Abstract
The prebiotic effect of different concentrations of inulin (0, 1 and 2%) on the growth and survival of Lactobacillus plantarum (LP) CECT 220 in blended carrot and orange juices was investigated after 24 h of fermentation, during 30 days of storage at 4 °C and through the phases of gastrointestinal digestion after different storage periods. Microbiological and chemical determinations were also carried out in all juices. The lactic fermentation increased the shelf life of the fermented juices with inulin. The hygienic-sanitary quality in fermented juices was better than the control juices. During storage, the inulin improved the viability of LP and the monosaccharide concentration remained higher with respect to the juice without inulin (40% lower). At 30 days, the fermented juices with 2% inulin after in vitro digestion presented the highest survival of L. plantarum.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Products containing probiotics and prebiotics are known as synbiotic foods. Probiotics consist mainly of Lactobacillus and Bifidobacterium strains together with other species such as Lactococcus, Enterococcus and Streptococcus [1, 2]. In addition, these bacteria should be resistant to processing and storage conditions and survive gastrointestinal digestion and be able to reach the colon in sufficient amount [3], hence, their concentration in foods has to be in the range of 106–107 UFC/mL or gram at the moment of consumption [1]. Lactobacillus plantarum is a homo-fermentative lactic acid bacteria (LAB) that produces only lactic acid but can also metabolize a variety of sugars, growing at the same time on different surfaces and substrates such as meat, dairy products and vegetables and frequently in the intestinal tract [4]. Prebiotics are non-digestible food ingredients that are fermented in the colon by beneficial bacteria (Lactobacillus and Bifidobacterium), stimulating their growth and metabolic activity [5, 6]. Inulin is considered to be a prebiotic and can be added to food without affecting taste to stimulate the LAB [7].
The development of non-dairy probiotic or synbiotic foods from fruits and vegetables has a high potential for the food industry, owing to the growing trend on the market for vegetarian foods, together with the high percentage of lactose intolerant people and the presence of cholesterol in dairy products [8]. Hence, there are nutritional reasons for testing lactic acid fermentation as a potential process for production of fermented juice from fruits and vegetables [9]. During storage of fermented drinks, the low pH, the nutrient depletion and the accumulation of lactic acid is a challenge for the survival of probiotic bacteria being difficult to keep the right microbial doses at the time of consumption [10].
The aim of the present study was to investigate the prebiotic effect of different concentrations of inulin on fermented blended carrot and orange juice, through the measurement of the growth of L. plantarum after 24 h of fermentation, during 30 days of refrigerated storage and during simulated human digestion in relation to the storage period. The amounts of inulin, sugars and organic acids were also determined in fermented juices during fermentation and storage.
Materials and Methods
Bacterial Strain and Culture Conditions
A lyophilized culture of Lactobacillus plantarum CECT 220 (LP) was obtained from the Spanish Type Culture Collection (CECT, Valencia, Spain). The bacterial strain was prepared according to the method described by Valero-Cases and Frutos [11]. The lyophilized microorganism was re-suspended in 10 mL of the Man Rogosa Sharpe (MRS) broth (Oxoid; Madrid, Spain) for 48 h at 37 °C (pre-inoculum). To obtain an initial microbial count of around 108 colony forming units per mL (CFU/mL), the pre-inoculum (1%, v/v) was inoculated in MRS broth and incubated during 24 h at 37 °C. The biomass was harvested by centrifugation at 2000×g for 10 min at 4 °C, and washed twice with sterile phosphate buffer saline (PBS) (Oxoid; Madrid, Spain) and stored in 10% (v/v) of glycerol at −80 °C until use.
Development and Fermentation of Blended Carrot and Orange Juice Fortified with Inulin
The juice was prepared using 2 kg of carrots (Daucus carota L. cv. Nantesa) and 1 kg of oranges (Citrus sinensis L. cv. Valencia-Late) from a local market in Orihuela (Alicante). The vegetable material was washed for 5 min with tap water and sodium hypochlorite at 90 °C and immediately rinsed with cold tap water. The carrot juice was obtained using an automatic juice extractor (Vitale Taurus, Taurus Group, Lleida, Spain). The orange juice was extracted with a hand squeezer (Taurus TC8, Taurus Group). The juices were clarified by centrifugation at 5000 × g for 5 min at 4 °C. The juice samples were prepared by blending 67% (v/v) of carrot juice with 33% (v/v) of orange juice, with a final pH of 4.9. Xanthan gum (0.25%, w/v) (Guinama; Valencia, Spain) was added as a stabilizer.
Artichoke inulin with a degree of polymerization (DP) 10 (Farma-química; Málaga, Spain), was added to the blended juice samples in different proportions to prepare juice without inulin (JIN0%), juice supplemented with 1% inulin (JIN1%) and with 2% inulin (JIN2%). The juices were transferred into sterile borosilicate glass bottles with polypropylene screw caps (250 mL), pasteurized at 90 °C for 5 min in a water bath and cooled to 37 °C in an ice bath. The JIN0%, JIN1% and JIN2% were inoculated with 2.5 mL/250 mL of juice of a suspension of previously prepared LP (106 viable cells/mL of juice), and incubated at 37 °C for 24 h. The non-fermented control juice (CJ) was kept in the same conditions during the incubation. After 24 h of incubation, the fermented juices and CJ were stored during 30 days at 4 °C. The samples were analyzed at 0, 1, 8, 10, 12, 15 and 30 days.
Determination of the Viability of Lactobacillus plantarum
The viability of LP in the fermented juices was determined by the plate count method. Aliquots (1 mL) of each sample were diluted with 9 mL of sterile peptone water in serial dilutions and they were spread plated in MRS agar (Oxoid; Madrid, Spain) for enumeration and incubated under aerobic conditions at 37 °C for 48 h. The results were expressed as Log10 colony forming unit per mL of juice (CFU/mL).
Determination of Yeasts and Moulds
The moulds and yeasts were determined in Petrifilm™ yeast and mould count plates (3 M; Madrid, Spain). The plates were incubated aerobically at 25 °C during 48–72 h for yeasts and 72–140 h for moulds. The results were expressed as Log10 CFU/mL.
Survival of L. plantarum under Simulated Gastric Juices (SGJ) and Simulated Intestinal Juices (SIJ)
The tolerance of LP to in vitro digestion during storage time was determined according to the method described by Valero-Cases and Frutos [11], through the exposition of 10 mL of JIN0%, JIN1% and JIN2% at 37 °C during 60 min to SGJ. These SGJ were prepared in 100 mL of MRS broth acidified to pH 3 with 1 M HCl (Panreac; Barcelona, Spain) and 3 g/L of pepsin (Farma-química; Málaga, Spain). For the preparation of SIJ, the reaction was stopped after 60 min, by adjusting the pH to 7 with 1 M NaOH (Panreac; Barcelona, Spain), 4.5 g/L of bile salts (Sigma-Aldrich; Madrid, Spain) and 1 g/L of pancreatin (Sigma-Aldrich; Madrid, Spain) were added and samples were incubated during 60 min at 37 °C. The survival of LP in each different fermented juice was calculated according to Eq. 1:
where N0 is the total number of viable cells in each different fermented juice (JIN0%, JIN1% or JIN2%) before in vitro digestion and Nf is the number of viable cells after in vitro digestion in each different fermented juice (JIN0%, JIN1% or JIN2%).
Chromatographic Analysis of Sugars, Inulin and Organic Acids
The sugars, inulin and organic acids were simultaneously analyzed using high performance liquid chromatography (HPLC). The determination was made using a Hewlett-Packard HPLC series 1100 instrument (Woldbronn, Germany) equipped with a Supelcogel C-610H (30 cm × 7.8 mm) column and a Supelcoguard C-610H (5 cm × 4.6 mm) guard column (Supelco, Sigma-Aldrich; Madrid, Spain). A refractive index detector (RID G1362A) was used for the sugars and inulin analysis. The acids were monitored at 210 nm with a visible-ultraviolet (UV-Vis-) diode array detector (DAD G1315A). The mobile phase was orthophosphoric acid at 0.1% and the injection volume was 20 μL with a flow of 0.5 mL/min in isocratic conditions. Identification and quantification were obtained through standard calibration curves for sugars (glucose, fructose, and sucrose), inulin and organic acids (malic, lactic, citric and oxalic acids) (Sigma-Aldrich; Madrid, Spain). The results were expressed as grams per litre.
Statistical Analysis
All experiments and analysis were conducted in triplicate. The results were expressed as mean ± standard deviation. The mean comparison was performed using analysis of variance (ANOVA) followed by a Duncan test (p < 0.05), using SPSS v 21.0 software package (SPSS Inc., Chicago, IL, USA).
Results and Discussion
Effect of Inulin on the Growth and Survival of L. plantarum and Changes in the Composition of Juices after Fermentation and during Storage
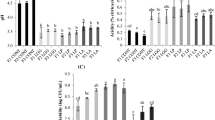
After 24 h of fermentation at 37 °C, the growth of LP was about 9.13 Log10 CFU/mL in all juices (Fig. 1a) regardless of the addition of inulin (Fig. 1b, c). At the same time an increase in lactic acid concentration was observed (2 g/L) without significant differences (p > 0.05) in samples with inulin and without inulin (Fig. 2). This was associated with a considerable reduction in fructose and glucose, with initial concentrations of 14 g/L decreasing to 9.5 g/L in both monosaccharides at the end of fermentation (Fig. 3). The concentrations of malic acid also decreased from 6.5 to 2 g/L (Fig. 2). This indicates the fast transformation of monosaccharides and the conversion of the malic acid into lactic acid through the malolactic fermentation pathway made by LP after 24 h [12]. Accordingly, the lactic acid production from the metabolism of these substrates resulted in a pH decrease from initial values of 4.9 to 3.9 in all fermented juices after 24 h of fermentation. However, the inulin remained unchanged during this fermentation period (Fig. 1b, c). These results indicate that during fermentation, the main carbon and energy sources for LP were glucose and fructose (Fig. 3), while inulin, with a DP ≥ 10, is fermented more slowly [13, 14]. Thus, the degradation of inulin during fermentation may be related to the food composition, the LAB strain and to the fermentation time. However, between 10 and 15 days of storage, the inulin concentration decreased 17% in JIN1% and JIN2% (p > 0.05) (Fig. 1b, c), while the monosaccharides concentrations remained constant during this period for JIN1% and JIN2%. The fermentation of inulin may be favored by the decrease of nutrients after a certain storage period [15]. In the present study, it can be stimulated by the decrease of monosaccharides during the fermentation time and the first eight days of storage. However, in the same storage period, the control samples JIN0% showed a decrease in the concentration of glucose and fructose as the only energy source. After 15 days, the viability of LP was higher for samples with inulin. Nevertheless, the survival of LP in JIN0% started to decrease progressively after 15 days of storage because of the lower concentration of monosaccharides at this stage (Fig. 1a).
a Effect of different concentrations of inulin on the survival of L. plantarum (Log10 CFU/mL) in blended carrot and orange juices during refrigerated storage. Evolution of inulin concentrations (g/L) during storage period: b Juices with 1% inulin (JIN1%) and c Juices with 2% inulin (JIN2%). Data represent average values ± standard deviation of three independent samples, JIN0%, juice without inulin; JIN1%, juice with 1% inulin; JIN2%, juice with 2% inulin
Changes in individual acid content in blended carrot and orange juices fermented with L. plantarum in the presence of different concentrations of inulin, during refrigerated storage, expressed as g/L of juice. Data represent average values ± standard deviation of three independent samples. CJ: control juice; JIN0%: juice without inulin; JIN1%: juice with 1% inulin; JIN2%: juice with 2% inulin. * denotes significant differences between different juices during storage period (p < 0.05)
Changes in sugar content in blended carrot and orange juices fermented with L. plantarum in the presence of different concentrations of inulin, during refrigerated storage, expressed as g/L of juice. Data represent average values ± standard deviation of three independent samples. CJ: control juice; JIN0%: juice without inulin; JIN1%: juice with 1% inulin; JIN2%: juice with 2% inulin. * denotes significant differences between different juices during storage period (p < 0.05)
During the last 15 days of storage, JIN1% and JIN2% had a lower reduction in the concentration of monosaccharides compared to control samples JIN0% (Fig. 3). This fact could be related to the continuous consumption of inulin by LP during this period, where a decrease in the concentration of inulin was observed in all the samples with inulin (Fig. 1b, c). After 30 days, the total monosaccharide concentration in JIN0% was 4 g/L lower with respect to juices with inulin (Fig. 3). This means that the fermentation of inulin by LP improved the monosaccharide concentration, probably having an impact on the sensory properties. In previous studies, non- fermented blended carrot and orange juices showed high sensory quality in relation to odour and flavour [16, 17]. However, Luckow and Delahunty [18] reported that regular consumers of orange juice detected differences in the odour and flavour of fermented orange juices without prebiotics, being preferred by 11% of consumers. Therefore, more sensory tests must be done during storage in fermented juices with and without inulin in future studies. Nevertheless, in all the fermented juices, the sucrose content did not change after the fermentation period and during the 30 days of refrigerated storage, indicating that sucrose is not metabolized by this strain of LP. However, Kun et al. [19] found that glucose and sucrose were the main carbon and energy sources in carrot juice fermented with Bifidobacterium strains. The knowledge on the metabolic use of the different carbon and energy sources is interesting as it depends on the probiotic strain and on the substrate composition.
The citric acid in all the blended carrot and orange juices remained without significant changes (p > 0.05) (ca. 3.5 g/L) during fermentation and storage (Fig. 2) meaning that it was not metabolized by LP. Similar results were found in orange juice fermented with LP NCIMB 8826 with the lactic acid being produced after fermentation from the metabolism of sugars, reaching values of 2.7 g/L after six weeks of storage but where the citric acid concentrations remained unchanged [20]. However, other authors reported that the lactic acid in pomegranate juice after 72 h of fermentation ranged between 2 and 6 g/L for different Lactobacillus strains (L. plantarum, L. delbrueckii, L. acidophilus and L. paracasei), the citric acid being the main energy source for them, due to low sugar content in pomegranate juice [21].
Hence, inulin improved the viability of LP during the last 20 days of refrigerated storage, without showing any significant differences (p > 0.05) between JIN1% and JIN2% (Fig. 1b, c). After 30 days of storage, the LP population was 9.2 Log10 CFU/mL in JIN1% and JIN2% (p > 0.05) and 8.95 Log10 CFU/mL in JIN0% (Fig. 1a). Therefore, inulin represents a carbon source available during storage for the LP strain tested and can also protect the microorganism during refrigerated storage avoiding cell damage, mainly through physical immobilization of the cells in the inulin structure as this polymer can form aggregates in aqueous media [22, 23]. This protection could be improved by the low precipitation of the semi-dilute particles of inulin during storage, together with the sedimentation of the LP that may increase their interaction leading to a higher protection of the microorganism [24]. Zimeri and Kokini [25], in previous studies reported a 5% of sedimentation of inulin in deionized water after three weeks of storage at room temperature [25]. Valero-Cases and Frutos [11], found similar results after 15 days of storage for the same strain of LP after being microencapsulated with inulin as the only source of energy and reported that 2% of inulin improved the microorganism survival during 30 days of storage compared with 1% of inulin. Paseephol and Sherkat [26] observed that yogurts with inulin improved the viability of L. casei but did not have any influence on the survival of L. delbruecki subsp. bulgaricus and S. thermophilus. In milk fermented by different co-cultures of Lactobacillus and Bifidobacterium, either in pure cultures or in binary co-cultures with S. thermophilus or in a cocktail containing all of them an improvement after cold storage in cell viability was observed due to the presence of inulin, depending on the type of microorganism [27], as the ability to ferment inulin is different for every LAB strain [28].
The microbiological analysis of moulds and yeasts showed that they were not detected during the assay in any of the JIN0%, JIN1% and JIN2% juices. Nevertheless, the unfermented juice showed mould and yeast concentrations higher than >3 Log10 CFU/mL after 15 days of refrigerated storage. The higher contents of lactic acid in fermented, blended carrot and orange juices contributed to a lower pH and to the increase in shelf life showing a good hygienic-sanitary quality of the samples during the 30 days of refrigerated storage.
Effect of Different Concentrations of Inulin on the Survival of L. plantarum in Vegetable Juices under In Vitro Digestion during Storage
The LP concentration in fermented juices after 30 days of storage was in the range of the recommended values (106–107 CFU/mL or gram) [1] for reaching the colon in sufficient concentration after consumption. Therefore, it is interesting to evaluate the effect of inulin on the survival of LP under simulated gastrointestinal digestion at different storage periods (1, 15 and 30 days) and to check the survival through LP concentrations observed during storage (Fig. 4).
Effect of different concentrations of inulin on the survival of L. plantarum (Log10 CFU/mL) in fermented carrot and orange juices during in vitro digestion at 1, 15 and 30 days. The error bars represent the standard deviation (n=3). Different letters above the bars denote significant difference on L. plantarum survival between three juices for the same step of in vitro digestion (p < 0.05). JIN0%: juice without inulin; JIN1%: juice with 1% inulin; JIN2%: juice with 2% inulin; BD: Before digestion (0 min); SGJ: after simulated gastric juices (60 min); SJI: after simulated intestinal juice conditions (60 min).
Comparing the LP survival in JIN0%, JIN1% and JIN2% under SGJ and SIJ on the first day (Fig. 4), the presence of inulin did not affect the percentage of survival of LP after 120 min of incubation, presenting values of 73% of survival in all fermented juices without significant differences (p > 0.05). This represents a decrease of ca. 2.5 logarithmic cycles at the end of the SGJ for this storage period in all samples. However, after in vitro digestion at 15 and 30 days of storage, LP showed the same resistance during SGJ, regardless of the presence of inulin. This effect can be due to the preference shown by LAB for simple carbohydrates to resist gastric digestive conditions [29, 30]. Nazzaro et al. [31] found the same results for L. acidophilus after SGJ with inulin, pectin and glucose. Nevertheless, during this period, at the end of SIJ, the survival of LP was higher in the juices with inulin. After 15 days, it was observed that the inulin after in vitro digestion improved the survival of cells without significant differences (p > 0.05) in the concentrations of inulin. However, at 30 days, after in vitro digestion it was observed that although the concentration of LP in the JIN0% was high (6.62 Log10 CFU/mL), the addition of 1 and 2% of inulin improved the survival of LP, reaching higher values in JIN2% (6.95 and 7.40 Log10 CFU/mL for concentrations of 1 and 2% of inulin, respectively). The results demonstrate that the presence of inulin could improve the survival of LP under intestinal conditions for long periods of storage. This could be due to the fact that the period of refrigerated storage time and the intestinal conditions could limit the available sugars [30], favouring the consumption of inulin by LP. The percentage of gastrointestinal survival of LP at the end of the storage was 80% for JIN2%, 75% for JIN1% and 73% for JIN0%. The effect of inulin on the survival of LP during in vitro digestion was the same as the one during refrigerated storage as discussed previously. Different food matrices supplemented with inulin have been used in previous studies. In refrigerated synbiotic guava mousses (4 °C) with different amounts of inulin DP 25 (0, 1.33, 2 and 4%) combined with FOS, it has been observed that the samples with inulin improved the survival of L. acidophilus after simulated gastrointestinal conditions after 1 and 7 days of storage but not after 14 days [28]. However, in fermented soy products the supplementation with 3% of inulin with a DP 10 did not improve the survival of L. acidophilus and B. animalis after the gastrointestinal simulation in different storage periods [23]. In the present study, the beneficial effect of inulin on LP survival during the storage and gastrointestinal digestion is mainly observed after long periods of storage. In all cases, there is a high amount of viable cells after the gastrointestinal digestion (> 106 CFU/mL).
Conclusion
The present study showed that the hygienic-sanitary quality in fermented juices was better than the control juices for long storage periods. The fermented, blended carrot and orange juice could be a good matrix for the delivery of L. plantarum at high concentrations (>106 CFU/mL) in the colon. However, during long storage periods, the fermented juices with 2% of inulin showed the best survival of L. plantarum after in vitro digestion. During storage, the inulin leads to the highest L. plantarum survival (regardless of the concentration) and to the highest monosaccharide content (40% higher).
References
FAO/WHO (2001) Evaluation of health and nutritional properties of probiotics in food including powder milk with live lactic acid bacteria. Report of a Joint FAO/WHO Expert Consultation, Córdoba, Argentina. ftp://ftp.fao.org/es/esn/food/probio_report_en.pdf. Accessed 30 Dec 2016
Prado FC, Parada JL, Pandey A, Soccol CR (2008) Trends in non-dairy probiotic beverages. Food Res Int 41(2):111–123. doi:10.1016/j.foodres.2007.10.010
Saarela M, Mogensen G, Fondén R, Mättö J, Mattila-Sandholm T (2000) Probiotic bacteria: safety, functional and technological properties. J Biotechnol 84(3):197–215
Kleerebezem M, Boekhorst J, van Kranenburg R, Molenaar D, Kuipers OP, Leer R, Tarchini R, Peters SA, Sandbrink HM, Fiers MW, Stiekema W, Lankhorst RM, Bron PA, Hoffer SM, Groot MN, Kerkhoven R, de Vries M, Ursing B, de Vos WM, Siezen RJ (2003) Complete genome sequence of Lactobacillus plantarum WCFS1. Proc Natl Acad Sci USA 100(4):1990–1995. doi:10.1073/pnas.0337704100
Al-Sheraji SH, Ismail A, Manap MY, Mustafa S, Yusof RM, Hassan FA (2013) Prebiotics as functional foods: a review. J Funct Foods 5(4):1542–1553. doi:10.1016/j.jff.2013.08.009
Forssten SD, Sindelar CW, Ouwehand AC (2011) Probiotics from an industrial perspective. Anaerobe 17(6):410–413. doi:10.1016/j.anaerobe.2011.04.014
El Nagar G, Clowes G, Tudorica CM, Kuri V, Brennan CS (2002) Rheological quality and stability of yog-ice cream with added inulin. Int J Dairy Technol 55:89–93
Martins EMF, Ramos AM, Vanzela ESL, Stringheta PC, de Oliveira Pinto CL, Martins JM (2013) Products of vegetable origin: a new alternative for the consumption of probiotic bacteria. Food Res Int 51:764–770. doi:10.1016/j.foodres.2013.01.047
Panda SH, Ray RC (2007) Lactic acid fermentation of beta-carotene rich sweet potato (Ipomoea batatas L.) into lacto-juice. Plant Foods Hum Nutr 62(2):65–70. doi:10.1007/s11130-007-0043-y
Tripathi MK, Giri SK (2014) Probiotic functional foods: survival of probiotics during processing and storage. J Funct Foods 9:225–241. doi:10.1016/j.jff.2014.04.030
Valero-Cases E, Frutos MJ (2015) Effect of different types of encapsulation on the survival of Lactobacillus plantarum during storage with inulin and in vitro digestion. LWT-Food Sci Technol 64(2):824–828. doi:10.1016/j.lwt.2015.06.049
Garcerá MJG, Campos MA, Zúñigaga M, Uruburu FB (2006) Growth and metabolism of L-malic acid by Lactobacillus plantarum CECT 220 in a defined medium. J Food Sci 57(3):778–780. doi:10.1111/j.1365-2621.1992.tb08096.x
Cummings JH, Macfarlane GT, Englyst HN (2001) Prebiotic digestion and fermentation. Am J Clin Nutr 73:715S–720S
Roberfroid MB, Van Loo JAE, Gibson GR (1998) The bifidogenic nature of chicory inulin and its hydrolysis products. J Nutr 128:11–19
Grimoud J, Durand H, Courtin C, Monsan P, Ouarne F, Theodorou V, Roques C (2010) In vitro screening of probiotic lactic acid bacteria and prebiotic glucooligosaccharides to select effective synbiotics. Anaerobe 16(5):493–500. doi:10.1016/j.anaerobe.2010.07.005
Fernández García A, Butz P, Bognàr A, Tauscher B (2001) Antioxidative capacity, nutrient content and sensory quality of orange juice and an orange-lemon-carrot juice product after high pressure treatment and storage in different packaging. Eur Food Res Technol 213(4–5):290–296. doi:10.1007/s002170100332
Rivas A, Rodrigo D, Martínez A, Barbosa-Cánovas GV, Rodrigo M (2006) Effect of PEF and heat pasteurization on the physical–chemical characteristics of blended orange and carrot juice. LWT-Food Sci Technol 39(10):1163–1170. doi:10.1016/j.lwt.2005.07.002
Luckow T, Delahunty C (2004) Consumer acceptance of orange juice containing functional ingredients. Food Res Int 37(8):805–814. doi:10.1016/j.foodres.2004.04.003
Kun S, Rezessy-Szabó JM, Nguyen QD, Hoschke Á (2008) Changes of microbial population and some components in carrot juice during fermentation with selected Bifidobacterium strains. Process Biochem 43(8):816–821. doi:10.1016/j.procbio.2008.03.008
Nualkaekul S, Charalampopoulos D (2011) Survival of Lactobacillus plantarum in model solutions and fruit juices. Int J Food Microbiol 146:111–117. doi:10.1016/j.ijfoodmicro.2011.01.040
Mousavi ZE, Mousavi SM, Razavi SH, Emam-Djomeh Z, Kiani H (2011) Fermentation of pomegranate juice by probiotic lactic acid bacteria. World J Microbiol Biotechnol 27(1):123–128. doi:10.1007/s11274-010-0436-1
Saarela M, Virkajarvi I, Nohynek L, Vaari A, Matto J (2006) Fibres as carriers for Lactobacillus rhamnosus during freeze-drying and storage in apple juice and chocolate-coated breakfast cereals. Int J Food Microbiol 112(2):171–178. doi:10.1016/j.ijfoodmicro.2006.05.019
Bedani R, Rossi EA, Isay Saad SM (2013) Impact of inulin and okara on Lactobacillus acidophilus La-5 and Bifidobacterium animalis bb-12 viability in a fermented soy product and probiotic survival under in vitro simulated gastrointestinal conditions. Food Microbiol 34(2):382–389. doi:10.1016/j.fm.2013.01.012
Chawla R, Patil GR (2010) Soluble dietary fiber. Compr Rev Food Sci Food Saf 9:178–196
Zimeri JE, Kokini JL (2003) Morphological characterization of the phase behavior of inulin-waxy maize starch systems in high moisture environments. Carbohydr Polym 52(3):225–236
Paseephol T, Sherkat F (2009) Probiotic stability of yoghurts containing Jerusalem artichoke inulins during refrigerated storage. J Funct Foods 1(3):311–318. doi:10.1016/j.jff.2009.07.001
Oliveira RPS, Perego P, Oliveira MN, Converti A (2011) Effect of inulin as prebiotic and synbiotic interactions between probiotics to improve fermented milk firmness. J Food Eng 107(1):36–40. doi:10.1016/j.jfoodeng.2011.06.005
Buriti FC, Castro IA, Saad SM (2010) Viability of Lactobacillus acidophilus in synbiotic guava mousses and its survival under in vitro simulated gastrointestinal conditions. Int J Food Microbiol 137(2–3):121–129. doi:10.1016/j.ijfoodmicro.2009.11.030
Vernazza CL, Gibson GR, Rastall RA (2006) Carbohydrate preference, acid tolerance and bile tolerance in five strains of Bifidobacterium. J Appl Microbiol 100(4):846–853. doi:10.1111/j.1365-2672.2006.02832.x
Kimoto-Nira H, Suzuki C, Sasaki K, Kobayashi M, Mizumachi K (2010) Survival of a Lactococcus lactis strain varies with its carbohydrate preference under in vitro conditions simulated gastrointestinal tract. Int J Food Microbiol 143(3):226–229. doi:10.1016/j.ijfoodmicro.2010.07.033
Nazzaro F, Fratianni F, Nicolaus B, Poli A, Orlando P (2012) The prebiotic source influences the growth, biochemical features and survival under simulated gastrointestinal conditions of the probiotic Lactobacillus acidophilus. Anaerobe 18(3):280–285. doi:10.1016/j.anaerobe.2012.03.002
Acknowledgements
Assistance of Graham Arnold during manuscript preparation is acknowledged.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no competing interests.
Rights and permissions
About this article
Cite this article
Valero-Cases, E., Frutos, M.J. Effect of Inulin on the Viability of L. plantarum during Storage and In Vitro Digestion and on Composition Parameters of Vegetable Fermented Juices. Plant Foods Hum Nutr 72, 161–167 (2017). https://doi.org/10.1007/s11130-017-0601-x
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11130-017-0601-x