Abstract
The objective of this study is to assess whether elevation of serum inflammatory markers levels may indicate the progression of clinical impairment in Parkinson’s disease (PD) patients. In 47 PD patients, the serum levels of the C3 and C4 part of the complement and Interleukin-6 (IL-6) were measured. The results at baseline and after 2 years were correlated with scales measuring memory, depression, motor symptoms, and quality of life. Patients with higher levels of C3 and C4 at baseline had decreased quality of life, verbal ability, and memory. Patients with higher IL-6 at baseline showed worse depression scores at 2 years. Patients with persistently higher levels of C3 and C4 at 2 years had worse quality of life and memory ability. Uncorrected p values are reported due to the exploratory nature of the study. The results indicate an impact of inflammation on non-motor signs and quality of life in PD. The increase of levels of serum inflammatory biomarkers may indicate the progression of non-motor impairment in PD.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
In the previous studies, we investigated serum biomarkers in PD patients. Decreased Mannan-binding lectin and elevated tumor necrosis factor alpha were present; no abnormalities of IL-6, acute-phase proteins, or factors of the complement system were observed. Our first results indicated that the inflammatory process may be detected in the serum of PD patients (Dufek et al. 2009). Although there was no significant correlation between the patient’s clinical state and the immunoproteins, in a follow-up study of the same cohort, we found that IL-6 elevation may be a marker of increased mortality in PD patients (Dufek et al. 2015). In the current study, the serum levels of the C3 and C4 part of the complement and IL-6 were measured. IL-6 was investigated because of its function in both innate and adaptive immunity. IL-6 stimulates the synthesis of acute-phase proteins by hepatocytes as well as the growth of antibody-producing B lymphocytes. IL-6 also promotes cell-mediated immune reactions by stimulating proinflammatory cytokines and by inhibiting the generation and actions of regulatory T cells (Kang et al. 2015). A meta-analysis of 25 studies demonstrated higher peripheral levels of interleukins in patients with PD than in healthy controls (Qin et al. 2016). Increased serum level concentrations of interleukins were found in idiopathic PD patients with and without vascular risk factors as well as in atypical parkinsonian syndromes (multiple system atrophy and progressive supranuclear palsy) as compared to the control healthy group (Brodacki et al. 2008). A postmortem study showed higher concentration of proinflammatory interleukins in the dopaminergic striatal region in PD patients than in controls (Mogi et al. 1994). In Mogi’s study (1996), authors showed elevated level of interleukins in ventricular cerebrospinal fluid in PD patients and patients with juvenile form of PD. Possible mechanisms that may explain elevated cytokine levels in PD patients is that activated lymphocytes in the blood produce several cytokines. An alternative mechanism through which cytokine levels could be elevated might include the direct efflux of cytokines from the brain through the bloodbrain barrier (BBB). It is known that cytokines may diffuse easily from the brain through the BBB (Brodacki et al. 2008). C3 is the central complement system protein; it is involved in both the classical and the alternative pathway cascades. C4 is, in addition to C3, a marker of the classical complement pathway. Both C3 and C4 are acute-phase proteins. Complement-mediated neuroinflammation was reported in neurodegenerative diseases (Bonifati and Kishore 2007). The complement-activated oligodendroglia revealed by anti-C3d and anti-C4d antibodies was displayed in the substantia nigra in PD, indicating the activation of a complement pathway (Yamada et al. 1992). The complement proteins C3b and C4b were observed as multiple isoforms in the cerebrospinal fluid (CSF) in PD. Changes in complement isoform concentrations were found in the CSF of PD patients when compared to normal subjects (Finehout et al. 2005). The volume of the C3b and C4b isoforms in PD patients were lower than those in normal subjects. A decrease in these components could indicate that they were being depleted by over-activation of the complement system (Finehout et al. 2005). Reactive microglia and reactive astrocytes was closely associated with complement positive extracellular neurofibrillary tangles, indicating an inflammatory response in Guam Parkinson-dementia syndrome (Schwab et al. 1996). As neuroinflammation is evidently present in PD, we hypothesized that it may have, in addition to the neurodegenerative process, an impact on clinical impairment in PD. We presumed that a longitudinal study of these serum markers in a larger cohort could reveal correlations with clinical data. We raised the question: Can elevation of serum levels of IL-6, C3, and C4 complement proteins indicate the progression of motor and non-motor impairment with an impact on quality of life in PD patients?
Subjects
In a longitudinal study, 47 patients who met the UK Brain Bank Criteria for PD (Hughes et al. 1992) (28 males, 19 females, mean age 65 ± 7.8; mean duration of PD 7.7 ± 4.2 at baseline, mean age at onset 58.5 ± 8.1 years) passed the assessment at baseline and after 2 years. We excluded patients with atypical and vascular parkinsonism, serious cognitive impairment, or major depression, or under advanced therapy (deep brain stimulation, apomorphine, and duodopa/carbidopa). None of the patients had systemic inflammatory disease or other serious comorbidity in their personal history; one patient was treated for rheumatoid arthritis with oral steroids. None of the patients took antidepressants, anticholinergics, and inhibitors of acetylcholinesterase. The clinical assessment, neuropsychological testing, and laboratory tests were assessed at the baseline enrollment visit in 2012/2013 in on state and on dopaminergic medication. All investigations were repeated after a 2-year period, in 2014/2015 also in on state and on dopaminergic medication. All patients signed an informed consent form. The ethics committee allocated to Faculty Hospital, Constantine the Philosopher University, Špitálska 6, 94901 Nitra, Slovak Republic approved the study protocol.
Methods
Clinical assessment of PD
The clinical status of PD patients was assessed using the Unified Parkinson’s Disease Rating Scale (UPDRS part I, II, III) (Fahn et al. 1987), Hoehn–Yahr Scale (Hoehn and Yahr 1967), Activity of Daily Living Scale (ADL) (Lawton and Brody 1969), and Barthel Index (Mahoney and Barthel 1965) (BI). A detailed medical history, including antiparkinsonian medication, was available for each patient.
Laboratory assessment
Laboratory tests were performed at the Faculty Hospital in Nitra, Slovakia. The normal reference data used in the hospital laboratory that were obtained by measuring the concentration of biomarkers from the healthy blood donors were used as reference values. All patients had blood tests for the serum level of, CRP, C3 and C4 part of the complement and IL-6. All patients were in a fasting state and in on state when the blood samples were taken. Level of IL-6 was measured by electrochemiluminescence immunoassay method (ECLIA) by commercial test (Elecsys Cobas®). Factors of complement system (C3, C4) and CRP were assessed by nephelometry (Dimension Vista® system Flex reagent® cartridge).
Neuropsychological assessment
Two trained psychologists, each blinded to other clinical and laboratory results, assessed the patients using a battery of tests. The patients underwent the mini-mental state examination (MMSE) (Folstein et al. 1975), Addenbrooke’s cognitive examination (ACE) (Reyes et al. 2009), Benton visual retention test (BVRT) (Benton 1983), the clock drawing test (CDT) (Freedman et al. 1994), the verbal fluency test (VFT) (Auriacombe et al. 1993), two subtests of Wechsler Memory Scale III (WMS III) (Wechsler 1997), and the Montgomery Asberg Depression Rating Scale (MADRS) (Montgomery and Asberg 1979).
Statistical analysis
An independent statistician evaluated the relation between immunological parameters and motor, cognition, depression, and quality of life variables using Spearman’s partial correlation, while controlling for the influence of age, gender, duration of PD, l-DOPA dosage (l-DOPA equivalent), and education. The statistical analysis was performed in four steps. First, immunological parameters were correlated with motor, cognition, depression, and quality of life variables at baseline. Second, the immunological values at baseline were correlated with the difference between the clinical data at baseline and after 2 years. Any significant correlations indicated that the changes in motor, clinical, quality of life, or cognitive data were associated with immunological data at baseline, i.e., the baseline immunological data predicted changes in clinical data. The third step was correlating the differences between the values of immunological and clinical data at baseline and the values after 2 years, indicating an association between the changes in immunological parameters and motor, clinical, quality of life, mood, or cognitive data. The fourth step was correlating the variables at the follow-up assessment after 2 years. Besides, difference between baseline and follow-up assessment was assessed using Wilcoxon test for paired samples. Due to the exploratory nature of the study, we report uncorrected p values.
Results
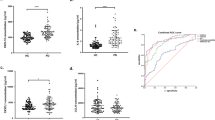
For descriptive statistics of data, see Table 1. After 2 years, there was increase in levels of C3, C4, and IL6 in 26, 17, and 14 patients, respectively. Six patients had increased all three immunomarkers. Other 9 patients had increased C3 and C4 levels, and simultaneous increase in C3 and IL6 was observed in other 5 patients. Significant difference between baseline and follow-up assessment was in l-DOPA equivalent (p = 0.03), UPDRS (p < 0.001), H–Y (p < 0.001), ADL (p = 0.036), and Barthel Index (p < 0.001). There were no statistically significant correlations between immunological factors and motor symptoms or psychological tests in the first and third step of analysis. Table 2 shows following significant results of the second step of analysis: higher values of C3 and C4 at baseline led to lower increases or even decreases in ADL (p = 0.012), VFT semantic (p = 0.026) and BVRT correct scores (p = 0.012). From a clinical point of view, patients who had higher levels of C3 and C4 at baseline presented less improved or even impaired of quality of life, verbal ability, and memory scores at 2 years. Higher values of IL-6 at baseline led to higher increases in MADRS (p = 0.02), indicating an increased risk for the worsening of depression after 2 years. As for the fourth step of analysis, persistently higher levels of C3 and C4 at the follow-up assessment after 2 years correlated with decreased quality of life (p = 0.024) and memory ability (p = 0.034, p = 0.037) after 2 years at follow-up assessment.
Discussion
A prospective 3-year study of unselected cohort of PD cases recruited close to diagnosis was published recently. A large more proinflammatory profile associated with lower MMSE scores and faster motor decline was shown (Williams-Gray et al. 2016). Our study performed on an unselected cohort on consecutive PD patients confirmed the association of proinflammatory with cognitive decline. The MMSE has several shortcomings; in our study, we used a broad set of methods to investigate various aspects of cognitive decline, depression and quality of life to demonstrate an association of proinflammatory markers and non-motor impairment in PD. We focused on basic orientation, visuospatial and constructional disabilities, auditory memory, semantic association, and depression. Our study showed that the patients who had higher levels of C3 and C4 at baseline had less improved or even impaired quality of life, verbal ability and memory, as assessed by a semantic verbal fluency test and BVRT correct score, in 2 years. Patients with higher levels of IL-6 at baseline displayed a worsening of their depression, as assessed by MADRS, after 2 years. These baseline immunological data indicated changes in clinical data. Patients with persistently higher levels of C3 after 2 years had worse quality of life. Patients with persistently higher levels of C4 after 2 years had worse memory ability, as assessed by Wechsler memory test, after 2 years at follow-up visit. Our results confirm a relationship between elevated IL-6 and depression. In a cross-sectional case–control study, IL-6 was significantly higher in the serum of PD patients with depression and fatigue (Lindqvist et al. 2012). Depression, fatigue, and cognitive impairment were also associated with higher CSF levels of inflammatory markers (Lindqvist et al. 2013). The cognitive impairment was not reflected by IL-6 serum level. It is possible that a correlation between cognition and serum IL-6 could be observed with longer observation period and further progression of cognitive impairment. However, lower CSF levels of IL-6 in depressed PD patients (Pålhagen et al. 2010) were also reported. Although we did not assess fatigue separately in our study, fatigue negatively influences quality of life; our results showed a negative influence of elevated complements on quality of life in PD patients. Cross-sectional study by Hoffmann et al. (2009) showed a negative correlation of IL-6 levels and the Activities of Daily Living Scale, indicating that patients with more severe disease had higher levels of IL-6. There were no significant correlations between immunological markers and other psychological tests (CDT, MMSE). We were unable to confirm the negative impact of elevated IL-6 serum levels on motor symptom. This is in contrast to a study demonstrating an impact on gait speed (Scalzo et al. 2010); in addition, association between higher plasma CRP levels and more rapid motor progression in PD has been reported in a cohort of 375 patients (Umemura et al. 2015); however, we did not study gait in detail. Higher CSF levels of IL-6 are associated with the severity of motor impairment (Müller et al. 1998). It is possible that with more detailed testing and/or higher numbers of PD patients, a connection with motor impairment would be observed. Moreover proinflammatory cytokine appeared elevated in idiopathic parkinsonians whose postural and psychomotor responses were abnormal, being suppressed where they were normal: trends which contrasted with those in controls (Dobbs et al. 1999). Our results support the hypothesis that neuroinflammation participates in non-motor impairment in PD. As the CRP levels were in normal range (except one above-mentioned patient with rheumatoid arthritis), a subclinical systemic inflammation that could have influenced the course of PD (Umemura et al. 2015) was probably not present in our cohort. There are many different potential mechanisms by which pro-inflammatory cytokines may generate symptoms of depression, fatigue, and cognitive impairment in PD (Lindqvist et al. 2013). Cytokines may have direct effects on monoaminergic neurotransmission (Lindqvist et al. 2013; Dunn 2006), the hypothalamic–pituitary–adrenal axis (Lindqvist et al. 2013; Berczi et al. 2009), and the kynurenine pathway of tryptophan degradation (Lindqvist et al. 2013; Dantzer et al. 2008). The pro-inflammatory cytokines and other factors secreted by reactive microglia may trigger a neurotoxic cascade that has detrimental effects within the CNS by exacerbating neuronal lesions (Olson and Gendelman 2016). Possible mechanisms for action of cytokines in the pathogenesis of parkinsonism are induction of acute-phase response in brain, antigen presentation, phagocytosis, augmentation of cell-mediated cytotoxicity, interaction with cortisol and TNF-alpha, inhibition of mitochondrial oxidoreductase activity, energy depletion, contribution to oxidative stress, stimulation of glial production of toxins, excitatory amino acids, nitric oxide, reactive oxygen species, increment level of auto-antibodies, and permeability of blood–brain barrier to iron (Dobbs et al. 1999). The present results cannot contribute to the debate whether the activated immune system, which can be measured by level of cytokines (IL-6 and C3 and C4 complements), is a causal factor or a response to neurodegeneration, but it indicates that neuroinflammation may have clinical relevance in non-motor PD symptoms. The limitation of our study was that the relatively small number of patients does not allow generalizing the findings; a confirmation by a larger study is needed. The follow-up period of 2 years is also a limiting factor; it is probable that over a longer period, the deterioration of PD would have been more expressed and correlations would be more significant. We are aware of the fact that correction for multiple testing would result in non-significant results. However, due to the exploratory nature of the study, we report and uncorrected p values. On the other hand, a progression of cognitive impairment (in some cases also improvement) over 2 years was shown in a larger study (Santangelo et al. 2015). Additional research with larger numbers of subjects, greater batteries of immunological tests, and longer periods of study in this field is needed. The question of whether an inflammatory treatment may have a positive impact on PD is not new. It was already shown that non-steroid anti-inflammatory drugs may prevent or delay the onset of PD (Honglei et al. 2003). Promising results were shown in experimental models of PD. Neuroinflammation associated with activated microglial responses contributes to the pathogenesis of PD, making reactive microglia an interesting therapeutic target (Olson and Gendelman 2016). However, there have not been clinical advances using anti-inflammatory agents. On the other hand, the full potential of modern anti-inflammatory treatment has not yet been explored. As we were able to demonstrate a sensitivity of non-motor symptoms to the elevation of inflammatory markers, we suggest that these symptoms, in particular cognition and mood disturbances, should be given more attention in further testing of the therapeutic potential of anti-inflammatory agents.
Abbreviations
- ACE:
-
Addenbrooke’s cognitive examination
- ADL:
-
Activity of Daily Living Scale
- BI:
-
Barthel Index
- BVRT:
-
Benton visual retention test
- CDT:
-
Clock drawing test
- CSF:
-
Cerebrospinal fluid
- ECLIA:
-
Electrochemiluminescence immunoassay
- H–Y:
-
Hoehn–Yahr Scale
- IL-6:
-
Interleukin 6
- MADRS:
-
Montgomery Asberg Depression Rating Scale
- MMSE:
-
Mini-mental state examination
- PD:
-
Parkinson’s disease
- UPDRS:
-
Unified Parkinson’s Disease Rating Scale
- VFT:
-
Verbal fluency test
- WMS:
-
Wechsler Memory Scale
References
Auriacombe S, Grossman M, Carvell S, Gollomp S (1993) Verbal fluency deficits in Parkinson’s disease. Neuropsychology 7:182–192
Benton AL (1983) Contributions to neuropsychological assessment. Oxford University Press, New York
Berczi I, Quintanar-Stephano A, Kovacs K (2009) Neuroimmune regulation in immunocompetence, acute illness, and healing. Ann N Y Acad Sci 1153:220–239
Bonifati DM, Kishore U (2007) Role of complement in neurodegeneration and neuroinflammation. Mol Immunol 44:999–1010
Brodacki B, Staszewski J, Toczyłowska B, Kozłowska E, Drela N, Chalimoniuk M, Stepien A (2008) Serum interleukin (IL-2, IL-10, IL-6, IL-4), TNF-alpha, and IFNgamma concentrations are elevated in patients with atypical and idiopathic parkinsonism. Neurosci Lett 441:158–162
Dantzer R, O’Connor JC, Freund GG, Johnson RW, Kelley KW (2008) From inflammation to sickness and depression: when the immune system subjugates the brain. Nat Rev Neurosci 9:46–56
Dobbs RJ, Charlett A, Purkiss AG, Dobbs SM, Weller C, Peterson DW (1999) Association of circulating TNF-alpha and IL-6 with aging and parkinsonism. Acta Neurol Scand 100:34–41
Dufek M, Hamanová M, Lokaj L, Goldemund D, Rektorová I, Michálková Z, Sheardová K, Rektor I (2009) Serum inflammatory biomarkers in Parkinson’s disease. Parkinsonism Relat Disord 15:318–320
Dufek M, Rektorová I, Thon V, Lokaj L, Rektor I (2015) Interleukin-6 may contribute to mortality in Parkinson’s disease patients: a 4 year prospective study. Parkinson’s Dis 2015:898192
Dunn AJ (2006) Effects of cytokines and infections on brain neurochemistry. Clin Neurosci Res 6:52–68
Fahn S, Elston RL, Members of the UPDRS Development Committee (1987) Unified Parkinson’s disease rating scale. In: Fahn S, Marsden CD, Goldstein M, Calne DB (eds) Recent developments in Parkinson’s disease, vol 2. Macmillan, New York, pp 153–163
Finehout EJ, Franck Z, Lee KH (2005) Complement proteins isoforms in CSF as possible biomarkers for neurodegenerative diseases. Dis Markers 11:93–101
Folstein MF, Folstein SE, McHugh PR (1975) “Mini-mental state”. A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res 12:189–198
Freedman M, Kaplan E, Delis D, Morris R (1994) Clock drawing: a neuropsychological analysis. Oxford University Press, New York
Hoehn MM, Yahr MD (1967) Parkinsonism: onset, progression and mortality. Neurology 17:427–442
Hoffmann KW, Schuh Schumacher AF, SauteJ Townsend R, Fricke D, Leke R, Souza DO, Valmor Portela L, Fagundes Chaves ML, Rieder CRM (2009) Interleukin-6 serum levels in patients with Parkinson’s disease. Neurochem Res 34:1401–1404
Honglei C, Shumin M, Ascherio A (2003) Nonsteroidal anti-inflammatory drugs and the risk of Parkinson disease. Arch Neurol 60:1059–1064
Hughes AJ, Daniel SE, Kilford L, Lees AJ (1992) Accuracy of clinical diagnosis of idiopathic Parkinson’s disease: a clinico-pathological study of 100 cases. J Neurol Neurosurg Psychiatry 55:181–184
Kang Sujin, Tanaka Toshio, Kishimoto Tadamitsu (2015) Therapeutic uses of anti-interleukin-6 receptor antibody. Int Immunol 27(1):21–29
Lawton MP, Brody EM (1969) Assessment of older people: self-maintaining and instrumental activities of daily living. Gerontologist 9:179–186
Lindqvist D, Kaufman E, Brundin L, Hall S, Surova Y, Hansson O (2012) Nonmotor symptoms in patients with Parkinson’s disease—correlations with inflammatory cytokines in serum. PLoS One 7:e47387
Lindqvist D, Hall S, Surova Y, Nielsen HM, Janelidze S, Brundin L, Hansson O (2013) Cerebrospinal fluid inflammatory markers in Parkinson’s disease-associations with depression, fatigue, and cognitive impairment. Brain Behav Immun 33:183–189
Mahoney FI, Barthel DW (1965) Functional evaluation: the BARTHEL index. MD State Med J 14:61–65
Mogi M, Harada M, Kondo T, Riederer P, Inagaki H, Minami M, Nagatsu T (1994) Interleukin-1beta, interleukin-6, epidermal growth factor and transforming growth factor-alpha are elevated in the brain from parkinsonian patients. Neurosci Lett 180:147–150
Mogi M, Harada M, Narabayashi H, Inagaki H, Minami M, Nagatsu T (1996) Interleukin (IL)-1 beta, IL-2, IL-4, IL-6 and transforming factor levels are elevated in ventricular cerebrospinal fluid in juvenile parkinsonism and Parkinson’s disease. Neurosci Lett 211:13–16
Montgomery SA, Asberg MA (1979) A new depression scale designed to be sensitive to change. Br J Psychiatry 134:382–389
Müller T, Blum-Degen D, Przuntek H, Kuhn W (1998) Interleukin-6 levels in cerebrospinal fluid inversely correlate to severity of Parkinson’s disease. Acta Neurol Scand 98:142–144
Olson KE, Gendelman HE (2016) Immunomodulation as a neuroprotective and therapeutic strategy for Parkinson’s disease. Curr Opin Pharmacol 26:87–95
Pålhagen S, Qi H, Mårtensson B, Wålinder J, Granérus AK, Svenningsson P (2010) Monoamines, BDNF, IL-6 and corticosterone in CSF in patients with Parkinson’s disease and major depression. J Neurol 257:524–532
Qin XY, Zhang SP, Cao C, Loh YP, Cheng Y (2016) Aberrations in peripheral inflammatory cytokine levels in Parkinson’s disease: a systematic review and meta-analysis. JAMA Neurol 73:1316–1324
Reyes MA, Perez-Lloret SP, Roldan Gerscovich EG, Martin ME, Leiguarda R, Merello M (2009) Addenbrooke’s Cognitive Examination validation in Parkinson’s disease. Eur J Neurol 16:142–147
Santangelo G, Vitale C, Picillo M, Moccia M, Cuoco S, Longo K, Barone P (2015) Mild Cognitive Impairment in newly diagnosed Parkinson’s disease: a longitudinal prospective study. Parkinsonism Relat Disord 21:1219–1226
Scalzo P, Kümmer A, Cardoso F, Teixeira AL (2010) Serum levels of interleukin-6 are elevated in patients with Parkinson’s disease and correlate with physical performance. Neurosci Lett 468:56–58
Schwab C, Steele JC, McGeer PL (1996) Neurofibrillary tangles of Guam Parkinson-dementia are associated with reactive microglia and complement proteins. Brain Res 707:196–205
Umemura A, Oeda T, Yamamoto K (2015) Baseline plasma C-reactive protein concentrations and motor prognosis in Parkinson disease. PLoS One 10(8):0136722
Wechsler D (1997) Wechsler memory scale the third edition, WMS-III. The Psychological Corporation, San Antonio
Williams-Gray CH, Wijeyekoon R, Yarnall AJ, Lawson RA, Breen DP, Evans JR, ICICLE-PD Study Group et al (2016) Serum immune markers and disease progression in an incident Parkinson’s disease cohort (ICICLE-PD). Mov Disord 31(7):995–1003
Yamada T, McGeer PL, McGeer EG (1992) Lewy bodies in Parkinson’s disease recognized by antibodies to complement proteins. Acta Neuropathol 84:100–104
Acknowledgements
We would like to thank to Prof. Egon Kurča, M.D., Ph.D., FESO, Prof. Peter Valkovič, M.D., Ph.D., and Ján Necpal, M.D., for kindly referring their patients for participation in this study and to Anne Johnson for grammatical assistance.
Funding
The study was supported by the Ministry of Education, Youth and Sports of the Czech Republic under the Project CEITEC 2020 (LQ1601).
Author information
Authors and Affiliations
Contributions
BV: research project: conception, organization, execution; statistical analysis: review and critique; manuscript: writing of the first draft. MD: research project: execution; manuscript: review and critique. VT: research project: execution; manuscript: review and critique. EK: statistical analysis: design, execution, review and critique. MB: research project: execution. SK: research project: execution. TH: research project: execution. IR: research project: conception, organization, execution; statistical analysis: design; manuscript: review and critique.
Corresponding author
Ethics declarations
Conflict of interest
None of the authors report any conflict of interest or financial disclosure.
Rights and permissions
About this article
Cite this article
Veselý, B., Dufek, M., Thon, V. et al. Interleukin 6 and complement serum level study in Parkinson’s disease. J Neural Transm 125, 875–881 (2018). https://doi.org/10.1007/s00702-018-1857-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00702-018-1857-5