Abstract
The fruit of Guamúchil is an excellent source of bioactive compounds for human health although their natural occurrence could be affected by the ripening process. The aim was to evaluate some physicochemical, chemical and antioxidant changes in guamúchil fruit during six ripening stages (I to VI). A defined trend (p ≤ 0.003) was observed for color [°Hue, 109 (light green) to 20 (dark red)], anthocyanins (+571 %), soluble solids (+0.33 oBrix), ash (+16 %), sucrose (−91 %), proanthocyanidins (63 %), ascorbic acid (−52 %) and hydrolysable PC (−21 %). Carotenoids were not detected and chlorogenic acid was the most abundant phenolic compound. Maximal availability of these bioactives per ripening stage (p ≤ 0.03) was as follows: I (protein/ lipids/ sucrose/ proanthocyanidins/ hydrolysable phenolics), II (total sugars/ascorbic acid), III (total phenolics), IV (flavonoids/ chlorogenic acid) and VI (fructose/ glucose/ anthocyanins). Color change was explained by sucrose (β = 0.47) and anthocyanin (β = 0.20) contents (p < 0.001). Radical scavenging capacity (ORAC, DPPH and TEAC) strongly correlated with total PC (r = 0.49–0.65, p ≤ 0.001) but 89 % of ORAC’s associated variance was explained by anthocyanin + sucrose + ascorbic acid (p ≤ 0.0001). Guamúchil fruit could be a more convenient source of specific bioactive compounds if harvested at different ripening stages.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Pithecellobium dulce (Roxb.) Benth is a semi-evergreen, small to medium size tree with a broad crown that belongs to the Leguminosae-Mimosaceae family. It is widely distributed in tropical and sub-tropical regions of India, Southeast Asia and Latin America where is known as Manilla tamarind, Madras thorn, Sweet tamarind, Makamted, Konapuli, Opiuma, Monkeypod, Khoya babla, Jilapi fol gach, Jungli jalebi, Kamachile, Guasima, Guamá americano, Pinzan or Guamúchil [1–4]. One tree produces ~40 kg of dehiscent pods (65–75 % fruit, 9–13 % seed, 17–19 % peel) during a very short fruiting season and one pod contains 5–12 black seeds surrounded by a spongy edible fruit (PDf) called aryl. Mexicans, Thais and Indians consume white, pink or red PDf, either raw or in traditional drinks [1, 3, 4].
PDf is a good source of vitamins, dietary fiber and high quality protein. One hundred grams (86–93 kcal) of fresh ripe PDf is enough to fulfill between 100 and 320 %, 23–44 % and 18–32 % of the recommended dietary allowance of ascorbic acid, thiamine and dietary fiber for Mexicans 4 to 50 years old [1, 3, 4]. Semi-dried (15–18 % moisture) PDf contains 12–15 % protein with a higher content of lysine (7.8 %) and sulfur amino acids (2.8 %) than Glycine max or Phaseolus vulgaris [4–7]. From a pharmacognostic standpoint, all plant parts have been used to prepare several folk remedies for centuries [2]. Its phytochemicals exhibit several bioactivities with anti-inflammatory, anti-bacterial, anti-convulsant and anti-ulcer effects [2, 8]; most of these biological actions involve PDf free radical scavenging capacity (RSC) and other antioxidant mechanisms [9–14]. However, Asian [1, 5] and Mexican [3, 4] ripe PDf seem to differ in its composition of essential nutrients (EN) and phenolic compounds (PC). Nevertheless, to our knowledge, there are no reports on EN/PC changes in PDf during ripening. The aim was to evaluate some physicochemical, chemical and antioxidant changes of PDf during six ripening stages and to explore, by means of linear regression analysis, the dynamic changes in color and RSC of this underutilized legume from Northern Mexico.
Material and Methods
Reagents
Pure (≥93 %) standards [D-[+] isomers of glucose, fructose and sucrose, (+)-α, β-carotenes, gallic, chlorogenic, and caffeic acid, (+)-catechin, rutin, cyanidin chloride, and Trolox], radicals (DPPH, ABTS▪ −, AAPH), Folin–Ciocalteau phenol reagent, fluorescein, and ACS-grade salts and acids (KH2PO4, NaNO2, Na2CO3, FeCl3.6H2O, AlCl3, NaOH, KCl, HCl, H2SO4, K2S2O8, C2H3O2Na) were obtained from Sigma-Aldrich-Fluka (St. Louis, MO, USA). HPLC- or analytical-grade solvents (acetonitrile, tetrahydrofuran, methanol, ethanol, acetone and butanol) were obtained from JT-Baker (Avantor Performance Materials S.A. de C.V., Mexico).
Plant Material
P. dulce pods were collected in four occasions (May to June) in a location nearby Hermosillo, Sonora, Mexico (29° 10′ 56″ N-110° 52′ 54″ W, 250 m ASL) and transported under refrigeration (4 °C) immediately to the laboratory. Pods were examined for integrity, insect contamination and absence of dust. Clean PDfs were carefully separated from their seeds and subjectively classified into six ripening stages according to their size and color (ESM 1): small/light green (I), medium/white (II), medium/pink (III), medium/50 % red (IV), medium/70 % red (V) and, medium/red ≥90 % (VI). 500 g of PDf per ripening stage were divided in two subsamples for further analyses in fresh- or dry-matter: 100 g fresh PDf (PDf-FM) were used to estimate all physicochemical parameters and 400 g were frozen (−80 °C) and freeze-dried (−42 °C, 114 militorr, 48 h; 12 XL Virtis-Sentry Freezemobile Freeze Dryer, Virtis Co., Inc. Gardiner, NY) to a constant weight in 1200 cm3 flasks protected from light. All six freeze-dried samples (PDf-DM) were grinded to a fine powder (≤0.40 μm) and kept at -20 °C until use.
PDf-DM Extracts
Methanol (5 mL), hydro-methanol (20:80; 20 mL), acidified methanol (20 mL), ethanol (10 mL), tetrahydrofuran (25 mL) or HPO3:CH3COOH:water (30:80:90, w/v/v, 20 mL) extracts, were obtained at room temperature from 0.5–1 g of each PDf-DM samples, following Palafox-Carlos et al. [15] extraction procedures. Air-dried precipitates obtained from hydro-methanol extractions were further hydrolysed with 10 mL of FeCl3: HCl 37 %: butanol (0.07:2.5:97.5 w/v/v) for 3 h at 100 °C to estimate the content of proanthocyanidins or with MetOH: H2SO4 (20:2, v/v) for 20 h at 85 °C to estimate hydrolysable PC content, under dark conditions and cooled at room temperature. All samples were either centrifuged (3000 rpm, 15 min, 2-5 °C) or directly filtered (0.22–0.45 μM) prior spectrophotometric or HPLC-analyses. Six replicates per sample (I-VI) were obtained.
Physicochemical Analysis
50 arils (PDf-FM) per stage (I to VI) were used to evaluate their weight (to the nearest ±0.001 g) and color using a colorimeter (CR-300, Konica Minolta Sensing, Inc., USA) and a Hunter Lab scale. Average L*, a* and b* values were used to calculate [16, 17] hue angle [oHue = Tang−1 (b */ a *)] and total color change as compared to stage I with the following formula: ΔE* = [(L o *-L *)2 + (a o *-a *)2 + (b o *-b *)2 ] ½. Also, 0.5 g of each PDf-DM samples were homogenized in distilled water (50 mL) and used to estimate total soluble solids (oBrix), pH and titratable acidity (g citric acid / 100 mL).
Proximate Analysis
Proximate analysis in triplicates (CV ≤ 10 %) was performed in PDf-DM samples, using official AOAC methods for ash, protein, fat, moisture and carbohydrate (by difference) content.
HPLC Analysis
Ethanol soluble sugars (glucose, fructose and sucrose, g/100g DM) were analyzed by normal phase-HPLC [isocratic: acetonitrile: water (80:20, w/v)]/ refractive index detection according to Gonzalez-Aguilar et al. [18]. Total carotenoids (α + β, μg/100 g DM) extracted with tetrahydrofuran were analyzed by reverse phase-HPLC [isocratic = acetonitrile: methanol: tetrahydrofuran (58:35:7)]/ UV-VIS detection (460 nm) according to Mejia et al. [19]. Ascorbic acid (mg/100g DM) extracted with HPO3: glacial acetic acid: water was analyzed by normal phase-HPLC [isocratic: KH2PO4: acetonitrile (25:75, w/v)]/ UV-VIS detection (268 nm) according to Donner and Hicks [20]. Methanol extractable-PC were analyzed by reverse phase-HPLC [gradient: acetonitrile (100 %): diluted acetic acid (1 %)]/ diode array detection (280 nm) according to Feregrino-Pérez et al. [21] with minor modifications in the gradient protocol and using gallic, caffeic and chlorogenic acids, (+)-catechin, rutin and quercetin as external standards.
Phenolic Compounds (PC)
Total PC and flavonoids were extracted from PDf-DM samples with hydro-methanol (20:80) and analyzed in a FLUOstar Omega multifunctional microplate reader (BMG LABTECH, Inc. Durham, NC, USA) in UV-VIS spectrometer operation mode. PC content was assayed according to Singleton and Rossi [22] with the Folin-Ciocalteu reagent and expressed as mg of gallic acid equivalents per gram of DM (mgGAE/g DM) and total flavonoids according to Kim et al. [23] and expressed as mg of quercetin equivalents per gram of dry matter (mgQE/g). Precipitates from hydro-methanol extraction were used to evaluate the content of proanthocyanidins according to Reed et al. [24] and expressed as mg of cyanidin equivalents [25] per gram (mgCyE/g) and hhydrolysable PC according to Hartzfeld et al. [26], treated as for total PC, and expressed as mgGAE/g. Monomeric anthocyanins extracted with acidified methanol were evaluated by the AOAC (2005.02)-pH differential method [27] and expressed as milligrams cyanidin-3-gycoside (Cy3G) equivalents (mg Cy3GE/ g).
Free Radical Scavenging Capacity (RSC)
DPPH (at 518 nm) and TEAC (at 734 nm) RSC assays were performed according to Brand-Williams et al. [28] and Re et al. [29] respectively, with minor modifications in reaction volumes as suggested by Palafox-Carlos et al. [15]. ORAC assay was performed according to Ou et al. [30] using fluorescein [10 nM, excitation (485 nm)/emission (520 nm)], AAPH (240 mM) and Trolox (0.006–0.2 μmol/mL, R2 ≥ 0.95) as standard. All values were expressed as mg (DPPH, TEAC) or μmol (ORAC) of trolox equivalents (TE)/g PDf-DM.
Statistics
All variables were expressed as mean ± standard deviation (SD). One-way ANOVA followed by Tukey’s post-hoc test was performed after testing data for skewness and kurtosis. Correlations were evaluated by Pearson’s product-moment correlation (r) and simple and multivariate linear regressions were used to explain changes in color (°Hue Ln) and RSC during PDf ripening, using response variables with a known role in color (L*, a*, b*, anthocyanins, ascorbic acid, sucrose and ethanol-soluble sugars) or RSC (PC, ascorbic acid). Statistical differences were considered at p < 0.05. Statistical analysis was performed using NCSS 2000 (NCSS Statistical Software, Kaysville, UT, USA).
Results and Discussion
Nowadays, nutrition-conscious consumers are seeking for organic and non-conventional plant foods in order to improve their daily intake of both EN and health-promoting phytochemicals, including PC. In order to fill this market gap, the food industry continuously searches for natural ingredients with a convenient combination of EN/PC within several ethnic crops. In this sense, PDf could be a very convenient source of EN/PC if it is harvested at different ripening stages as it will be explained in the following paragraphs.
The assessment of physicochemical changes during ripening is extremely important to decide when a given fruit should be harvested to ensure an acceptable sensorial quality [15]. Fruit’s texture, flavor and color have a remarkable influence on consumer’s acceptance. Mexican ripe PDf should have a smooth and fresh texture, sweet flavor and a pinkish-to-red hue [3, 4], associated with several physicochemical and functional changes during ripening. In this study, except for moisture (~72.5 %, p = 0.12), blueing (b *) and weight loss (−45 %, only noticeable from stage V to VI), all other physicochemical parameters showed significant changes (p trend ≤ 0.001) during ripening (ESM 2a,b): pH (−0.3 units), total soluble solids (+0.33 oBrix) and color (°Hue, 109 to 20; ΔE* = 71.6) from a lighter-green (L o * = 83.9, a o * = −10.2) to a darker-red (L * = 31.2, a * = 33.7). Mexican white and red [3] or Indian white and pink [5] ripe PDf have almost the same physicochemical characteristics as PDf at stages III to V reported here. However, average titratable acidity was lower than that reported by Pio-León et al. [3] in PDf samples collected 580 km south (24° 46′ 02″ N-107° 41′ 40″ W) at a higher altitude (450 m ASL). Nevertheless, even when the time of harvest is different due to differences in altitude, these results indicate that both crops are quite similar.
From a nutritional standpoint, the apparent energy content of PDf-DM did not change during ripening (394 ± 1 kcal/100 g). However, except for total carbohydrate (81–83 % as dry matter, p = 0.17), all other chemical parameters changed during ripening (p ≤ 0.03; ESM 3): protein (−11 %), lipids (−42 %), sucrose (−92 %) and ashes (+16 %). The latter could be explained by a significant increment in certain minerals such as calcium, iron, potassium, sodium and zinc, as reported by Pio-León et al. [3] in reddish PDf. Most changes occurred between the first two stages, just as the pod opens (shatering) and its photosynthetic capacity decreases, as judged by PDf’s green color disappearance (ESM 1). PDf’s protein content at any stage seems to be higher than previously reported [4] but lower than that of soybean [6] or dry beans [7] or Pitecellobium jiringa [31]. Also, despite the fact that PDf loses 252 mg of ascorbic acid during ripening (−52 %), the average content (mg/100 g) in PDf-FM (calculated from PDf-DM values) is many times higher than that of apples, plums, mangoes, oranges and litchis and active vitamin C (ascorbic acid + dehydroascorbic acid) could be higher than previously reported [1, 3]. Gathering all these findings plus the fact that non-ethanol-soluble sugars represent more than 50 % of all carbohydrates (most of them dietary fiber) at any ripening stage [4], the low calorie/nutrient dense nature of PDf-DM makes it an ideal ready-to-eat snack or a food ingredient (if grinded) for individuals with insulin-resistance or dyslipidemias [32], particularly in its first two ripening stages.
The reduction in ascorbic acid and sucrose contents with an apparently steady-state in glucose and fructose levels is noteworthy (ESM 3). It seems likely that the observed peak level of ascorbic acid, from stage I to II and the steady state level in sucrose at the same time, could be the result of a lesser pod’s photosynthetic capacity and the exposure of PDf to environmental stressors since ascorbic acid increases for plant defense in absence of more complex antioxidant molecules produced during phase II metabolism. From stage III-VI, the channeling of fructose and glucose (from sucrose breakdown) to produce cell wall polymers to support PDf growth (explained by a higher size, ESM 1) in semi-open pods plus the production of specialized metabolites during phase II metabolism (e.g., anthocyanins), seems to reduce the ascorbic acid and sucrose pools. Lastly, the total carotenoid content was below detection level (≤0.1 μg/g) at any stage, as commonly found in raw legumes.
PC have been considered the most important and ubiquitous molecules in the plant kingdom, responsible for the RSC of many edible plants. While many EN are produced, accumulated and metabolized during phase I plant metabolism, PC are produced during phase II. In this study, changes in total PC and subgroups (total flavonoids, proanthocyanidins, hydrolysable PC, and anthocyanins) were statistically different between stages (p ≤ 0.003). However, a positive or negative trend associated with ripening for total PC and total flavonoids was not as evident (ESM 4) as for anthocyanins (0.21 to 1.41 mg Cy3GE, +571 %), proanthocyanidins (1.66 to 0.71 mgCyE, −63 %) or hydrolysable PC (0.58 to 0.46 mgGAE, −21 %). Pearson’s product-moment correlation (r) evidenced a strong relationship (p ≤ 0.001) between L * and °Hue with anthocyanins (r = −0.92 and −0.71), ascorbic acid (r = 0.91 and 0.71) or sucrose (r = 0.99 and 0.89) while the opposite was observed for a* (r = 0.65, −0.66, −0.88, p ≤ 0.01). L * was mildly associated with ethanol-soluble sugars changes (r = 0.57) and blueing (b*) with anthocyanins (r = −0.60) or °Hue (r = 0.50). The logarithmic (Ln) transformation of °Hue values (°Hue Ln) to improve its normality showed that 88 % (p ≤ 0.001) of its associated variance was explained by a* (Table 1) and an additional 9 % by b* (a* + b*, Table 2) but L *, anthocyanins and ascorbic acid lost statistical significance in this model. However, anthocyanins alone (Table 1) or combined with sucrose (Table 2) explained 67 to 89 % of °HueLn associated variance (p < 0.001). Anthocyanins are probably the most important flavonoid subgroup in ripe PDf, although they have been scarcely reported in scientific literature and our results are consistent with these studies [3, 5]. Nevertheless, anthocyanins changes during the ripening of PDf are reported here for the first time.
The apparent steady state in total PC and total flavonoids may resulted from de novo biosynthesis and/or from polymeric PC breakdown, as judged by the significant increments in chlorogenic acid, catechin (possibly from breakdown of proanthocyanidins) and gallic acid (from breakdown of hydrolysable PC) contents from stage II to V (ESM 4). Regardless of intrinsic methodological differences, many authors have reported gallic, mandelic, ellagic, ferulic and p-coumaric acids and naringin, quercetin, kaempferol, daidzein and rutin but no chlorogenic acid or catechin [11, 13]. Moreover, the ratio of phenolic acids (~60 mg/g DM, mainly gallic, caffeic and p-coumaric acids) and flavonoids (~203 mg/g DM, mainly luteolin and myricitin) present in Thai ripe PDf [1] seems to be inverted in the ripe (V-VI) PDf studied here. Many factors are involved in these differences including, but not restricted to, environmental and agronomical factors and plant’s genomic programming that can modify PDf nutraceutical potential. This fact deserves a more detailed comparison in a future study. For now, Mexican PDf is an excellent source of chlorogenic acid, a phenolic acid with anti-diabetic, anti-lipidemic and fatty liver attenuating properties [32, 33].
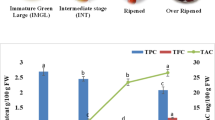
Lastly, RSC is the result of a concerted action of all antioxidant species present in a sample. However, the selection of a single method to evaluate RSC would not accurately reflect the complexity of all chemical interactions in vivo and thus more than one method should be selected [15]. Three RSC methods were selected here: DPPH (stable radical), TEAC (radical cation) and ORAC (peroxyl radicals) which have been extensively used in plant biochemistry and to evaluate other biological specimens. In this study, two interesting findings emerged from RSC assays: a) PDf at stage III (ESM 5) showed the highest RSC [22.3 (DPPH) and 19.9 (TEAC) mg TE and 159.7 (ORAC) μmol TE/g DM] and b) ORAC’s associated variance was explained by total PC or ascorbic acid alone (49 %, p ≤ 0.05; Table 1) or both (54 %, p = 0.04; Table 2) by anthocyanins + sucrose + ascorbic acid (89 %, p ≤ 0.0001; Table 2). These results could be explained by the same argument: PC with mild (ascorbic acid, proanthocyanidins and hydrolysable PC) and high (anthocyanins, chlorogenic and gallic acid) RSC counteract at stage III (ESM 4) resulting in the highest value. In agreement with our results, Pio-León et al. [3] reported values of DPPH and TEAC (as mg equivalents of ascorbic acid /100 g PF) much higher in red (223 and 225) than white (171and 156) PDf. It is well known that RSC of a complex mixture of PC can be enhanced or reduced by several synergistic or antagonistic actions between molecular species and their structural elements, particularly the number and position of hydroxyl groups [34]. However, the extent to which this phenomenon occurs during PDf ripening deserves a future study.
Conclusion
Maximal availability of essential nutrients (EN) and phenolic compounds (PC) in PDf is affected by the ripening process (p ≤ 0.03), being more nutrient-dense at earlier stages (I-II) but richer in monomeric PC afterwards (III-VI) and so, PDf could be a more convenient source of these bioactives if it is harvested at different ripening stages. This evidence could be translated into a more profitable agronomic exploitation of this ethnic fruit, by using more standardized and sophisticated horticultural procedures instead of its manual collection with hooks and bags. However, further studies are needed to evaluate the biological impact (either pro- or anti-physiological) of all chemical, physicochemical and antioxidant changes in order to formulate novel nutraceuticals based on PDf bioactives.
Abbreviations
- AAPH:
-
2,2'-azobis-2-amidinopropane
- ABTS▪ − :
-
2,2'-azinobis-3-ethylbenzothiazoline-6-sulfonate
- DPPH:
-
2-diphenyl-2-picrylhydrazyl hydrate
- EN:
-
Essential nutrient
- ORAC:
-
Oxygen radical absorbance capacity
- PC:
-
Phenolic compounds
- PDf :
-
Pithecellobium dulce fruit
- RSC:
-
Radical scavenging capacity
- Trolox:
-
6-hydroxy.2.5.7.8-tetramethyl-s-dicarboxylic acid
- TE:
-
Trolox equivalents
References
Kubola J, Siriarmornpun S, Meeso N (2011) Phytochemicals, vitamin C and sugar content of Thai wild fruits. Food Chem 126:972–981
Rasingam L (2012). Ethnobotanical studies on the wild edible plants of Irula tribes of Pilur Valley, Coimbatore district, Tamil Nadu India. Asian Pac J Trop Biomed 2012: S1493–S1497
Pio-León JF, Díaz-Camacho SP, Montes-Ávila J, et al. (2013) Nutritional and nutraceutical characteristics of white and red Pithecellobium dulce (Roxb.) Benth fruits. Fruits 68:397–408
Chaparro-Santiago A, Osuna-Fernández HR, Aguilón-Arenas J, et al. (2015) Nutritional composition of Pithecellobium dulce, Guamuchil aril. Pakistan. J Nutr 14(9):611–613
Rao GN, Nagender A, Satyanarayana A, et al. (2011) Preparation, chemical composition and storage studies of quamuchil (Pithecellobium dulce L.) aril powder. J Food Sci Technol 48(1):90–95
Mohamed AI, Rangappa M Screening soybean (grain and vegetable) genotypes for nutrients and anti-nutritional factors. Plant foods Hum Nutr 42(1):87–96
Mojica L, de Mejía EG (2015) Characterization and comparison of protein and peptide profiles and their biological activities of improved common bean cultivars (Phaseolus vulgaris L.) from Mexico and Brazil. Plant Foods Hum Nutr 70(2):105–112
Juárez-Vázquez MC, Carranza-Álvarez C, Alonso-Castro AJ, et al. (2013) Ethnobotany of medicinal plants used in Xalpatlahuac Guerrero, Mexico. J Ethnopharm 148:521–527
Nagmoti DM, Khatri DK, Juvekar PR, et al. (2012) Antioxidant activity and free-radical scavenging potential of Pithecellobium dulce Benth seed extracts. Free Rad Antiox 2(2):37–43
Katekhaye SD, Kale MS (2011) Antiopxidant and free radical scavenging activity of Pithecellobium dulce (Roxb.) Benth wood bark and leaves. Free Rad Antiox 2(3):47–57
Megala J, Geetha A (2010) Free radical-scavenging and H+, K + −ATPase activities of Pithecellobium dulce. Food Chem 121:1120–1128
Megala J, Geetha A (2012) Antiulcerogenic activity of hydroalcoholic fruit extract of Pithecellobium dulce in different experimental ulcer models in rats. J Ethnopharm 142(2):415–421
Manna P, Bhattacharyya S, Das J et al (2011). Phytomedicinal role of Pithecellobium dulce against CCl4-mediated hepatic oxidative impairments and necrotic cell death. Ev Based Compl Altern Med 2011:1–17
Pal PB, Pal S, Manna P, et al. (2012) Traditional extract of Pithecellobium dulce fruits protects mice against CCl4 induced renal oxidative impairments and necrotic cell death. Pathophysiol 19:101–114
Palafox-Carlos H, Yahia E, Islas-Osuna MA, et al. (2012) Effect of ripeness stage of mango fruit (Mangifera indica L. cv. Ataulfo) on physiological parameters and antioxidant activity. Sci Hortic 135:7–13
Nsonzi F, Ramaswamy HS (1998) Quality evaluation of osmo-convective dried blueberries. Dry Technol 6:705–723
McLellan MR, Lind LR, Kime RW (1995) Hue angle determinations and statistical analysis multiquadrant hunter L, a, b data. J Food Quality 18(3):235–240
González-Aguilar GA, Celis J, Sotelo-Mundo RR, et al. (2008) Physiological and biochemical changes of different fresh cut mango cultivars stored at 5oC. Int J Food Sci Technol 43(1):91–101
Mejía LA, Hudson E, González de Mejía E, et al. (1988) Carotenoid content and vitamin a activity of some common cultivars of Mexican peppers (Capsicum annum) as determined by HPLC. J Food Sci 53:1448–1451
Donner LW, Hicks KB (1981) High-performance liquid chromatographic separation of ascorbic acid, erytrorbic acid, dehydroascorbic acid, dehydroerythorbic acid, dikelogulonic acid and dikelogluconic acid. Anal Biochem 115:225–230
Feregrino-Pérez AA, Torres-Pacheco I, Vargas-Hernández M, et al. (2011) Antioxidant and antimutagenic activities of Acacia pennatula pods. J Sci Ind Res India 70:859–864 http://nopr.niscair.res.in/handle/123456789/12681
Singleton VL, Rossi JA (1965) Colorimetry of total phenolics with phosphomolybdic-phosphotungstic acid reagents. Am J Enol Vitic 16(3):144–158 http://www.ajevonline.org/content/16/3/144
Kim DO, Jeong SW, Lee CY (2003) Antioxidant capacity of phenolic phytochemicals from various cultivars of plums. Food Chem 81(3):321–326
Reed J, McDowell RE, Van Soest PJ, et al. (1982) Condensed tannins: a factor limiting the use of cassava forage. J Sci Food Agric 33:213–220
Schofield P, Mbugua DM, Pell AN (2001) Analysis of condensed tannins: a review. Animal Feed Sci Technol 91(1–2):21–40
Hartzfeld PW, Forkner R, Hunter DM, et al. (2002) Determination of hydrolyzable tannins (gallotannins and ellagitanins) after reaction with potassium iodate. J Agric Food Chem 50:1785–1790
Lee J, Durst, RW, Wrolstad, RE (2005). Determination of total monomeric anthocyanin pigment content of fruit juices, beverages, natural colorants, and wines by the pH differential method: Collaborative study. J AOAC Int 88(5): 1269–1278. http://citeseerx.ist.psu.edu/viewdoc/download?doi=10.1.1.487.1462&rep=rep1&type=pdf
Brand-Williams W, Cuvelier ME, Berset CLWT (1995) Use of a free radical method to evaluate antioxidant activity. LWT-Food Sci Technol 28(1):25–30
Re R, Pellegrini N, Proteggente A, Pannala A, Yang M, Rice-Evans C (1999) Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radic Biol Med 26(9):1231–1237
Ou B, Hampsch-Woodill M, Prior RL (2001) Development and validation of an improved oxygen radical absorbance assay using fluorescein as the fluorescent probe. J Agric Food Chem 49:4619–4626
Nilakshi V, Gambhir V, Bhaskar VV (2011) HPTLC analysis of vitamin C from Pithecellobium dulce, benth (fabaceae. J Pharm Res 4(4):1197–1198 http://connection.ebscohost.com/c/articles/74250428/hptlc-analysis-vitamin-c-from-pithecellobium-dulce-benth-fabaceae
Ong KW, Hsu A, Tan BKH (2013) Anti-diabetic and anti-lipidemic effects of chlorogenic acid are mediated by ampk activation. Biochem Pharmacol 85(9):1341–1351
Wan CW, Wong CNY, Pin WK, et al. (2013) Chlorogenic acid exhibits cholesterol lowering and fatty liver attenuating properties by up-regulating the gene expression of PPAR-α in hypercholesterolemic rats induced with a high-cholesterol diet. Phytother Res 27(4):545–551
López-Martínez LM, Santacruz-Ortega H, Navarro RE, et al. (2015) A 1H NMR investigation of the interaction between mango (Mangifera indica cv Ataulfo) and papaya (Carica papaya cv Maradol) and 1,1-diphenil-2-picrylhydrazyl (DPPH) free radicals. PLos ONE 10(11):e0140242
Acknowledgments
The authors are grateful to Prof. Arturo Velazquez-Salazar for providing PDf samples, to Dr. Fernando Ayala-Zavala for reviewing this manuscript and to all authorities from CIAD, UACJ and UAQ for their support during this study.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no conflict of interest
Electronic supplementary material
ESM 1
Pithecellobium dulce (Roxb.) Benth fruit (PDf) during ripening. Left to right: stages I to III (up), stages IV to VI (down). JPEG file (JPEG 68 kb)
ESM 2
a. Physicochemical changes in PDf during ripening. JPEG file (JPEG 205 kb)
ESM 2
b (JPEG 183 kb)
ESM 3
Chemical changes in PDf during ripening. JPEG file (JPEG 274 kb)
ESM 4
Phenolic compounds changes in PDf during ripening. JPEG file (JPEG 324 kb)
ESM 5
Radical scavenging capacity (RSC) changes in PDf during ripening. JPEG file (JPEG 154 kb)
Rights and permissions
About this article
Cite this article
Wall-Medrano, A., González-Aguilar, G.A., Loarca-Piña, G.F. et al. Ripening of Pithecellobium dulce (Roxb.) Benth. [Guamúchil] Fruit: Physicochemical, Chemical and Antioxidant Changes. Plant Foods Hum Nutr 71, 396–401 (2016). https://doi.org/10.1007/s11130-016-0575-0
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11130-016-0575-0