Abstract
Ripening and growing location are important factors that can impact fruit quality characteristics. In this study, the influence of these factors on physicochemical characteristics, carbohydrates, aliphatic organic acids, phenolic compounds, and antioxidant capacity of red guava (Psidium cattleianum Sabine) was evaluated. Fruit ripening increased fructose and glucose (up to 22.83 and 16.42 g 100 g− 1 dry matter (DM), respectively), and decreased citric acid, the major organic acid (up to 135.35 mg g− 1 DM). Ripening and growing location also influenced the concentration of phenolic compounds and antioxidant capacity of red guava, in which a dependency between both factors was observed in most cases. Apigenin, galangin, isoquercitrin, among other phenolic compounds were quantified for the first time in red guava, in which isoquercitrin was the major (up to 13409.81 mg kg− 1 DM). The antioxidant potential of red guava was also confirmed by ferric reducing antioxidant power assay (up to 82.63 µmol Fe+ 2 g− 1 DM), Folin-Ciocalteu reducing capacity assay (up to 17.79 mg gallic acid equivalent g− 1 DM), and DPPH free radical scavenging assay (up to 25.36 mg ascorbic acid equivalent g− 1 DM). These results especially demonstrated the bioactive potential of red guava and provided knowledge regarding the influence of ripening and growing location on chemical and bioactive components encouraging its industrial exploitation.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Ripening is a coordinated and genetically programmed process that results in physiological and biochemical changes which modify fruit characteristics. Transformations during the ripening stages include modifications in carbohydrate and aliphatic organic acids (AOA) metabolism, color changes, cell wall restructuring, cuticle properties alteration, and organoleptic compound formation, promoting in the fruit a combination of color, flavor, and texture [1, 2]. In this sense, ripening has been an important factor studied in fruits to select the most suitable ripening stage for consumption and/or processing, in which sensorial characteristics, nutritional and bioactive potential are generally found at their highest values [1,2,3]. The growing region is another important factor that affects fruit characteristics, since different geographical locations have different edaphoclimatic conditions, which can affect the biosynthesis pathways of fruit compounds [3, 4]. Additionally, the fruit variety also can impact the composition and bioactive characteristics of fruits, since different metabolic pathways may be acting and promoting the production of certain compounds in a variety that are not synthesized or are synthesized in low concentrations in other varieties of the same fruit [5, 6].
Psidium cattleianum Sabine species (Myrtaceae) is native to the Brazilian Atlantic coast with wide occurrence in southern region. It is a small tree (1–4 tall) that produces guava fruits (also named araçá), which are obovoid (2–4 cm in diameter), pyriformis, and crowned by the chalice [7,8,9]. The average fruit productivity of this crop is estimated at 4.3 tons per hectare [10]. There are two morphotypes of P. cattleianum guava fruits: one with red epicarp (red guava) and another with yellow epicarp (yellow guava). Both types contain a pulp whitish, aromatic, juicy, and with small seeds. P. cattleianum guava fruits have a sweet-acid flavor and good acceptance by consumers in natura and processed forms (jams and juices) [7,8,9].
Regarding its composition, there is still a lack of studies carried out with P. cattleianum guava fruits, especially on red epicarp morphotype. Most of the literature has focused on the characterization of bioactive compounds, mainly phenolics, and evaluation of the antioxidant capacity, which demonstrated that these fruits are an excellent source of these compounds and have antioxidant potential [8, 9, 11,12,13]. However, scientific literature lacks data on AOA and carbohydrate profiles in red guava fruit, as well as information on ripening and growing location effects on chemical constituents and antioxidant capacity of this fruit have not yet been published. Therefore, this study seeks to advance knowledge about the composition and antioxidant potential of red guava through unprecedented data on the influence of pre-harvest factors on its physicochemical, chemical, and bioactive characteristics. These data can boost the industry’s interest in the commercial exploitation of red guava, as well as contributing to a better understanding of the influence of ripening and growing location on its composition, which impacts the selection of the most suitable fruit for direct consumption and/or processing.
In this context, this work aimed to determine the changes in physicochemical parameters (moisture, TTA, TSS, and pH), carbohydrates, AOA, phenolic compounds, and antioxidant capacity of red guava fruit in three edible ripening stages harvested in two Brazilian growing locations.
Materials and Methods
The Materials and methods section is presented in Supplementary material 1.
Results and Discussion
Physicochemical Characterization, Carbohydrates, and AOA
For moisture, the interaction between ripening and growing location was observed as prevalent than the isolated factors (p < 0.05; Supplementary material 2). This indicates the existence of a degree of dependence between the factors (ripening and growing location), jointly and dependently influencing the moisture of red guava fruit. In general, higher values were observed for fruits from Pinhalzinho city (76.97 to 80.27 g 100 g− 1) compared to the fruits from Osório city (75.35 to 76.39 g 100 g− 1). These variations may be associated with variations in plant nutrition, edaphoclimatic conditions, and other factors involved in each fruit cultivation [1, 2]. However, the moisture content found in the present study agree with previous report (81.6 g 100 g− 1) for red guava (P. cattleianum) [11].
Generally, the consumption of any fruit is strongly correlated with its flavor, which involves the balance between sweetness and acidity [14]. Soluble carbohydrates are the main contributors of the fruit sweetness and can be estimated by TSS; while the organic acids, due to their acidic characteristic, strongly influence the fruit acidity and can be estimated by TTA [1, 15]. During fruit ripening, several metabolic processes involving biosynthesis, degradation, and accumulation pathways of compounds occur simultaneously [1, 15, 16], which affect, for example, the TSS, TTA, and pH values of fruits: it is expected the TSS increase, due to the synthesis of simple carbohydrates; associated with the TTA decrease due to the reduction in the AOA content of the fruit, which consequently also promote the pH increase [3, 11]. This behavior was observed during ripening of red guava fruit for both growing locations (Supplementary material 2), with higher TTA (up to 2.03 mg citric acid 100 g− 1) and lower TSS (up to 15.10 °Brix) and pH levels (up to 3.41) observed in the ripening stage 1. The interaction between ripening and growing location was observed as prevalent for TSS (p < 0.05; Supplementary material 2), suggesting the existence of dependence between the factors on TSS values. Despite no significant interaction has been verified for TTA and pH, these parameters were significant influenced (p < 0.05; Supplementary material 2) for both isolated factors, which indicate the lack of dependence between these factors on TTA and pH values.
Considering TSS/TTA ratio as an important indicator of fruit maturity and more representative of flavor than isolated values of TSS and TTA [11], this parameter was assessed for red guava fruits. As shown in Supplementary material 2, the highest TSS/TTA ratio was observed for the ripening stage 3 (9.7 and 10.3) compared to the other ripening stages studied (6.0 to 9.0), with significant interaction between both factors (p < 0.05). The TSS/TTA ratio values found for red guava in the present study were higher than those found in red guava (P. cattleianum) fruits (5.1) reported by Dalla Nora et al. [11]. This is due to the low TSS value (6 °Brix) observed by Dalla Nora et al. [11] compared to those found in the present study (12.2 to 18.1 °Brix), which strongly affected the TSS/TTA ratio result, since the TTA (1.2 g citric acid 100 g− 1) reported by Dalla Nora et al. [11] was closer to those found in the present study (1.76 to 2.03 g citric acid 100 g− 1). These different physicochemical characteristics resulted in a fruit with a more acidic and less sweet characteristic than the fruits evaluated in the present study and are possibly associated with the influence especially of edaphoclimatic factors associated with the growing location (Estrela Velha city/Rio Grande do Sul state) on the composition of the fruit, since this fruit was collected as fully ripe [11].
As shown in Supplementary material 2, fructose and glucose were found in all samples. Although there was no significant influence of the interaction between the factors or of the isolated growing location on carbohydrates, the ripening alone significantly influenced the fructose and glucose values (p < 0.05; Supplementary material 2). Fructose was the major and both simple carbohydrates were higher in the ripening stage 3. Fructose and glucose contents of red guava fruits were higher (3.42 to 5.53 g 100 g− 1 FM for fructose and 2.67 to 3.90 g 100 g− 1 FM for glucose) than those found for yellow guava (P. cattleianum) (1.4 and 1.3 g 100 g− 1 FM for glucose and fructose, respectively) [17]. The gradual accumulation of these soluble monosaccharides during red guava ripening is associated with metabolic processes of starch, the fruit’s carbon reserve, which is gradually hydrolyzed to simpler carbohydrates by metabolic pathways during the fruit ripening [1, 7, 16].
In relation to AOA, citric acid was predominant with 90% of total AOA found in red guava fruits, followed by malic, propionic, and formic acids (Supplementary material 2). Citric and malic acids are commonly the most abundant AOA in fruits, including yellow guava (P. cattleianum) [1, 15, 17]. Malic acid has a smooth tart taste similar to citric acid, but slightly more stimulating and continuous. Thus, the presence of malic acid, even in low concentrations, can positively impact the sensorial characteristics of red guava fruit, contributing to a sensation of prolongation of the smooth tart flavor [18, 19]. The higher values found in the present study for citric acid (23.03 to 27.87 mg g− 1 FM) and malic acid (1.56 to 2.76 mg g− 1 FM) compared to those found in yellow guava (P. cattleianum) fruit (0.7 and 0.04 mg g− 1 FM for citric and malic acids, respectively) [17] suggest the red guava variety as a more acidic fruit than the yellow guava variety. Normally, AOA in fruits are influenced by ripening, besides cultivation conditions and environmental factors [15]. In this study, a dependency between ripening and growing location may be suggested due to the observation of a significant interaction (p < 0.05; Supplementary material 2) between both factors for citric, formic, and propionic acids. For malic acid, both isolated factors significant (p < 0.05; Supplementary material 2) affected its content, indicating the non-existence of dependence between ripening and growing location on malic acid content. Except for propionic acid, there was a decrease in AOA content with the ripening of red guava fruits (Supplementary material 2). This behavior is expected in fruits, which commonly accumulate AOA at the early stages of development with a further decrease since are usually used as substrates for respiration during fruit ripening, as well as for biosynthesis of carbohydrates and aromatic amino acids [2, 15, 20]. Decrease in the content of malic and citric acids was also observed during ripening of apple (Malus domestica) [21] and blueberry (Vaccinium corymbosum) fruits [20].
Phenolic Compounds
As shown in Table 1, eight flavonoids, two phenolic acids, and one lignin-derived aldehyde were quantified in red guava fruits. Except for pinocembrin and sinapaldehyde, the other phenolic compounds found in red guava were quantified in all ripening stages in both growing locations. Isoquercitrin was the major, followed by isorhamnetin, and catechin. This is the first report of quantification of apigenin, galangin, isoquercitrin, isorhamnetin, kaempferol, pinocembrin, and sinapaldehyde in red guava fruits.
The content of phenolic compounds found in this study (up to 319.89 mg kg− 1 DM for catechin, up to 18.96 mg kg− 1 DM for gallic acid, up to 2.80 mg kg− 1 FM for quercetin, and up to 2.30 mg kg− 1 DM for sinapic acid) was in many cases similar to those reported in red guava (P. cattleianum) fruits for catechin (nd to 168.7 mg kg− 1 DM), gallic acid (9.9 to 34.1 mg kg− 1 DM), quercetin (nd to 6.6 mg kg− 1 FM), and sinapic acid (nd to 10.4 mg kg− 1 DM) [8, 12]. Additionally, the content of some compounds, such as isoquercitrin (up to 13409.81 mg kg− 1 DM), isorhamnetin (up to 1864.22 mg kg− 1 DM), and catechin found in this study was also higher than those found for other fruits such as acerola (Malpighia emarginata), jabuticaba (Myrciaria cauliflora), blackberry (Rubus ulmifolius), and jambolan (Syzygium cumini) (nd to 431.9 mg kg− 1 DM for isoquercitrin, nd to 9.9 mg kg− 1 DM for isorhamnetin, and nd to 101.0 mg kg− 1 DM for catechin) [22]. Considering the sum of quantified phenolic compounds (up to 15633.36 mg kg− 1 DM), these contents were also higher than those reported for other fruits such as yellow guava (P. cattleianum), guabiju (Myrcianthes pungens), blackberry (R. ulmifolius), jabuticaba (M. cauliflora), acerola (M. emarginata), and jambolan (S. cumini) (152.2 to 1282.1 mg kg− 1 DM) [22], as well as to those reported for red guava (P. cattleianum) fruits (939.2 to 1549.5 mg kg− 1 DM) [8]. Phenolic compounds, in addition to being involved in attracting pollinators and in the plant protection system, have several health-promoting biological activities. Both flavonoid and non-flavonoid phenolic compounds are recognized for their antioxidant properties [23, 24]. Additionally, quercetin and their derivatives (including isoquercitrin and isorhamnetin) also have antiviral, anti-inflammatory, and antitumor potential [25, 26], while phenolic acids such as gallic and sinapic have been associated to antidiabetic, chemopreventive, and anti-inflammatory effects [27]. In this sense, the phenolic composition data revealed in this study demonstrate that the consumption of red guava may have positive effects on health, in addition to being a possible natural ingredient for dietary supplements, pharmaceutical, and cosmetics industries.
Production and accumulation of different secondary metabolites in fruits, such as phenolic compounds, are associated with ripening process and other factors such as species and variety, growing conditions, and biotic and abiotic stress conditions, such as UV light, pathogens, water precipitation, altitude, among others [22, 28, 29]. Indeed, a significant interaction (p < 0.05; Supplementary material 2 and Table 1) between ripening and growing location was verified for all phenolic compounds, indicating that there is a degree of dependence between both factors on the concentration of phenolic compounds in red guava fruits. However, the effect of this interaction on phenolic compound concentration was distinct for each compound. For example, catechin, isoquercitrin, gallic acid, and quercetin showed the highest concentration in the ripening stage 2 from Osório city, while the lower concentrations were verified in the ripening stages from Pinhalzinho city. In contrast, for apigenin, galangin, kaempferol, and sinapic acid, the highest concentration was found in the ripening stage 1 from Pinhalzinho city, while the lowest concentration was verified in the ripening stage 3 from Osório city. Due to the major contribution of isoquercitrin, highest sum of phenolic compounds was verified in the ripening stage 2 from Osório city, and the lowest values in the ripening stages from Pinhalzinho city. Therefore, although both ripening and growing location jointly affect (interaction) the phenolic compounds quantified in red guava fruit, this influence does not result in similar behaviors between the compounds.
Significant interaction (p < 0.05; Supplementary material 2) was also found between growing location and ripening for TMA content, in which the lowest concentration was found in the ripening stage 1 from Osório city and the highest concentration was verified in the ripening stage 3 of both growing locations (Supplementary material 2). TMA values found in this study (up to 9.74 mg cya-3-glu 100 g− 1 DM and up to 2.40 mg cya-3-glu 100 g− 1 FM) were lower than those found in red guava (P. cattleianum) fruits reported by Pereira et al. [13] (42.2 to 43.7 mg cya-3-glu 100 g− 1 DM), but higher than those found by Chaves et al. [30] (0.17 mg cya-3-glu 100 g− 1 FM). The increase in TMA values during ripening is expected in reddish and purple fruits due to the stimulation of anthocyanin biosynthetic pathways impacting the tissue pigmentation during fruit development [4, 28, 29].
Phenylpropanoid and flavonoid pathways are relevant biosynthetic pathways of phenolic compounds, including anthocyanins, in fruits during their ripening and are impacted by biotic and abiotic conditions. Pathways regulation is complex involving many enzymes and distinct genes that modulate the production of different compounds at different stages of fruit development and ripening [31]. For example, reduction of FaLAR and FaANR genes expression during strawberry fruit ripening can promote the reduction of catechin and epicatechin concentration, respectively, as well as the reduction of FaANR gene expression can promote a decrease in phenolic acids’ concentration. Downregulation of these genes seems to occur especially in later stages of strawberry ripening and is associated with the increase in the expression of other genes such as FaANS and FaUFGT, stimulating anthocyanin biosynthesis pathway [31, 32]. These genes may also be involved in regulation of phenolic compounds in red guava fruits, especially for apigenin, galangin, kaempferol, and sinapic acid, in which there was a tendency of concentration decreasing of these compounds during ripening. However, other genes as well as stress conditions that affect the metabolic system of fruits are possibly involved in production and accumulation of phenolic compounds quantified in red guava fruits, resulting in different behaviors reported in this study.
Antioxidant Capacity
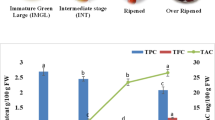
Antioxidant capacity of red guava fruits is shown in Supplementary material 2. For FC (Folin-Ciocalteu reducing capacity) and FRAP (ferric reducing antioxidant power) assays, there was a significant interaction (p < 0.05; Supplementary material 2) between growing location and ripening, indicating a dependence between both factors on the results of FC and FRAP assays. For both assays, ripening stages 2 and 3 from Osório city showed the highest value; while the ripening stages 1 and 2 from Pinhalzinho city showed the lowest value for FRAP assay, and the ripening stage 2 the lowest value for FC assay. For DPPH free radical scavenging assay, no significant interaction (p > 0.05; Supplementary material 2) between growing location and ripening was verified. However, a significant difference (p < 0.05; Supplementary material 2) was found for each isolated factor, confirming the isolated influence of growing location and ripening on DPPH values of red guava fruits. In this sense, the highest DPPH value was verified for the ripening stages from Osório city compared to Pinhalzinho city; and the ripening stages 1 and 2 showed the highest DPPH values compared to the ripening stage 3.
The influence of both parameters at distinct levels on antioxidant capacity was also reported for grumixama (Eugenia brasiliensis) and maqui berries (Aristotelia chilensis) and are possibly result of the variability in the profile and concentration of compounds with antioxidant potential that respond differently to each antioxidant assays since they involve different experimental conditions and reaction mechanisms [3, 4]. This set of factors is possibly related to the trend towards higher DPPH values in the first ripening stages of red guava fruits evaluated in this study and higher FC values in the later ones. Considering the FC values found in this study (17.79 mg gallic acid equivalent g− 1 DM), they were higher than that found by Rosário et al. [9] (3.98 mg gallic acid equivalent g− 1 DM) for red guava (P. cattleianum) fruits, while the DPPH results found in the present study (25.36 mg ascorbic acid equivalent g− 1 DM) were similar to those found for yellow guava (P. cattleianum) fruits (18.46 to 20.01 mg ascorbic acid equivalent g− 1 DM) reported by Schulz et al. [33]. For FRAP assay, Luximon-Ramma et al. [34] reported a higher FRAP value (34 µmol Fe+ 2 g− 1 FM) in red guava (P. cattleianum) fruit than those found in the present study (19.47 µmol Fe+ 2 g− 1 FM).
Among antioxidant compounds present in fruits, phenolic compounds are the most important group. Indeed, a positive and significant correlation (p < 0.05) was found among the sum of quantified phenolic compounds and FC (r = 0.7756), DPPH (r = 0.8275), and FRAP assays (r = 0.8892). Despite anthocyanins being an important polyphenolic group with antioxidant potential, in this study, there was not a significant correlation (p > 0.05) between TMA and antioxidant assays (-0.1458 ≤ r ≤ 0.4505). Therefore, the absence of a significant correlation between TMA and antioxidant capacity, but the existence of a significant correlation between the sum of phenolic compounds quantified (mostly phenolic acids and non-anthocyanin flavonoids) and the antioxidant capacity suggests that there is a greater contribution of non-anthocyanin flavonoids and phenolic acids than anthocyanins on the antioxidant potential of red guava fruits.
Conclusion
In this study was possible to confirm that ripening and growing location affect the compositional and bioactive parameters of red guava fruit, showing in many cases the existence of dependence between the factors. Therefore, the results found in this study serve as a basis to boost the commercial exploitation of red guava fruit and demonstrate that care should be taken while selecting fruit mainly for processing proposes because the biochemical changes resulting from ripening and growing location are important for fruit industrialization.
Data Availability
Data and material may be provided on request by the corresponding authors.
References
Yun Z, Gao H, Jiang Y (2022) Insights into metabolomics in quality attributes of postharvest fruit. Curr Opin Food Sci 45:100836
Perotti VE, Moreno AS, Podestá FE (2014) Physiological aspects of fruit ripening: the mitochondrial connection. Mitochondrion 17:1–6
Nehring P, Seraglio SKT, Schulz M et al (2022) Grumixama (Eugenia brasiliensis Lamarck) functional phytochemicals: effect of environmental conditions and ripening process. Food Res Int 157:111460
Pinto AA, Fuentealba-Sandoval V, López MD et al (2022) Accumulation of delphinidin derivatives and other bioactive compound in wild maqui under different environmental conditions and fruit ripening stages. Ind Crops Prod 184:115064
Lu X, Zhao C, Shi H et al (2023) Nutrients and bioactives in citrus fruits: different citrus varieties, fruit parts, and growth stages. Crit Rev Food Sci Nutr 63:2018–2041
Pereira ES, Vinholes J, Franzon RC et al (2018) Psidium cattleianum fruits: a review on its composition and bioactivity. Food Chem 258:95–103
Lopes MMA, Silva EO (2018) Araça — Psidium cattleyanum Sabine. In: Rodrigues S, Silva EO, Brito ES (eds) Exotic fruits, 1st edn. Academic Press, London, pp 31–36
Mallmann LP, Tischer B, Vizzotto M et al (2020) Comprehensive identification and quantification of unexploited phenolic compounds from red and yellow araçá (Psidium cattleianum Sabine) by LC-DAD-ESI-MS/MS. Food Res Int 131:108978
Rosário FM, Biduski B, Santos DF et al (2021) Red araçá pulp microencapsulation by hydrolyzed pinhão starch, and tara and arabic gums. J Sci Food Agric 101:2052–2062
Gomes GC, Gomes JCC, Cunha LF (2023) Produtividade do araçá-amarelo (Psidium cattleyanum L.) em sistema de produção ecológico aos seis anos da implantação. https://www.alice.cnptia.embrapa.br/bitstream/doc/868374/1/029.pdf. Accessed 27 Nov 2023
Dalla Nora C, Jablonski A, Rios AO et al (2014) The characterisation and profile of the bioactive compounds in red guava (Psidium Cattleyanum Sabine) and guabiju (Myrcianthes Pungens (O. Berg) D. Legrand). Int J Food Sci Technol 49:1842–1849
Medina AL, Haas LIR, Chaves FC et al (2011) Araçá (Psidium cattleianum Sabine) fruit extracts with antioxidant and antimicrobial activities and antiproliferative effect on human cancer cells. Food Chem 128:916–922
Pereira ES, Vinholes JR, Camargo TM et al (2021) Araçá (Psidium cattleianum Sabine): bioactive compounds, antioxidant activity and pancreatic lipase inhibition. Cienc Rural 51:20200778
Shi J, Xiao Y, Jia C et al (2023) Physiological and biochemical changes during fruit maturation and ripening in highbush blueberry (Vaccinium corymbosum L). Food Chem 410:135299
García-Gómez BE, Salazar JA, Nicolás-Almansa M et al (2020) Molecular bases of fruit quality in Prunus species: an integrated genomic, transcriptomic, and metabolic review with a breeding perspective. Int J Mol Sci 22:333
Tian X, Zhu L, Yang N et al (2021) Proteomics and metabolomics reveal the regulatory pathways of ripening and quality in post-harvest kiwifruits. J Agric Food Chem 69:824–835
Cardoso PC, Sviech F, Reis MFA et al (2021) Development and application of a liquid chromatography-mass spectrometry method for the determination of sugars and organics acids in araza, ceriguela, guava, mango and pitanga. Braz J Food Technol 24:2020169
Søltoft-Jensen J, Hansen F (2005) 15 – new chemical and biochemical hurdles. In: Da-Wen S (ed) Emerging technologies for food processing, 1st edn. Academic Press, London, pp 387–416
Izawa K, Amino Y, Kohmura M et al (2010) 4.16 – human–environment interactions – taste. In: Hung-Wen L, Mander L (eds) Comprehensive natural products II, 1st edn. Elsevier, New York, pp 631–671
Li X, Li C, Sun J, Jackson A (2020) Dynamic changes of enzymes involved in sugar and organic acid level modification during blueberry fruit maturation. Food Chem 309:125617
Yang S, Meng Z, Li Y et al (2021) Evaluation of physiological characteristics, soluble sugars, organic acids and volatile compounds in ‘orin’ apples (Malus domestica) at different ripening stages. Molecules 26:807
Betta FD, Nehring P, Seraglio SKT et al (2018) Phenolic compounds determined by LC-MS/MS and in vitro antioxidant capacity of Brazilian fruits in two edible ripening stages. Plant Foods Hum Nutr 73:302–307
Pereira-Netto AB (2018) Tropical fruits as natural, exceptionally rich, sources of bioactive compounds. Int J Fruit Sci 18:231–242
Abbas M, Saeed F, Anjum FM et al (2017) Natural polyphenols: an overview. Int J Food Prop 20:1689–1699
Septembre-Malaterre A, Boumendjel A, Seteyen ALS et al (2022) Focus on the high therapeutic potentials of quercetin and its derivatives. Phytomed Plus 2:100220
Alsharairi NA (2023) Quercetin derivatives as potential therapeutic agents: an updated perspective on the treatment of nicotine-induced non-small cell lung cancer. Int J Mol Sci 24:15208
Sun W, Shahrajabian MH (2023) Therapeutic potential of phenolic compounds in medicinal plants—natural health products for human health. Molecules 28:1845
Günther CS, Plunkett BJ, Cooney JM et al (2022) Biotic stress-induced and ripening-related anthocyanin biosynthesis are regulated by alternate phytohormone signals in blueberries. Environ Exp Bot 203:105065
Shahab M, Roberto SR, Ahmed S et al (2020) Relationship between anthocyanins and skin color of table grapes treated with abscisic acid at different stages of berry ripening. Sci Hortic 259:108859
Chaves VC, Boff L, Vizzotto M et al (2018) Berries grown in Brazil: anthocyanin profiles and biological properties. J Sci Food Agric 98:4331–4338
Siebeneichler TJ, Crizel RL, Reisser PL et al (2022) Changes in the abscisic acid, phenylpropanoids and ascorbic acid metabolism during strawberry fruit growth and ripening. J Food Compos Anal 108:104398
Baldi P, Orsucci S, Moser M et al (2018) Gene expression and metabolite accumulation during strawberry (Fragaria × ananassa) fruit development and ripening. Planta 248:1143–1157
Schulz M, Seraglio SKT, Della Betta F et al (2020) Determination of phenolic compounds in three edible ripening stages of yellow guava (Psidium cattleianum Sabine) after acidic hydrolysis by LC-MS/MS. Plant Foods Hum Nutr 75:110–115
Luximon-Ramma A, Bahorun T, Crozier A (2003) Antioxidant actions and phenolic and vitamin C contents of common Mauritian exotic fruits. J Sci Food Agric 83:496–502
Funding
The authors would like to thank CAPES – Brazil (Finance code 001) and CNPq – Brazil (Grant numbers 165753/2020-0, 150915/2022-5, 150069/2021-9, and 309702/2022-4) for financial support.
Author information
Authors and Affiliations
Contributions
Conceptualization: SKTS and MS; Investigation: SKTS, MS, BS, CTPD; Writing – original draft: SKTS, MS, and BS; Writing – review and editing: SKTS, MS, BS, CTPD, RBH, LVG, RF, and ACOC; Supervising: RBH, LVG, RF, and ACOC.
Corresponding authors
Ethics declarations
Ethical Approval
Not applicable.
Conflict of Interest
The authors declare no conflict of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic Supplementary Material
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Seraglio, S.T., Schulz, M., Silva, B. et al. Chemical Constituents and Antioxidant Potential of Red Guava (Psidium cattleianum Sabine) from Southern Brazil in Different Edible Ripening Stages. Plant Foods Hum Nutr 79, 166–172 (2024). https://doi.org/10.1007/s11130-024-01141-6
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11130-024-01141-6