of 60. Currently, there are no highly effective preventive or curative pharmacological or nutritional treatments for PD. PD is characterized by the selective loss of dopaminergic neurons in the striatum and substantia nigra pars compacta (SNpc). As dopamine is transported by dopaminergic projections from the substantia nigra to the striatum, this loss leads to dopamine deficiency in the striatum, which, in turn, causes prominent motor function disturbances [1, 2]. Multiple mechanisms contribute to neurodegeneration in PD, including oxidative stress, neuroinflammation, microglial activation and elevated levels of IL-1β and IL-6. Therefore, the inhibition of microglia-mediated neuroinflammation bears potential as a protective strategy against PD [3,4,5,6,7,8,9]. It has also been widely accepted that excessive reactive oxygen species (ROS) are key mediators of PD pathogenesis and dopaminergic neuron death, as they induce the oxidation of cell proteins, lipids and DNA [10, 11]. In recent years, there has been increased interest in the therapeutic effects of phytochemicals. At the same time, the increasing number of patients with PD has resulted in growing interest in phytochemicals that can support neuronal health and help to prevent PD [12,13,14]. Essential oils are multicomponent mixtures containing hundreds of low-MW phytochemicals. They are used as folk medicines to treat various kinds of inflammatory diseases, organ dysfunction and systemic disorders [15]. The advantage of plant essential oils for the treatment of multifactorial diseases such as PD lies in the variety of compounds that they include, which act synergistically and simultaneously on several targets [16,17,18]. Geranium oil is obtained by steam distillation of Pelargonium graveolens l’Heritier ex Aiton [19] and is defined by the FDA as a generally recognized as safe (GRAS) substance. The main constituents (≥ 10%) of geranium oil are citronellol (26%), citronellyl formate (16%) and linalool (10%) [20]. Geranium oil has been used for many years in traditional medicine for various purposes [21] and has been shown to exhibit anti-inflammatory activity in mice [20, 22,23,24]. The components of geranium oil are lipophilic and have even low molecular weights (~ 150 g/mol) which means that they are likely to be capable of crossing the blood–brain barrier and acting synergistically on various targets. However, the safety of geranium oil for brain cells and its effects in mice models of neurodegenerative diseases, such as PD, have not been studied previously. In a previous study, we showed that geranium oil down-regulates microglial activation [20]. In light of that finding and the fact that robust microglial activation has been observed in the SNpc in PD patients and animal models for PD [7, 9], we set out to determine whether daily consumption of geranium oil is safe and whether it can mitigate behavioral impairment and dopaminergic damage in a mouse model of PD. Among the various toxic models of PD, the MPTP model has become the most commonly used, since MPTP is the only known dopaminergic neurotoxin capable of inducing a clinical picture indistinguishable from PD in both humans and primates [25]. In the current study, we assessed the anti-inflammatory and antioxidant effects of geranium oil in primary cultures of microglial cells, as well as its therapeutic effect and the safety of daily consumption of geranium oil in the 1-methyl-4-phenyl-1,2,3,6-tetra-hydropyridine (MPTP) mouse model for PD.
Materials and Methods
The Materials and Methods section is presented as supplementary material.
Results and Discussion
Geranium Oil Attenuates the Secretion of IL-1β and IL-6 From LPS-Stimulated Microglial Cells
Given the robust microglial activation that has been found in the SNpc in PD patients and animal PD models [7, 9], the potential inhibitory effect of geranium oil on the release of the inflammatory cytokines IL-1β and IL-6 was examined in activated microglial cells. We described the gas chromatography-mass spectrometry (GC-MS) profile of geranium oil in a previous publication [20]. Microglial cells were activated by LPS, in the presence or absence of the geranium oil and cytokine levels in the conditioned media were later determined by ELISA. In unstimulated microglial cells, levels of IL-1β and IL-6 were very low or undetectable (Fig. 1a, b). In contrast, stimulation of the cells with LPS resulted in a remarkable increase in the secretion of these cytokines, which were dose-dependently reduced by 83% (IL-1β) and 85% (IL-6), following treatment with 20 µg/mL of geranium oil (Fig. 1a, b). The anti-inflammatory steroid dexamethasone, which served as a positive control, inhibited the cytokines to similar levels, although lower concentrations of dexamethasone were needed to obtain the inhibitory effect. These effects were not the result of any cytotoxic activity of the oil, as was confirmed by a cell-viability assay (Fig. 1c).
Down-regulation of IL-1β and IL-6 secretion from LPS-stimulated microglial cells by geranium oil. Microglial cells were treated with the indicated concentrations of geranium oil and then stimulated with LPS (100 ng/mL) for 24 h. Conditioned medium was then collected and (a) IL-6 and (b) IL-1β levels were measured by ELISA. Data represent the means ± SEM of three (for IL-1β) or two (for IL-6) independent experiments (n = 6 for IL-1β; n = 4 for IL-6). * p < 0.05; *** p < 0.001 relative to LPS-treated cells. (c) Microglial cell viability was tested after 20 h of incubation with geranium oil using the crystal-violet assay. Each point on the graph represents the mean ± SEM of one experiment (n = 4). No statistical differences were observed between treatments
Geranium Oil Reduces Levels of Peroxyl Radicals in Microglial Cells
Excessive ROS are key mediators of PD pathogenesis [10, 11]. To measure the ability of geranium oil to reduce intracellular ROS levels in microglial cells, we treated the cells with ABAP, which is a generator of peroxyl radicals [26]. Subsequent treatment of microglial cells with geranium oil reduced ABAP-induced ROS levels (Fig. 2a). We used a cell-viability assay to verify that the reduced levels of ROS were not due to any cytotoxic effect of the oil (Fig. 2b).
Geranium oil reduced ROS levels in microglial cells. Cells were incubated for 1 h with various concentrations of geranium oil. (a) Cells were then loaded with the non-fluorescent cell-permeating compound, 2’7’- dichlorofluorescein diacetate (DCF-DA) for 30 min and washed with PBS. 2,2’-Azobis(amidinopropane) (ABAP, 0.6 mM) was added and fluorescence was measured 3 h later. (b) Viability was tested after 20 h using the crystal-violet method. No statistical differences were observed between treatments. Each point on the graph represents the mean ± SEM of two experiments (n = 8). **p < 0.01 relative to cells treated with ABAP alone
Effects of Geranium Oil on the Viability of SH-SY5Y Human Neuroblastoma Cells
We also examined whether geranium oil affects the viability of SH-SY5Y human neuroblastoma cells [27]. Our results demonstrate that geranium oil did not affect cell viability and no toxic effects were observed even at a concentration of 100 µg/mL, which was the highest concentration tested (Fig. 3).
Effects of Geranium Oil on the Rotarod Performance of the Mice
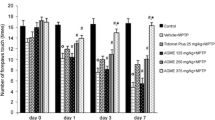
Geranium oil has been used for many years in traditional medicine for various purposes [21] and has been shown to have anti-inflammatory activity in mice [22,23,24]. In an earlier study, we determined the components of this essential oil, which are lipophilic and have low molecular weights (~ 150 g/mol) and are, therefore, predicted to cross the blood-brain barrier [20]. Given our results regarding the lack of toxicity of geranium oil to microglial and neuronal cells, as well as our finding that geranium oil down-regulates microglial activation, we set out to determine whether daily consumption of geranium oil is safe and whether it can mitigate the behavioral impairments and dopaminergic damage caused by the administration of MPTP to mice. To that end, we studied the effect of 11 days of oral treatment with geranium oil on motor coordination by challenging MPTP-induced acute PD C57BL/6 mice with the rotarod task (Fig. 4).
As compared to the healthy control mice, the MPTP PD mice treated with vehicle alone traveled significantly shorter distances (Fig. 5a) and spent significantly less time (Fig. 5b) on the rotarod on Day 11 of the study (Fig. 5). Treatment with geranium oil significantly increased the distance the mice traveled on the rotarod (Fig. 5a) and the amount of time they spent on the bar before they fell (i.e., latency; Fig. 5b), as compared to the values recorded for MPTP mice treated with vehicle alone. No statistically significant differences in body weights were found between the different treatment groups (Fig. 5c). In this study, the effective dose of geranium oil that was shown to mitigate the behavioral impairment of PD mice was 500 mg/kg. The human equivalent dose (HED) is estimated to be 40.5 mg/kg, calculated as described previously [28].
In previous studies, various plant extracts and phytochemicals, such as chlorogenic acid (MW 354.31 g/mol), were shown to improve neurobehavioral activity in Parkinsonian mice models [12, 13, 29]. Our work differs from that previous research in several important ways. First, the molecular weights of those compounds are greater than those of the components of geranium oil, which is an essential oil. Second, our study also differed from those earlier studies in that we exclusively studied the effects of oral administration of the substance of interest (i.e., geranium oil); whereas previous studies did not always examine that method of administration. Third, we have used the MPTP model and not other models (e.g. paraquat or rotenone). Finally, we applied our treatment concomitant with PD induction; whereas some of that previous work examined potential preventative effects (i.e., treatment before the induction of PD). It remains to be determined whether the effects of geranium oil are elicited by a single constituent or whether they are the outcome of the synergistic activities of several components. Some of the neuroprotective activities of the bioactive phytochemicals present in geranium oil might be attributable to citronellol, which is the main (~ 26%) low-MW (MW = 156.26 g/mol) substance in this oil [20].
Geranium oil improved motor performance in Parkinson’s disease model mice. Mice (10–12 per group) were trained on the rotarod (RR), 3 trials/day, 7, 5, and 3 days before induction of PD by 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP). On Day 11, mice were placed on the rotarod to measure motor performance. Data are presented as (a) the mean time on the rotating bar until the first fall and (b) the distance traveled before the fall. Each mouse was tested three times. Data are expressed as the mean ± SD (n = 12 animals/group). Results were analyzed using two-way ANOVA repeated measurements and post hoc Bonferroni post-tests. *** p < 0.001; **p < 0.01. (c) Body weight of experimental mice. No statistical differences were observed between treatments. Each point represents the mean ± SEM of 11–13 mice/group
Effect of Geranium-Oil Treatment on the Number of Dopaminergic Neurons in the Substantia Nigra
To determine whether geranium oil prevents neuronal mortality in the SNpc of MPTP mice, mice were sacrificed after 11 days of treatment with the geranium oil or vehicle and the brains of all of the sacrificed mice were fixed and sectioned. As there is a direct relationship between the reduction in DAT expression and nigral cell loss [30], samples were immunostained for DAT and the dopaminergic neurons in the substantia nigra were counted. As shown in Fig. 6, the number of dopaminergic neurons in the SNc was markedly reduced in the brains of the MPTP PD mice, as compared to the control group of healthy mice. In contrast, daily consumption of geranium oil significantly enhanced the number of DAT-positive dopaminergic neuronal cells in the SNpc, relative to the SNpc of untreated MPTP mice.
Effect of the geranium-oil treatment on the number of dopamine transporter-positive neurons in the SNpc of PD model mice. At the end of the study, on Day 12, brains were immunostained for DAT. Three fields were sampled from each mouse. In each field, the dopaminergic neurons were counted and the area of SNc was measured. SNpc area was included in the analysis of variance as a covariable, to remove any possible contribution. Data are expressed as mean ± SD. n = 33. *** p < 0.001
Repeated-Dose Oral Toxicity in Mice
Neither the safety of geranium oil for brain cells nor its effects in mice models of PD or any other neurodegenerative diseases have been studied previously. Although the essential oil of geranium is defined by the FDA as GRAS, we specifically tested the safety of the oil we produced from our specific cultivar in a repeated oral toxicity experiment in mice. Geranium oil or vehicle was administered by oral gavage for 14 consecutive days. Mice from all groups showed stable food consumption during the study, with no significant differences between the treatment groups. No changes in body weight (Table 1) and no adverse clinical symptoms were observed among any of the tested mice in any of the tested groups.
We did not observe any changes in the skin, fur, eyes, mucous membranes, incidence of secretions and excretions (e.g., diarrhea), autonomic activity (e.g., lacrimation, salivation, piloerection, pupil size, unusual respiratory pattern), gait, posture or response to handling. We did not observe the presence of any bizarre behavior, tremors, convulsions, unusual sleep behavior or coma. No animal was found in a moribund or severely distressed condition. There were no observations of animals presenting severe pain. No gross pathological findings were observed in any of the mice at the end of the study. Hematology and clinical chemistry parameters at the end of the study are presented in Table 2. All of the data obtained were within the normal accepted range for mice and no statistically significant differences were observed between the group treated with geranium oil and the vehicle-treated group.
Conclusions
Our data show that daily consumption of geranium oil significantly and almost completely inhibited the impairment of motor performance and degeneration of dopaminergic neurons in the tested PD model. These therapeutic effects can be partially attributed to the antioxidant and anti-inflammatory properties of geranium oil in microglial cells, as demonstrated by the attenuated accumulation of ROS in microglial cells and the inhibition of the secretion of the proinflammatory cytokines IL-1β and IL-6 from microglial cells that had been treated with geranium oil. Neither the safety of geranium oil for brain cells nor its effects in mice models of PD or any other neurodegenerative diseases have been studied previously. Our findings provide proof of the safety and neuroprotective effects of geranium oil against MPTP-induced neurotoxicity. Further studies will be needed to confirm that geranium oil can be used to prevent or treat PD.
Data Availability
Data will be made available upon request.
Abbreviations
- ABAP:
-
2,2’-azobis(amidinopropane)
- DAT:
-
Dopamine transporter
- DCF-DA:
-
2’7’-dichlorofluorescein diacetate
- IL-1β:
-
Interleukin 1β
- IL-6:
-
Interleukin 6
- GC-MS:
-
Gas chromatography-mass spectrometry
- GRAS:
-
Generally recognized as safe
- LPS:
-
Lipopolysaccharide
- MCT:
-
Medium-chain triglyceride
- MPTP:
-
1-methyl-4-phenyl-1,2,3,6-tetra-hydropyridine
- MTT:
-
3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide
- MW:
-
Molecular weight
- PD:
-
Parkinson’s disease
- ROS:
-
Reactive oxygen species
- SNc:
-
Substantia nigra zona compacta
References
Simon DK, Tanner CM, Brundin P (2020) Parkinson disease epidemiology, pathology, genetics, and pathophysiology. Clin Geriatr Med 36:1–12. https://doi.org/10.1016/j.cger.2019.08.002
Vijiaratnam N, Simuni T, Bandmann O, Morris HR, Foltynie T (2021) Progress towards therapies for disease modification in Parkinson’s disease. Lancet Neurol 20:559–572. https://doi.org/10.1016/S1474-4422(21)00061-2
Karpenko MN, Vasilishina AA, Gromova EA, Muruzheva ZM, Miliukhina IV, Bernadotte A (2018) Interleukin-1beta, interleukin-1 receptor antagonist, interleukin-6, interleukin-10, and tumor necrosis factor-alpha levels in CSF and serum in relation to the clinical diversity of Parkinson’s disease. Cell Immunol 327:77–82. https://doi.org/10.1016/j.cellimm.2018.02.011
Leal MC, Casabona JC, Puntel M, Pitossi FJ (2013) Interleukin-1beta and tumor necrosis factor-alpha: reliable targets for protective therapies in Parkinson’s disease? Front Cell Neurosci 7:53. https://doi.org/10.3389/fncel.2013.00053
More SV, Kumar H, Kim IS, Song SY, Choi DK (2013) Cellular and molecular mediators of neuroinflammation in the pathogenesis of Parkinson’s disease. Mediators Inflamm 2013:952375. https://doi.org/10.1155/2013/952375
Pajares M, A IR, Manda G, Bosca L, Cuadrado A (2020) Inflammation in Parkinson’s disease mechanisms and therapeutic implications. Cells 9. https://doi.org/10.3390/cells9071687
Sanchez-Guajardo V, Tentillier N, Romero-Ramos M (2015) The relation between alpha-synuclein and microglia in Parkinson’s disease: recent developments. Neuroscience 302:47–58. https://doi.org/10.1016/j.neuroscience.2015.02.008
Vesely B, Dufek M, Thon V, Brozman M, Kiralova S, Halaszova T, Koritakova E, Rektor I (2018) Interleukin 6 and complement serum level study in Parkinson’s disease. J Neural Transm (Vienna) 125:885–881. https://doi.org/10.1007/s00702-018-1857-5
Zhang QS, Heng Y, Yuan YH, Chen NH (2017) Pathological alpha-synuclein exacerbates the progression of Parkinson’s disease through microglial activation. Toxicol Lett 265:30–37. https://doi.org/10.1016/j.toxlet.2016.11.002
Weng M, Xie X, Liu C, Lim KL, Zhang CW, Li L (2018) The sources of reactive oxygen species and its possible role in the pathogenesis of Parkinson’s Disease. Parkinsons Dis 2018:9163040. https://doi.org/10.1155/2018/9163040
Puspita L, Chung SY, Shim JW (2017) Oxidative stress and cellular pathologies in Parkinson’s disease. Mol Brain 10:53. https://doi.org/10.1186/s13041-017-0340-9
Prakash J, Chouhan S, Yadav SK, Westfall S, Rai SN, Singh SP (2014) Withania somnifera alleviates parkinsonian phenotypes by inhibiting apoptotic pathways in dopaminergic neurons. Neurochem Res 39:2527–2536. https://doi.org/10.1007/s11064-014-1443-7
Yadav SK, Rai SN, Singh SP (2017) Mucuna pruriens reduces inducible nitric oxide synthase expression in parkinsonian mice model. J Chem Neuroanat 80:1–10. https://doi.org/10.1016/j.jchemneu.2016.11.00
Rai SN, Yadav SK, Singh D, Singh SP (2016) Ursolic acid attenuates oxidative stress in nigrostriatal tissue and improves neurobehavioral activity in MPTP-induced parkinsonian mouse model. J Chem Neuroanat 71:41–49. https://doi.org/10.1016/j.jchemneu.2015.12.002
Ramsey JT, Shropshire BC, Nagy TR, Chambers KD, Li Y, Korach KS (2020) Essential oils and health. Yale J Biol Med 93:291–305
Javed H, Nagoor Meeran MF, Azimullah S, Adem A, Sadek B, Ojha SK (2018) Plant extracts and phytochemicals targeting alpha-synuclein aggregation in Parkinson’s Disease Models. Front Pharmacol 9:1555. https://doi.org/10.3389/fphar.2018.01555
Shahpiri Z, Bahramsoltani R, Hosein Farzaei M, Farzaei F, Rahimi R (2016) Phytochemicals as future drugs for Parkinson’s disease: a comprehensive review. Rev Neurosci 27:651–668. https://doi.org/10.1515/revneuro-2016-0004
Williamson EM (2001) Synergy and other interactions in phytomedicines. Phytomedicine 8:401–409
Fekri N, El Amir D, Owis A, AbouZid S (2021) Studies on essential oil from rose-scented geranium, Pelargonium graveolens L’Herit. (Geraniaceae). Nat Prod Res 35:2593–2597. https://doi.org/10.1080/14786419.2019.1682581
Elmann A, Mordechay S, Rindner M, Ravid U (2010) Anti-neuroinflammatory effects of geranium oil in microglial cells. J Funct Foods 2:17–22. https://doi.org/10.1016/j.jff.2009.12.001
Lis-Balchin M (2002) Geranium and pelargonium. Taylor & Francis Inc, London and Ney York
Abe S, Maruyama N, Hayama K, Inouye S, Oshima H, Yamaguchi H (2004) Suppression of neutrophil recruitment in mice by geranium essential oil. Mediators Inflamm 2004 13:21–24. https://doi.org/10.1080/09629350410001664798
Maruyama N, Ishibashi H, Hu W, Morofuji S, Inouye S, Yamaguchi H, Abe S (2006) Suppression of carrageenan- and collagen II-induced inflammation in mice by geranium oil. Mediators Inflamm 2006:62537. https://doi.org/10.1155/MI/2006/62537
Maruyama N, Sekimoto Y, Ishibashi H, Inouye S, Oshima H, Yamaguchi H, Abe S (2005) Suppression of neutrophil accumulation in mice by cutaneous application of geranium essential oil. J Inflamm (Lond) 2:1. https://doi.org/10.1186/1476-9255-2-1
Rai SN, Singh P (2020) Advancement in the modelling and therapeutics of Parkinson’s disease. J Chem Neuroanat 104:101752. https://doi.org/10.1016/j.jchemneu.2020.101752
Wolfe KL, Liu RH (2007) Cellular antioxidant activity (CAA) assay for assessing antioxidants, foods, and dietary supplements. J Agric Food Chem 55:8896–8907
Ferrari E, Cardinale A, Picconi B, Gardoni F (2020) From cell lines to pluripotent stem cells for modelling Parkinson’s Disease. J Neurosci Methods 340:108741. https://doi.org/10.1016/j.jneumeth.2020.108741
Nair AB, Jacob S (2016) A simple practice guide for dose conversion between animals and human. J Basic and clin Pharmacy 7:27–31
Shan S, Tian L, Fang R (2019) Chlorogenic acid exerts beneficial effects in 6-hydroxydopamine-induced neurotoxicity by inhibition of endoplasmic reticulum stress. Med Sci Monit 25:453–459.
Palermo G, Giannoni S, Bellini G, Siciliano G, Ceravolo R (2021) Dopamine transporter imaging, current status of a potential biomarker: a comprehensive review. Int J Mol Sci 22. https://doi.org/10.3390/ijms222011234
Funding
This work was supported by the Chief Scientist of the Ministry of Agriculture, Israel (grant No. 421-0135-09).
Author information
Authors and Affiliations
Contributions
Anat Elmann: Conceptualization, Data curation, Formal analysis, Funding acquisition, Investigation, Methodology, Project administration, Resources, Supervision, Validation, Visualization, Writing - original draft, Writing - review & editing. Alona Telerman: Formal analysis, Investigation. Nativ Dudai: Funding acquisition, Formal analysis, Supervision. Uzi Ravid: Funding acquisition, Formal analysis, Supervision.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethics Approval
1. Preparation of primary cultures: The research was conducted in accordance with the US National Institute of Health (NIH) guidelines for the care and use of laboratory animals and was approved by the National Permit Committee for Animal Science (IL-135/07). 2. In vivo experiments: All animal experiments were carried out in accordance with the US National Institute of Health (NIH) Guidelines for the Care and Use of Laboratory Animals and were approved by the National Permit Committee for Animal Science (IL-10-12-111 and IL-12-12-223).
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic Supplementary Material
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Telerman, A., Ravid, U., Dudai, N. et al. Therapeutic Effects of Geranium Oil in MPTP-Induced Parkinsonian Mouse Model. Plant Foods Hum Nutr 78, 768–775 (2023). https://doi.org/10.1007/s11130-023-01112-3
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11130-023-01112-3