Abstract
The aim of this study was to assess the efficiency of different drying methods (room, sun, oven, microwave, cross-flow, infra-red, dehumidifier, and freeze-drying) on maximum retention of the nutritional and bioactive compounds profiling of Talinum triangulare, which is a less-known perishable leafy-vegetable. The evaluation of various drying methods is to learn the best appropriate strategy for a post-harvest drying method for retaining all the potential benefits with minor loss. Microwave and freeze-dried samples held the maximum ascorbic acid quantified by HPLC with 1.36 and 1.11 g/100 g DW, respectively. The main carotenoid compounds identified were violaxanthin, lutein, zeaxanthin, β-carotene isomers, trans-β-carotene, and cis-β-carotenes. Gallic, protocatechuic, catechin, para-coumaric, ferulic, rutin, trans-cinnamic, and quercetin are the significant phenolics and flavonoids identified and quantified by liquid chromatography. The efficiency of different solvents on bioactive extractions uncovered that the methanol and 80% aqueous ethanol were good for retention of total phenolics, total flavonoids, and antioxidant compounds, which was affirmed through phosphomolybdate, DPPH, and FRAP assays. Dried T. triangulare foliage could be productively utilized as a promising raw material for food and pharma businesses because of its rich bioactive composition.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Rapid changes in diets and lifestyles have a significant impact on the health and nutritional status of populations, particularly in developing countries. India, being a tropical country with varying climates and seasons, grows a variety of leafy vegetables (LVs), some of which are locally grown and utilized. LVs are most often from short-lived herbaceous plants and are rich sources of vitamins, especially β-carotene, riboflavin, folic acid, ascorbic acid, and minerals such as calcium, iron, and phosphorous [1]. Intake of LVs in our daily diet may enrich the essential micronutrients as well as dietary fiber with other vital nutrients. LVs wilt within a short span after harvesting due to transpiration and respiration activities; consequently, this leads to the forfeiture in quality of nutritional components. Therefore, optimization of suitable preservation methods such as blanching, dehydration, or drying can overcome the loss of essential nutraceutical molecules in the LVs for effective utilization and preservation. Moreover, the optimization of these regime steps can help in the exploitation of LVs to bring down the micronutrient malnutrition in developing countries to increase the occurrence of chronic degenerative diseases globally [2].
Talinum triangulare L., commonly known as ‘Common man’s spinach’ or ‘Ceylon Spinach’, is a non-conventional LV crop belonging to the family Talinaceae, originating from tropical Africa and widely grown in Asia, West Africa, and South America. T. triangulare leaves are spirally arranged with often crowded leaves at the top of the stem and bright pink flowers [3]. It is a fast-growing plant (35–45 days) in water lodged places, hence its being popularly known as waterleaf [3, 4]. Nutritionally, the leaves are a good source of fiber and minerals, especially potassium, calcium, and magnesium, which contribute to assembling the micronutrients for daily nutritional requirements. It is reported that T. triangulare had the highest amount of riboflavin (0.18 g/100 g FW) [5]. A good number of reports explained its rich phytoconstituents profile, vitamins, considerably high crude protein (22.1%), crude fiber (11.12%), cardiac glycosides, omega-3-fatty acids, flavonoids, and polyphenols [6]. The antioxidant activities and high kaempferol content from leaf extracts of T. triangulare have been reported recently [7]. The use of leaves and young shoots to thicken the sauce and their consumption in large quantities is known in the southern part of Nigeria [8]. Conferring to the traditional medicine system of India, T. triangulare leaves are used to treat polyuria, inflammation, heat, gastrointestinal disorders, measles, hepatic ailments, kidney disorders, gout, rheumatoid arthritis, cancer, and anemia in pregnant women and growing children [9]. Though there are numerous health and nutritional benefits of T. triangulare, the knowledge on effective preservation, phytoconstituents evaluation, and sustainable utilization of dehydrated leaves are limited.
Therefore, the present work was focused on the consequences of eight different drying methods (room, sun, oven, microwave, cross-flow, infra-red, and freeze and dehumidifier drying methods) and fresh leaves evaluation along with the extraction efficacy of different solvents on the extraction of nutritional and nutraceutical components from T. triangulare.
Materials and Methods
Plant Material
T. triangulare leaves were harvested from garden grown plants of the CFTRI campus from June to October 2018. The temperature variation during this period were 30 and 25 °C, respectively, with an average rainfall of 2–4 cm. The herbarium specimen of T. triangulare plant was submitted at Herbarium Center, Yuvaraja’s College, Department of Botany (Accession No: 0274). The leaves were sorted and washed with tap water thrice, followed by spreading on hand made blotting paper to remove water. Later on the leaves were used for different drying methods.
Drying Methods
Freshly harvested leaves of T. triangulare (1 kg) were exposed to eight different drying methods such as room, sun, oven, microwave, cross-flow, dehumidifier, Infra-red, and freeze drying, and fresh leaves (FL) were also analyzed for phytoconstituents. In room-drying (RD), the leaves were spread over the floor in a room at 27–32 °C for five days for complete drying of the leaves. In sun-drying (SD), the leaves were spread under sunlight at 35–38 °C mid-day temperature for two days. Oven drying (OD) (Ecocell, MMM group, Germany) involves drying of leaves for 5 h at 55 °C. Microwave drying (MWD) (Samsung, M183DN, with Triple Distribution System) was done at 850 W for 3 min. Cross-flow drying (CFD) (Technico Lab Products Pvt. Ltd) was performed for 16 h at 50–55 °C. In dehumidifier drying (DHD), the leaves were dried for 6 h at 55 °C. Infra-red drying (IRD) was done in CSIR-CFTRI developed machine with 1.1 to 1.3 μm wavelength or radiation for 5 h at 50–55 °C. In freeze-drying (FD), the leaves were lyophilized overnight in a vacuum tray at 0.012 mbar at −110 °C freeze dryer (Scanvac coolsafe™, Denmark). The dried leaf material was collected and grinded using a mixer (Maharaja Whiteline Perfect W&R 500 Mixer grinder) for 20 min at high speed. The particle size of powdered leaf material was analyzed by using a particle size analyzer (Microtrac Inc. Blue Wave, USA) to maintain a uniform particle size of almost 250–270 μm in all the dried leaves and stored in polythene air tight glass containers until further use. For comparative analysis, the fresh leaves were also simultaneously analyzed with the different dried leaf samples.
Determination of Moisture Content
The moisture content of fresh and different dried leaf powder material was measured according to the AOCS method to know the ultimate moisture content [10].
Color Measurement
The color of the fresh and different dried leaf powder samples were monitored by measuring L, a* and b* values using a color measurement instrument (Konica Minolta CM-5, VA, USA). The L values represent the lightness or darkness, a* values refer to the red-green color, and b* values refer to blue-yellow color [11]. The L, a*, and b* values were used to calculate the chroma and Hue angle.
Determination of Chlorophyll Content
The fresh and different dried leaf powder samples were extracted using mortar and pestle with acetone (1:10 w/v) and centrifuged at 7,000×g for 10 min, and the clear supernatant was collected. The absorbance of the collected supernatant was measured at 661.5, 663, and 645 nm with a double beam spectrophotometer (UV-160 A, Shimadzu Corporation, Kyoto, Japan), and the concentration of chlorophyll a, b, and total were calculated and compared with two standard methods [12, 13].
Extraction and Estimation of Ascorbic Acid by Using HPLC
Ascorbic acid from fresh and various dried leaf powder samples were extracted under subdued light with some modification [14]. A known quantity of leaf sample (1 g) was extracted with 2 ml of methanol and 10 ml of cold extraction solution, containing 3% meta phosphoric acid (w/v), 0.05% ethylenediaminetetraacetic acid (w/v) and 0.8% glacial acetic acid (v/v). The homogenate was centrifuged at 7,000×g for 15 min at 4 °C. Then the supernatant was collected and filtered (0.45 μm), and chromatographic analysis of ascorbic acid was carried out in LC 20 AD (M/s Shimadzu Corporation, Kyoto, Japan), Chromatopak C18 (150 × 4.6 mm i.d., 5 μ particle size) was used for separation, and the mobile phase was 50 mM K2HPO4 adjusted to pH 7 (Solvent A) and 100% methanol (Solvent B), with a linear gradient at a flow rate of 1 ml/min. Ascorbic acid was detected at 254 nm and quantified with standard ascrobic acid calibration curve (R2 = 0.992).
HPLC Analysis of Carotenoids Compounds
HPLC identification of carotenoids in HPLC grade acetone extracts (1:10, w/v) was carried out by an LC 20 AD HPLC with Princeton C-30 (ODS) column (250 × 4.6 mm; 5 μm) without saponification. The mobile phase consists of acetonitrile/methanol/dichloromethane (60:20:20, v/v/v) containing 0.1% ammonium acetate at a flow rate of 1 ml/min, and carotenoids were detected at 450 nm. The carotenoids were identified by comparing the retention times reported earlier [15].
Sample Extraction for Antioxidant Assays
The extraction efficiency of antioxidant compounds in different solvents, namely methanol and 80% ethanol, hot water, and cold water, was tested against fresh and dried leaf powders of T. triangulare following an earlier reported method [16]. Briefly, about 2.5 g leaf powder and fresh leaves were extracted with 50 ml of solvent by using mortar and pestle, shaken at 120 rpm in a gyro rotary shaker for 30 min, and centrifuged at 10,000 rpm for 10 min. The pellet was collected and re-extracted, and both the supernatants were pooled and stored in amber tubes to avoid light interference.
Determination of Total Phenolics Content (TPC)
Total phenolic content (TPC) of the above-prepared extracts was determined using the Folin-Ciocalteu method [17]. The extract (0.2 ml) was pipetted out into a test tube and used for further analysis. The volume in all the tubes was made up to 3 ml with distilled water. Of the Folin-Ciocalteu reagent (1:1 diluted with distilled water), 0.5 ml was added into each test tube and incubated for 3 min. Of the 20% Na2CO3 solution, 2 ml was added to each tube. The tubes were vortexed and placed in a boiling water bath for exactly 1 min. The absorbance was measured at 650 nm, and the amount of phenolics present in the samples was obtained by plotting against the standard graph. The TPC was expressed as gallic acid equivalent (GAEq.) in g/100 g of dry leaf material.
Estimation of Total Flavonoid Content (TFC)
To the 1 ml of diluted sample extract with absolute ethanol, 1 ml of 2% (w/v) methanolic solution of AlCl3 was added [18]. After incubation at room temperature for 15 min, the absorbance of the reaction mixture was read at 430 nm. The results were expressed as rutin equivalent (REq.) in g/100 g of dry leaf material.
Separation of Phenolics and Flavonoids by HPLC
Phenolics and flavonoids were extracted with ethyl acetate until the macerate became colorless and passed through anhydrous sodium sulfate. The obtained clear filtrate was completely evaporated (Hei-VAP Advantage, Heidolph Instrument GmbH & Co. KG, Schwabach, Germany) to dryness and re-dissolved in HPLC grade methanol and filtered through a 0.22 μm filter before HPLC analysis. The separation was performed by reverse phase HPLC (Shimadzu LC 20A; Shimadzu Corp.) fitted with a C18 column (250 × 4.6, 5 μm, Waters Corporation, Milford, MA, USA) and UV detector [19]. A binary solvent system, consisting of filtered Milli-Q water adjusted to pH 2.6 with acetic acid as solvent A and 80% acetonitrile as solvent B, was run for 60 min at a flow rate of 1.2 ml/min. The phenolics and flavonoids were detected at 280 and 320 nm using standards gallic, protocatechuic, catechin, syringic, para-coumaric, chlorogenic, ferulic, sinapic, rutin, trans-cinnamic acid, and quercetin.
Determination of Total Antioxidant Assay (TAA) by Phosphomolybdenum Method
The TAA of a known quantity of extract (2 mg/ml) was combined with sodium phosphate and ammonium molybdate, and the mixture was incubated at 95 °C for 90 min, then the absorbance of the mixture was measured at 695 nm against blank after cooling to room temperature [20]. The TAA was expressed as the number of gram equivalents of ascorbic acid (AA) in g/100 g of dry leaf material.
DPPH Free Radical Scavenging Activity
Different dilutions of extract were taken in different test tubes. Accordingly, DPPH (3.94 mg/100 ml methanol) was added to make the total volume 2 ml. The reaction mixture was vortexed properly and incubated in dark for 15 min, and absorbance was measured at 517 nm [21].
Ferric Reducing Antioxidant Power (FRAP)7
The FRAP assay was done according to the method described by Oyiazu [22]. The fresh, different dried, and solvent extracts were diluted and analyzed. Different dilutions of each extract (1 ml) were mixed with 2.5 ml of phosphate buffer (0.2 M, pH 6.6) and 2.5 ml of potassium ferricyanide [K3Fe (CN)6] (1%, w/v), and then the mixture was incubated for 30 min at 50 °C. Then, 2.5 ml of 10% trichloro acetic acid (TCA) was added to this mixture, which was centrifuged at 3,000 rpm for 10 min. Finally, 2.5 ml of the upper layer solution was taken and mixed with 2.5 ml of distilled water and 0.5 ml of 0.1% ferric chloride. The procedure was carried in triplicate and absorbance was measured at 700 nm. The absorbance obtained was converted to gram equivalent of ascorbic acid for 100 g of dry leaf material.
Statistical Analysis
The data in the manuscript have been presented in mean ± S.D. of three replicates (n = 3). One-way ANOVA was performed by using Microsoft Excel. The values with P < 0.05 were considered significant.
Results and Discussion
Determination of Moisture Content
The moisture content in the fresh leaf samples was found to be 95.7%. Similarly, after drying, the final moisture content of various dying methods was recorded in the range of 1.32 to 3.91%, respectively (Fig. 1a). The lowest moisture content was recorded in the sun-dried sample (1.32%) and the highest in oven-dried leaf (3.91%). The results were concomitant with other popular LVs, viz. Hibiscus sabdariffa L. and Moringa oleifera that were subjected to various drying methods [16, 23].
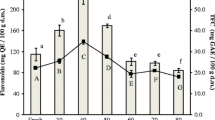
Moisture content (a) colour measurement values (b) and HPLC quantified ascorbic acid content (c) of the different dried leaf powders and fresh leaves of T. triangulare. FL-Fresh leaf, RD-Room dried, SD-Sun dried, OD-Oven dried, MWD-Microwave dried, CFD-Cross-flow dried, DHD-Dehumidifier dried, IRD-Infra-red dried, FD-Freeze dried. Values are mean ± SD and values with different superscript were found significant difference from each other
Color Measurement
The color is an important factor to be observed during dehydration to check the color changes (chroma values) of different dried leaf material. It indicates the pigment degradation such as chlorophyll and carotenoids, etc. The fresh and oven and cross-flow dried leaves have better retentions of green color in T. triangulare, which was evidenced by the L, chroma, and hue values (Fig. 1b). Similarly, the room and sun-dried samples showed less chroma values due to the dark green color of the powders. The changes in pigment color were caused by thermal treatment and oxidation during drying [11]. A longer drying time and higher temperature will lead to significantly greater pigment losses. Similar color measurement observation was also reported in various H. sabdariffa dried leaf powders recently [16].
Determination of Chlorophyll
Chlorophyll content of various dried and fresh leaf material is presented in Table 1. The temperature treated samples had greater degradation of chlorophyll pigment than the non-thermal drying. The freeze and infra-red dried samples retained maximum total chlorophyll content with 584 and 413 mg/100 g by the Lictenthaler method. When compared to Lictenthaler, the Arnon method showed higher chlorophyll content (12–18%), and this data was supported by earlier reports to compare both the methods advocated in H. sabdariffa dried leaves [16].
Estimation of Ascorbic Acid by Using HPLC
Ascorbic acid is a sensitive antioxidant compound, and the retention of it will be served as a quality parameter in evaluating the influence of post-harvest preservation techniques. Drying methods have varied effects on the retention of ascorbic acid in T. triangulare. Microwave and freeze-dried samples have maximum ascorbic acid retention with 1.36 and 1.11 g/100 g DW, respectively (Fig. 1c). However, the room and sun-drying have the lowest retention of ascorbic acid, with 0.19 and 0.25 g/100 g DW, respectively. The difference in ascorbic acid content is attributed to the drying time, temperature of drying, and effect of light that affected the volatilization of acids [11]. Ascorbic acid is an important compound that is helpful in regulating blood pressure, lowering and removing cholesterol from the arterial walls, hence preventing arteriosclerosis [24]. Earlier reports stated that 15–20% degradation of ascorbic acid during blenching, drying, and storage [25].
HPLC Analysis of Carotenoids Compounds
The carotenoid compounds such as antheraxanthin, lutein, violaxanthin, zeaxanthin, neoxanthin, isomers of β-carotene, trans-β-carotene, and cis-β-carotene were detected in HPLC of various dried and fresh leaves of T. triangulare, respectively (Fig. 2). A similar trend in the carotenoid profile was also observed in Basella spp. leaves reported earlier [16, 26]. These identified carotenoids, and isomers of carotenoids were detected earlier in fruits and green leafy vegetables [27].
HPLC chromatogram of carotenoids identified in microwave dried-MWD (a) and cross-flow dried-CFD (b) samples of the different dried leaf powders of T. triangulare. 1. Violaxanthin, 2. antheraxanthin, 3. neoxanthin, 4. lutein, 5. zeaxanthin, 6. isomers of β-carotene, 7. isomers of α-carotene, 8. transβ-carotene and 9. cisβ-carotene
Determination of Total Phenolics Content (TPC)
Phenolics compounds are plant secondary metabolites with an aromatic ring structure widely distributed in the plant kingdom that are linked with the color, sensory qualities, and antioxidant activities of the foods. The redox potentials of the phenolic compounds are the key factors for its antioxidant properties. The TPC profiles of the various dried and different solvent-based extraction quantities of the T. triangulare have been shown (Fig. 3a). There is a significant variation in TPC content in different solvent extractives and various dried leaf material. The methanol microwave (0.67 g/100 g DW) and infra-red (0.61 g/100 g DW) dried leaf samples showed the highest TPC content compared to other drying methods used. Similarly, the 80% aqueous ethanol extracts of microwave (1.09 g/100 g DW) and dehumidifier (0.94 g/100 g DW) dried material showed higher TPC content. The solvent based extraction denatures the polyphenol oxidase in the plant cell wall, and thus the extraction efficiency is more in methanol and aqueous ethanol compared with hot and cold water, respectively [16].
Total phenolics (a), total flavonoids (b) and HPLC separation (c, d) quantification profile of the different dried leaf powders and fresh leaves of T. triangulare. FL-Fresh leaf, RD-Room dried, SD-Sun dried, OD-Oven dried, MWD-Microwave dried, CFD-Cross-flow dried, DHD-Dehumidifier dried, IRD-Infra-red dried, FD-Freeze dried. Values are mean ± SD and values with different superscript were found significant difference from each other
Estimation of Total Flavonoid Content (TFC)
Figure 3b shows the TFC of the various dried and different solvent extraction efficiencies of T. triangulare. The TFC is more soluble in organic solvents than in water. The microwave (3.52 g/100 g DW), freeze (2.99 g/100 g DW), and oven (2.22 g/100 g DW) dried methanol extractives exhibited the highest TFC contents. However, the fresh leaf showed 0.33 g/100 g FW TFC content in methanol extracts. Similarly, the cold and hot water extraction did not much affect the extraction of TFC for all the dried T. triangulare samples. A similar trend of TFC in different H. sabdariffa dried leaf powders was reported recently [16].
Separation of Phenolics and Flavonoids by HPLC
The HPLC separation of individual phenolics and flavonoids in the various dried leaf samples are shown in Fig. 3c, d. The main phenolics and flavonoids quantified were gallic, protocatechuic, catechin, syringic, para-coumaric, chlorogenic, ferulic, sinapic, rutin, trans-cinnamic acid, and quercetin, respectively. However, catechin, gallic, syringic, and rutin were major in various dried leaf materials. In infra-red drying, the catechin (23.63 mg/100 g DW) was found to be highest.
Determination of Total Antioxidant Assay by Phosphomolybdenum Method
All the sample extractives were used for the determination of phosphomolybdenum complexes by the samples to know the antioxidant capacities. This method is mainly based on the reduction of Mo(VI) to Mo(V) by antioxidant compounds, which in turn, form the green color complex that has an absorbance maxima of 695 nm. The different dried leaf samples and various solvents showed various degrees of antioxidant activities (Fig. 4a). The microwave samples showed the highest retention of antioxidant compounds in all the extracts such as methanol (2.27 g/100 g DW), aqueous ethanol (2.8 g/100 g DW), hot water (2.11 g/100 g DW), and cold water (2.23 g/100 g DW). A similar trend in phosphomolybdenum reduction antioxidant assay was also reported for H. sabdariffa foliage dried using different methods [16].
Total antioxidant assay (a), DPPH radical scavenging activity (EC50) (b) and ferric reducing antioxidant activity (c) of the different dried leaf powders and fresh leaves of T. triangulare. FL-Fresh leaf, RD-Room dried, SD-Sun dried, OD-Oven dried, MWD-Microwave dried, CFD-Cross-flow dried, DHD-Dehumidifier dried, IRD-Infra-red dried, FD-Freeze dried. Values are mean ± SD and values with different superscript were found significant difference from each other
DPPH Free Radical Scavenging Activity
It is a widely used rapid and reproducible method for the analysis of the antioxidant activity in plant and foliage extracts [16]. The reduction of DPPH free radicals by the plant extracts in different solvents, which will reduce the color of DPPH to colorless based on the content of antioxidant capacity of the extracts, which will be read at 517 nm. The DPPH scavenging activity assay is shown in Fig. 4b. The oven, microwave, and cross-flow dried samples showed lesser EC50 values, indicating that they have good retention of antioxidant compounds. The oven dried methanol extract exhibited a 0.82 mg/ml EC50 value. Similarly, the microwave 80% ethanol extract showed still a lower 0.64 mg/ml EC50 value. The variation in antioxidant activity of the ginger leaves and H. sabdariffa leaves of various extracts have been reported [16, 28]. Phenolic and flavonoid compounds contribute mostly to the antioxidant activity, and the present results also correlated with their content. The variation in bioactive profiles during cooking of T. triangulare leaves was reported [29].
Ferric Reducing Antioxidant Power (FRAP)
The FRAP capability to reduce the ferric ions by various extracts of different dried leaf samples of T. triangulare is shown in Fig. 4c. The 80% aqueous ethanol dehumidifier (7.81 g/100 g), cross-flow (7.49 g/100 g), microwave (6.99 g/100 g), and oven (6.62 g/100 g) dried samples showed the highest FRAP activity compared to the other samples dried. However, the hot water extracts also showed a good FRAP activity in an oven, cross-flow, and dehumidifier drier. In food systems, the ferrous iron is most common, and its redox potential play an important role in cellular oxidative stress leading to various physiological dysfunctions. The T. triangulare showed a good chelating activity by capturing the ferrous ions before ferrozine. A similar trend was noticed in other leafy vegetables such as H. sabdariffa [2, 16].
Conclusion
The plant foliage processing, preservation, and post-harvest effective utilization of T. triangulare could be efficiently used as a promising raw material for food and pharma industries due to its rich bioactive composition. The oven, microwave, and infra-red drying of leaves showed good quality retention of nutrients and antioxidant activities to use throughout the year unlike fresh LVs, which are perishable. The efficiency of different solvents used for extraction of bioactive compounds, such as carotenoids, phenolics, flavonoids, and in-vitro antioxidant potentials, will have implications for their presence in dried leaf powders for preparing ready-to-serve food formulations.
References
Gupta S, Lakshmi AJ, Manjunath MN, Prakash J (2005) Analysis of nutrient and anti-nutrient content of underutilized green leafy vegetables. LWT-Food Sci Technol 38:339–345. https://doi.org/10.1016/j.lwt.2004.06.012
Gupta S, Prakash J (2009) Studies on Indian green leafy vegetables for their antioxidant activity. Plant Foods Hum Nutr 64:39–45. https://doi.org/10.1007/s11130-008-0096-6
Ezekwe CI, Uzomba CR, Ugwu OPC (2013) The effect of methanol extract of Talinum triangulare (water leaf) on the hematology and some liver parameters of experimental rats. Glob J Biotechnol Biochem 8:51–60. https://doi.org/10.5829/idosi.gjbb.2013.8.2.1109
Fontem DA, Schippers RR (2010) Talinum triangulare (Jacq.) Willd [internet] record from Protabase: Grubben GJH, Denton OA (editors). PROTA (Plant Resources of Tropical Africa/Ressources végétales de l’Afrique tropicale), Wageningen, Netherlands
Ogbonnaya EC, Chinedum EK (2013) Vitamin and carotenoid composition of raw and decoctions of water leaf (Talinum triangulare). Biochem Pharmacol 2:1–3. https://doi.org/10.4172/2167-0501.1000121
Yang RY, Tsou SC, Lee TC, Wu WJ, Hanson PM, Kuo G, Lai PY (2006) Distribution of 127 edible plant species for antioxidant activities by two assays. J Sci Food Agric 86:2395–2403. https://doi.org/10.1002/jsfa.2630
Ezekwe MO, Besong SA, Igbokwe PE, Ezekwe EI (2002) Beneficial influence of Purslane and waterleaf supplementation on cardiovascular disease risk in humans. FASEB J 16:A639–A639
Ibeawuchi II, Nwufo MI, Oti NN, Opara CC, Eshett ET (2007) Productivity of intercropped green (Amaranthus cruentus)/waterleaf (Talinum triangulare) with poultry manure rates in southeastern Nigeria. Plant Sci 2:222–227. https://doi.org/10.3923/jps.2007.222.227
Swarna J, Ravindhran R (2013) Pharmacognostical and phytochemical evaluation of Talinum triangulare (Jacq.) Willd. Int J Pharm Pharm Sci 2:249
AOCS (2003) Official methods and recommended practices of the American Oil Chemist’s Society. In: Moisture content: Da 2a-48, 5th edn. AOCS Press, Champaign
Vega-Galvez A, Di Scala K, Rodriguez K, Lemus-Mondaca R, Miranda M, López J, Pérez-Won M (2009) Effect of air-drying temperature on physico-chemical properties, antioxidant capacity, colour and total phenolic content of red pepper (Capsicum annuum, L. var. Hungarian). Food Chem 117:647–653. https://doi.org/10.1016/j.foodchem.2009.04.066
Arnon DI (1949) Copper enzymes in isolated chloroplasts. Polyphenol oxidase in Beta vulgaris. Plant Physiol 24:1. https://doi.org/10.1104/pp.24.1.1
Lichtenthaler HK (1996) Vegetation stress: an introduction to the stress concept in plants. J Plant Physiol 148:4–14. https://doi.org/10.1016/S0176-1617(96)80287-2
Vanderslice JT, Higgs DJ, Hayes JM, Block G (1990) Ascorbic acid and dehydroascorbic acid content of foods-as-eaten. J Food Compos Anal 3:105–118. https://doi.org/10.1016/0889-1575(90)90018-H
Simon DP, Anila N, Gayathri K, Sarada R (2016) Heterologous expression of β-carotene hydroxylase in Dunaliella salina by agrobacterium-mediated genetic transformation. Algal Res 18:257–265. https://doi.org/10.1016/j.algal.2016.06.017
Kumar SS, Manoj P, Shetty NP, Giridhar P (2015) Effect of different drying methods on chlorophyll, ascorbic acid and antioxidant compounds retention of leaves of Hibiscus sabdariffa L. J Sci Food Agric 95:1812–1820. https://doi.org/10.1002/jsfa.6879
Sadasivam S, Manickam A (2008) Biochemical methods, 8th edn. New Age International Publisher, New Delhi
Djeridane A, Yousfi M, Nadjemi B, Boutassouna D, Stocker P, Vidal N (2006) Antioxidant activity of some Algerian medicinal plants extracts containing phenolic compounds. Food Chem 97:654–660. https://doi.org/10.1016/j.foodchem.2005.04.028
Burin VM, Arcari SG, Bordignon-Luiz AMT, Costa LLF (2011) Determination of some phenolic compounds in red wine by RP-HPLC: method development and validation. J Chromatogr Sci 49:647–651. https://doi.org/10.1093/chrsci/49.8.647
Prieto P, Pineda M, Aguilar M (1999) Spectrophotometric quantitation of antioxidant capacity through the formation of a phosphomolybdenum complex: specific application to the determination of vitamin E. Anal Biochem 269:337–341. https://doi.org/10.1006/abio.1999.4019
Khan MI, Harsha PS, Giridhar P, Ravishankar GA (2012) Pigment identification, nutritional composition, bioactivity, and in vitro cancer cell cytotoxicity of Rivina humilis L. berries, potential source of betalains. LWT-Food Sci Technol 47:315–323. https://doi.org/10.1016/j.lwt.2012.01.025
Oyiazu M (1986) Studies on products of browning reactions: anti-oxidative activities of products of browning reaction prepared from glucosamine. Jpn J Nutr 44:307–315. https://doi.org/10.5264/eiyogakuzashi.44.307
Saini RK, Shetty NP, Prakash M, Giridhar P (2014) Effect of dehydration methods on retention of carotenoids, tocopherols, ascorbic acid and antioxidant activity in Moringa oleifera leaves and preparation of a RTE product. J Food Sci Technol 51:2176–2182. https://doi.org/10.1007/s13197-014-1264-3
Addo AA (2004) Seasonal availability of dietary ascorbic acid and incidence of scurvy in northern state of Nigeria. PhD Thesis, Department of Biochemistry, Ahmadu Bello University, Zaria
Van Duyne FO, Bruckart SM, Chase JT, Simpson JI (1945) Ascorbic acid content of freshly harvested vegetables. J Am Diet Assoc 21:153–154. https://www.cabdirect.org/cabdirect/abstract/19451400391
Raju M, Varakumar S, Lakshminarayana R, Krishnakantha TP, Baskaran V (2007) Carotenoid composition and vitamin a activity of medicinally important green leafy vegetables. Food Chem 101:1598–1605. https://doi.org/10.1016/j.foodchem.2006.04.015
Khoo HE, Prasad KN, Kong KW, Jiang Y, Ismail A (2011) Carotenoids and their isomers: color pigments in fruits and vegetables. Molecules 16:1710–1738. https://doi.org/10.3390/molecules16021710
Daniel DL, Huerta BB, Sosa IA, Mendoza MV (2012) Effect of fixed bed drying on the retention of phenolic compounds, anthocyanins and antioxidant activity of roselle (Hibiscus sabdariffa L.). Ind Crop Prod 40:268–276. https://doi.org/10.1016/j.indcrop.2012.03.015
Eleazu CO, Eleazu KC (2013) Bioactive constituents and in vitro antioxidant capacity of water leaf (Talinum triangulare) as affected by domestic cooking. Eur J Med Plants 3:540
Acknowledgements
The author (SSK) is grateful to CSIR New Delhi for the award of SRF fellowship and Department of Biotechnology (BT/PR16902/NER/95/422/2015), Government of India, New Delhi, for financial assistance. We greatly acknowledge the Director, CSIR-CFTRI, for his kind support.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare no conflict of interest.
Human and Animal Participants
This article does not contain any studies with human or animal subjects.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Kumar, S.S., Arya, M., Nagbhushan, P. et al. Evaluation of Various Drying Methods on Bioactives, Ascorbic Acid and Antioxidant Potentials of Talinum triangulare L., foliage. Plant Foods Hum Nutr 75, 283–291 (2020). https://doi.org/10.1007/s11130-020-00804-4
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11130-020-00804-4