Abstract
Lacto-juices processed by lactic acid fermentation bring about a change in the beverage assortment for their high nutritive value, vitamins and minerals which are beneficial to human health when consumed. Sweet potato roots (non-boiled/ fully-boiled) were fermented with Lactobacillus plantarum MTCC 1407 at 28 ± 2°C for 48 h to make lacto- juice. During fermentation both analytical [pH, titratable acidity, lactic acid, starch, total sugar, reducing sugar (g/kg roots), total phenol and β-carotene (mg/kg roots)] and sensory (texture, taste, aroma, flavour and after taste) analyses of sweet potato lacto-juice were evaluated. The fermented juice was subjected to panelist evaluation for acceptability. There were no significant variations in biochemical constituents (pH, 2.2–3.3; lactic acid, 1.19–1.27 g/kg root; titratable acidity, 1.23–1.46 g/kg root, etc.) of lacto-juices prepared from non-boiled and fully-boiled sweet potato roots except β-carotene concentration [130 ± 7.5 mg/kg (fully-boiled roots) and 165 ± 8.1 mg/kg (non-boiled roots)]. The panelist evaluation scores ranged from 3–4.8 (in a hedonic scale of 1–5) from moderate liking to very much liking of sweet potato lacto-juice. Principal component analyses reduced the eight original analytical variables to three independent components (factors), which accounted for 99.9% of the total variations. Similarly, five original sensory variables were reduced to two independent components, which accounted for 83.1% of the total variations.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Fermented beverages produced by controlled fermentation through lactic acid bacterial strains and yeasts that manifest probiotics characteristics [1] are new and in demand. The most frequently manufactured beverages and preparations containing probiotics lactic acid bacteria are based on milk and its derivatives. Fermented horticultural products such as vegetable and fruit juices are alternative to the group of consumers with an intolerance or allergenic to milk protein [2].
Lactic acid fermented vegetable juices can occur ‘spontaneously’ by the natural lactic acid bacterial microflora, i.e. Lactobacillus, Leuconostoc, Pediococcus, etc. [3]; however, the addition of ‘starter culture’ provides consistency and reliability of performance [4]. Lactobacillus plantarum is the ‘starter’ most frequently used in lactic acid fermentation of plant materials [5, 6]. Lactic acid fermented juices are mainly produced from cabbage, carrot, celery and tomato. There are agricultural, nutritional, sensory and preservation reasons for evaluating lactic acid fermentation as a potential process for production of lacto-juice from other vegetables such as sweet potato (SP).
SP is the world's seventh most important food crop after wheat, rice, maize, potato, barley and cassava [7]. India is considered as one of the leading producers of SP in world with production of 1.35 million tonnes of roots annually [7]. The roots are rich in starch, sugar, vitamin C, provitamin A, iron and minerals and some varieties contain coloured pigments such as β-carotene and anthocyanin [8]. These pigments are considered as anti-oxidant having physiological attributes such as anti-oxidation, anti-cancer, protection against night blindness, ageing and liver injury [9].
Principal component analysis (PCA) is used in all scientific branches [10]. This method is advantageously applied for the evaluation in food products analyses. PCA is used for the reduction of information on a large number of variables into a small set while losing only a small amount of information [11]. The major feature of this method is a reduction of the dimensionality in a set of variables by constructing an uncorrelated linear combination of them. The combinations are computed in such a way that the first component accounts for the major part of variance that is the major axis of the points in the p-dimensional space [10]. For the evaluation of the results of the biochemical analyses of sweet potato lacto-juice, the multivariate statistical methods (correlation analysis and principal component analysis) were studied.
In this study, β-carotene-rich SP roots were processed to produce lactic fermented juice using Lb. plantarum as the ‘starter culture’ and the sensory attributes (texture, taste, aroma, flavor and after taste) of the final product were evaluated. For the evaluation of the results of the biochemical and sensory analyses of sweet potato lacto-juice, the multivariate statistical methods (correlation analysis and principal component analysis) were studied.
Materials and Methods
Lactobacillus plantarum
Lb. plantarum was procured from Institute of Microbial Technology, Chandigarh, India. The bacterial culture was maintained on Mann Rogassa Sharpe (MRS) [12] agar slants at room temperature (28 ± 2°C).
Preparation of Starter Culture
Hundred grams of good quality of sweet grapes (Var. Bangalore Blue) were selected and washed under running tap water. These grapes were mashed in a Mixer-cum Grinder (TTK Prestige Ltd., Bangalore, India) and the juice was extracted by using a juice squeezer. The volume of juice (filtered through cheese cotton cloth) was measured and equal volume of water was added. The mixture was boiled for 10–15 min on a hot plate and was cooled at room temperature of 28 ± 2°C. After cooling the grape juice was inoculated with Lb. plantarum culture from the stock culture under laminar flow (Klenzoides, Bombay, India) and was incubated in an incubator (Beautex Instruments, New Delhi, India) at 30°C for 24 h to prepare the starter culture.
SP Roots
Freshly harvested and unbruished β-carotene rich SP (Var. ST 14) roots having following biochemical compositions (starch, 148.0±3.5 g kg−1; total sugar, 23.5± 0.34 g kg−1; dietary fiber, 18.9±0.29 g kg−1; ascorbic acid, 200±4.1 mg kg−1 and β-carotene, 180±4.1 mg kg−1) [13] were collected from the experimental farm of Regional Centre of Central Tuber Crops Research Institute, Bhubaneswar during the month of February, 2006 (day temperature, 28±2°C and night temperature, 24±2°C). These roots were harvested randomly from three sub-plots (4 m×4 m) of a larger plot (40 m × 40 m) and mixed. The roots were used within 24 h after harvest.
Preparation of SP Lacto-juice
SP roots were properly washed in running tap water, peeled and grated by a Mixer-cum-Grinder (TTK Prestige Ltd., Bangalore, India). These grated roots were subjected to three types of treatments: non-boiled, half-boiled (60°C for 5 min) and fully- boiled (100°C for 15 min). Again, each treatment was divided into two lots: one lot was not treated with commercial pectinase and the other lot was treated with pectinase (Pectinex, Novozymes, Denmark) for extraction of juice.
Extraction of Juice
Hundred grams of grated SP roots (<2 mm) (non-boiled, half-boiled or fully- boiled) were dispensed in 500 ml plastic jars (Polypet®, Bombay, India). Fifty ml of sterilized water was added (2:1 ratio) to the grated SP roots and four different concentrations (0.025–0.5%) of pectinase enzyme were added into each bottle and the contents were thoroughly mixed to facilitate juice extraction. The bottles were incubated on the laboratory bench at room temperature (28±2°C) for 24 h. The bottles (three replicates per treatment) containing grated SP and sterilized water but without pectinase served as control. After incubation for 24 h, the juice was extracted by pressing the grated roots by a cheese cotton cloth.
Fermentation
Only juice extracted from non-boiled and fully-boiled SP was used for fermentation. The extracted SP juice was inoculated with a teaspoon (3 ml) of Lb. plantarum starter culture (1 × 107 CFU/ml) and incubated for 48 h on the laboratory bench at room temperature. Three replicates were maintained for each treatment. The flowchart for SP lacto- juice preparation is given in Figure 1.
Biochemical Analyses of Lacto-Juice
Data were collected for several physical (juice yield and pH) and biochemicals (starch, total sugar, reducing sugar, lactic acid, titratable acidity, total phenols and β-carotene) constituents of SP lacto-juices prepared as described above.
The pH was measured by a pH meter [Model 351, Systronics (India) Pvt. Ltd., Ahmadabad, India] using glass electrode. Titratable acidity and lactic acid contents were measured by titration and spectrophotometric (UV-VIS spectrophotometer, Model 7250, Cecil Instruments, UK) methods, respectively [14] and the values were expressed in g/kg SP roots. Starch, total sugar, reducing sugar, total phenol and β-carotene contents were estimated by spectrophotometric method [15] and the values were expressed in g/kg SP roots (expect β-carotene and total phenol expressed in mg/kg SP roots).
Microbial Count
Coliform bacteria in SP lacto-juice were enumerated in Mac Conkey's broth (HiMedia Laboratories Pvt. Ltd., Mumbai, India) by Most Probable Number (MPN) technique [16].
Sensory Evaluation
Sensory attributes (such as texture, taste, aroma, flavour and after taste) were evaluated using a 5 point Hedonic scale (where 1= dislike extremely and 5= like extremely) by 24 panelists (gender: 8 women: 16 men; age group: 25–40) selected from students and staff members of the Institute. In one set, lacto-juice prepared from non-boiled and fully-boiled SP roots was added with 10% sugar cane, and the other set was evaluated without addition of sugar cane.
Lacto-juice was served in polypropylene transparent cups which had been labeled with 3-digit random number. Questionnaires and water for mouth rinsing between each tasting were provided. Panelists were asked to read through the questionnaires and the meaning of each attribute was explained to the panelists to avoid any misinterpretation [17]. Prior to evaluation, a session was held to familiarize panelists with the product. The sensory evaluation data were presented as the means of the panelists’ score. A standard t-test was used to test for the statistical significance of the differences observed between the scores of the two tests [18].
Statistical Methods
For the evaluation of analytical results, the multivariate statistical methods: correlation analysis and PCA were applied. The data matrix of the analytical results by SPSS (SPSS Software for Windows release 10.0, SPSS Inc., USA) was analyzed.
Results and Discussion
Fermented vegetables and vegetable juices contribute significantly to the diet of people in a number of countries [6]. Vegetable juice processed by lactic acid fermentation brings about a change in the beverage assortment for their high nutritive value, vitamins and minerals [5]. In several countries of Europe, the consumption of lactic acid fermented vegetable juice has increased. Vegetables like Chinese cabbage, tomato, carrot and spinach juice gave relatively high fermentability than other vegetables as they have more fermentable sugars [19].
Vegetable root crop such as SP is rich in starch, sugars and phenols and can serve as good substrate for lacto-juice preparation by fermentating with lactic acid bacteria [8]. SP lacto-juice is a new product with beneficial probiotics properties of lactic acid bacteria, and enriched with phenolics and β-carotene (anti-oxidants) contents. Further, there was no physiological and microbiological deterioration of SP lacto-juice for 30 days. The biochemical and sensory analyses of SP lacto-juice are described below.
Enzymatic Extraction of SP Lacto-juice
Juice yield is a critical factor in the vegetable fermentation process [20]. The cell walls of SP roots are rich in cellulose, hemicellulose and pectin, which obstruct in the process of juice extraction. Figure 2 shows the application of commercial pectinase for extraction of juice from SP roots. With application of pectinase (0.025–0.5%), there was an increase of juice yield over non-treated samples. Further, there was more yield of juice from fully-boiled SP in comparison with non-boiled or half-boiled roots. For example, in 0.05% pectinase treatment, juice extraction was 44 and 29% more in fully-boiled SP roots than non-boiled and half-boiled roots, respectively. This was obvious because boiling at 100°C for 15 min partially gelatinized the starch and broke down the cross-linkage of cellulose-hemicellulose-pectin complex of cell wall which further disintegrated with the application of pectinase. However, there was a decline in juice yield beyond 0.25% pectinase treatment; this might be due to mashiness of the grated root which affected juice extraction [21]. Most of the vegetables such as carrot and beet roots are fermented without boiling [22]. SP is consumed in tropics either as ‘baked’ or ‘boiled’. Although the juice yield from non-boiled roots was low in comparison to fully-boiled roots, further experiments were conducted using both non-boiled and fully-boiled roots.
SP Lacto-juice of Fully-boiled and Non-boiled Roots
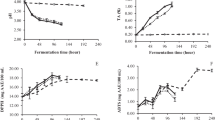
Table 1 shows the biochemical changes during lacto-juice preparation from SP fully-boiled and non-boiled roots. After 48 h fermentation, the pH dropped from 6.1 (prior to fermentation) to 3.3 and from 5.8 to 2.2 in fully boiled and non-boiled roots, respectively. Simultaneously, there was a decrease in starch, total sugar and reducing sugar content; concomitantly, there was an increase in titratable acidity and lactic acid concentration in both the above treatments. However, there was not significant variation in total phenolic contents. pH is a critical factor in developing flavour and aroma of fermented products [4]. The optimal pH of fermented vegetable juices like cabbage and carrot were reported to be 3.8–4.5 [23]. The decrease in pH during lactic acid fermentation was due to accumulation of organic acids especially lactic acid [10, 19, 24]. Further, the decrease in concentration of starch and sugar during fermentation was due to their bioconversion into lactic acid to a larger extent and utilization during growth and metabolism of Lb. plantarum to a lesser extent [22]. Klewicka et al. [2] reported a similar increase in lactic acid concentration during fermentation of beet juice using strains of Lactobacillus spp. (Lb. plantarum, Lb. acidophilus and Lb. delbrueckii). The situation was also similar to other vegetable juice fermentation [25].
Boiling at 100°C for 15 min substantially reduced β-carotene content from 180 mg/kg in fresh tuber to 140 mg/kg in fully-boiled tuber [26]. This amount remained more or less same after fermentation (Table 1). There were least variations in biochemical constituents of lacto-juices prepared from fully-boiled and non-boiled SP roots except β-carotene concentration.
Sensory Analysis
The consumer panel evaluated the SP lacto-juice prepared from non-boiled and fully-boiled roots with or without addition of 10% sugar cane. The sensory analysis showed that panelists rated the lacto-juice prepared from fully-boiled roots which was added with 10% cane sugar, was quite high in acceptability in comparison to other preparations (Table 2). However, SP lacto-juice prepared from non-boiled roots would be preferred nutritionally over fully-boiled roots because of the presence of comparatively higher β-carotene concentration that gave also an attractive yellow colour to lacto-juice.
Lactic acid fermentation has distinct advantages; for example the food becomes resistant to microbial spoilage and to development of toxins [27]. Lactic acid bacteria grow readily on most food substrates and lower the pH to a point where other competing organisms can no longer grow. In the present study, toxic intestinal coliform bacteria such as Escherichia coli were not found. Further, the lacto-juice was stable up to 30 days without any deterioration in quality and taste.
Statistical Evaluation of Results of Biochemical Analyses
The correlation analysis was used for the measurement of the linear association between variables. Pearson's correlation coefficients (r 2) among the analytical variables have revealed that the pH was significantly correlated with titratable acidity (−0.660), lactic acid (−0.544) and β-carotene (−0.837). The other significant correlations were titratable acidity-lactic acid (0.909), total sugar-phenol (0.923), total sugar-β-carotene (0.849) and β-carotene-phenol (0.940).
Using PCA, the eight original variables were reduced to three principal components (PC1, PC2 and PC3), which had eigenvalues larger than 1 and retained for rotation. PC1 accounted for 52.04%, whereas PC2 and PC3 accounted for 25.98% and 21.89% of the total variations, respectively. When combined, PC1–PC3 together accounted for 99.91% of the total variations. To assist interpretation of dimensions, the factor pattern was rotated using varimax method. Based on the guidelines provided by Stevens [28], an attribute was considered to load heavily on a given component if the factor loading was greater than 0.72. All the eight analytical variables loaded heavily on three dimensions. Three analytical variables, i.e. total sugar (+ve), total phenol (+ve) and β-carotene (+ve) were loaded heavily on PC1, indicating strong correlations among these attributes. Substantial factor loading of titratable acidity (+ve) and lactic acid (+ve) on PC2 and pH (−ve), starch (+ve) and reducing sugar (+ve) on PC3 was observed. Plotting PC1 against PC2 and PC3 shows the same trend (figures not shown).
Statistical Evaluation of Results of Sensory Analyses
Pearson correlation coefficients (r 2) among the sensory data show that all the sensory parameters were significantly correlated with each other. For example, taste was significantly correlated to texture (0.276), aroma (0.465) and flavour (0.537). The other important correlations were aroma-texture (0.584), flavour–aroma (0.758), etc.
Using PCA, the 5 original variables were reduced to two principal components (PC1 and PC2), which had eigenvalues larger than 1 and retained for rotation. PC1 accounted for 61.1% whereas PC2 accounted for 21.96% of the total variations. When combined, PC1 and PC2 together accounted for 83.1% of the total variations. These five sensory attributes loaded heavily on two dimensions. Texture, aroma and flavour were loaded positively on PC1, whereas taste and aftertaste were loaded positively on PC2. Plotting PC1 against PC2 shows the similar trend (figures not shown).
There was a few numbers of studies when PCA was applied for evaluation of lactic fermented food product analysis [10]. Karovićovă et al. [29] have evaluated vegetable juices processed by lactic fermentation using PCA and reduced seven original analytical variables to just one independent component (factor), which accounted for 96.9% of the total variances. Similarly, Panda et al. [13] applied PCA for evaluating biochemical analysis data for lactic acid fermentation of sweet potato into pickle. The original six analytical variables were reduced to two independent components, which accounted for 92% of the total variations.
Conclusion
SP lacto-juice was prepared by fermenting grated SP roots with Lb. plantarum. It provided additional minerals, dietary fiber, Vitamin C, total phenols and starch (carbohydrate source), which functioned as a thickner. It also provided significant β-carotene which is considered as an anti-oxidant and anti-cancer compound and the precursor of Vitamin A. Night blindness is a major physiological disorder among these people due to Vitamin A deficiency [30] which can be alleviated by regular consumption of orange flesh (β-carotene rich) SP either fresh, boiled or as a supplement in fermented vegetables [13] and vegetable juices as in the present study.
References
Kaur IP, Chopra K, Saini A (2002) Probiotics: Potential pharmaceutical applications. Eur J Pharm Sci 151: 1–9.
Klewicka E, Motyl I, Libudzisz Z (2004) Fermentation of beet juice by bacteria of genus Lactobacillus sp. Eur Food Technol 218: 178–183.
Nychas GJE, Panagou EZ, Parker ML, Waldron KW, Tassou CC (2002) Microbial colonization of naturally black olives during fermentation and associated biochemical activities in the cover brine. Lett Appl Microbiol 34: 173–177.
McFeeters RF (2004) Fermentation microorganisms and flavor changes in fermented food. J Food Sci 69(1): 35–37.
Molin G (2001) Probiotics in foods not containing milk or milk constituents, with special reference to Lactobacillus plantarum 299v. Am J Clin Nutr 73(Suppl): 380S–385S.
Montet D, Loiseau G, Zakhia-Rozis N (2006) Microbial technology of fermented vegetables. In: Ray RC, Ward OP (eds), Microbial Biotechnology in Horticulture, Vol 1. Science Publishers Inc, Enfield, NH, USA, pp 309–343.
Ray RC, Ravi V (2005) Post harvest spoilage of sweet potato in tropics and control measures. Crit Rev Food Sci Nutr 45: 623–644.
Ray RC, Ward OP (2006) Post harvest microbial biotechnology of tropical root and tuber crops. In: Ray RC, Ward OP (eds), Microbial Biotechnology in Horticulture, Vol 1. Science Publishers Inc., Enfield, NH, USA, pp 345–395.
Kaur C, Kapoor HC (2001) Antioxidants in fruits and vegetables- the millenium's health. Int J Food Sci Technol 36: 703–725.
Arvanitoyannis IS, Tzouros NE (2005) Implementation of quality control methods in conjunction with chemometrics toward authentication of dairy products. Crit Rev Food Sci Nutr 45: 231–249.
Semmar N, Jay M, Farman M, Chemli R (2005) Chemotaxonomic analysis of Astragalus caprinus (Fabaceae) based on the flavonic patterns. Biochem Syst Ecol 33(2): 187–200.
Sharpe M, Elisabeth Pyer TF (1996) Identification of the lactic acid bacteria in identification method for Microbiologists part A. In: Gibbs BM, Skinner FA (eds), Academic Press, London and New York, pp 65–79.
Panda SH, Parmanick M, Ray RC (2007) Lactic acid fermentation of sweet potato (Ipomoea batatas L.) into pickles. J Food Process and Presv 31(1): 83--101.
Amerine MA, Ough C (1984) Methods for Analysis of Musts and Wines. Wiley-Inter Science Publication, John Wiley and Sons, New York, pp 447.
Mahadevan A, Sridhar R (1993) Methods in Physiological Plant Pathology, 5th edn. Sivakami Publication, Madras, India.
Cochran WG (1950) Estimation of bacterial densities by means of the “most probable number”. Biometrics 6: 105–116.
Kilcast D, Subramanian P (2000) The Stability and Shelf-life of Food. Woodhead Publishing Ltd, Cambridge, UK.
Cass T (1980) Statistical Methods in Management, Vol 2. Casel Ltd, London, UK.
Kim HY, Min JH, Lee JH, Ji GE (2000) Growth of lactic acid bacteria and Bifidobacteria in natural media using vegetables, seaweeds, grains and potatoes. Food Sci Biotechnol 9: 322–324.
Demir N, Acar J, Sar K, Mutlu M (2001) The use of commercial pectinase in fruit industry. J Food Eng 47: 275–280.
Angelova MB (2007) Microbial pectinases: Application in horticultural industries. In: Ray RC, Ward OP (eds), Microbial Biotechnology in Horticulture, Vol 2. Science Publishers Inc. Enfield, NH, USA, In press.
Gardner NJ, Savard T, Obermeier P, Caldwell G, Champagne CP (2001) Selection and characterization of mixed starter cultures for lactic acid fermentation of carrot, cabbage, beet and onion vegetable mixtures. Int J Food Microbiol 64: 261–275.
Adams MR, Nicolaides L (1997) Review of the sensitivity of different food borne pathogens to fermentation. Food Control 8: 227–239.
Lee CHH (1999) Fermentation of rice using amylolytic Bifidobacterium. Int J Food Microbiol 50: 155–161.
Ogunjobi AA, Adebayo-Tayo BC, Ogunshe AA (2005) Microbiological, proximate analysis and sensory evaluation of processed Irish potato fermented in brine solution. Afr J Biotech 4(12): 1409–1412.
Haralampu SG, Karel M (1982) Kinetic models for moisture dependence of ascorbic acid and β-carotene degradation in dehydrated sweet potato. J Food Sci 48(6): 1872–1873.
Panda SH, Naskar SK, Ray RC (2006) Production, proximate and nutritional evaluation of sweet potato curd. J Food Agric Environ 4(1): 124–127.
Stevens J (1992) Applied multivariate statistics for the social sciences. Lawrence Erlbaum Associates Publishers Inc., Hillsdale, NJ, USA.
Karovićovă J, Kohajdovă Z (2002) The use of PCA, FA, CA for the evaluation of vegetable juices processed by lactic acid fermentation. Czech J Food Sci 20(4): 135–143.
Mayne ST (1996) β-carotenoids and disease prevention in humans. FASEB J 10: 690–701.
Acknowledgments
The authors thank Dr S. Edison, Director of CTCRI, for valuable suggestions and facilities.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Panda, S.H., Ray, R.C. Lactic Acid Fermentation of β-Carotene Rich Sweet Potato (Ipomoea batatas L.) into Lacto-juice. Plant Foods Hum Nutr 62, 65–70 (2007). https://doi.org/10.1007/s11130-007-0043-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11130-007-0043-y