Abstract
Co-delivery of edible proteins with health-protective fruit (muscadine grape) and vegetable (kale) phytoactive compounds was accomplished in a biofortified ingredient for use in convenient, portable food formulations. Polyphenolics were concentrated (10–42 mg/g range) in dry muscadine-protein matrices. Kale-fortified protein matrices also captured polyphenolics (8 mg/g), carotenoids (69 μg/g) and glucosinolates (7 μmol/g). Neither total phenolics nor glucosinolates were significantly diminished even after long term (6 months) storage at 4, 20, or 37 °C, whereas carotenoids degraded over time, particularly at higher temperatures. Dry biofortified phytoactive-protein ingredients allowed delivery of immunoprotective compounds from fruits and vegetables in a stable, lightweight matrix.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Dietary phytochemicals (phytoactives) from fruits and vegetables can reduce risks of chronic human diseases such as diabetes and cardiovascular disease [1–3], and provide adaptogenic, weight management, cognitive, and immunoprotective benefits [4–6]. However, most consumers fall far short of achieving the recommended daily consumption, due in part to the bulk and inconvenience of storing and preparing fresh produce, the high perishability, and the seasonal access. Hurdles to incorporating adequate fruits and vegetables into the diet are further exacerbated when meals are consumed in transit such as in school lunchboxes, or by motorists, cyclists, backpackers, or campers. A particularly heightened challenge is provision of health-protective phytoactives from produce into field combat rations for military personnel, in a form that will be lightweight, modular, portable, and have taste appeal, yet also maintain stability, shelf-life, and health-protective functionality [7]. Soldiers encounter demanding mental and physical challenges, and exposure to harsh environments combined with the physical requirements of combat lead to increased turnover and loss of nutrients, and impeded mental and physical performance [7, 8]. The relationship between strenuous physical activity, mental stress, and inflammation and immune system response is well established [8–10], and prompts a need to supplement rations with nutrients and nutraceuticals that heighten alertness, improve immune function, and bolster metabolism.
Recently, we reported on a straightforward food grade technology for biofortification of edible proteins with fruit phytoactives [11, 12]. Both bioavailability and health-protective efficacy of the phytoactive constituents were enhanced by the co-delivery with edible proteins in a complexed aggregate matrix [13]. In this report, we used muscadine grape (a source of polyphenols) [14] and kale (a source of glucosinolates, carotenoids, and polyphenols) [15] as representative model candidates to evaluate the utility of this novel biofortification strategy to capture and concentrate the health benefits from both fruit and vegetables into shelf-stable, dry, lightweight ingredient matrices, amenable to formulation into a variety of foods.
Materials and Methods
Reagents and Ingredients
All reagent-grade solvents were purchased from Fisher Scientific (Pittsburgh, PA). Sodium carbonate, gallic acid, β-carotene (synthetic, 93 %, powder), Folin-Ciocalteau reagent, butylated hydroxytoluene (BHT), sulfatase, and barium acetate were purchased from Sigma-Aldrich (St. Louis, MO), and β-cryptoxanthin from CaroteNature GmbH (Lupsingen, Switzerland). Benzyl glucosinolate was purchased from POS Pilot Plant Corp. (Saskatchewan, Canada). Fruits (Noble muscadine grape, The Muscadine Group, LLC, Pine Level, NC) and vegetables (curly kale, from a North Carolina grower, Kannapolis, NC) were harvested at grower-determined peak ripeness/stage of maturity for sale at local markets. Edible proteins included: soy protein isolate (SPI, 90 % protein, Archer Daniels Midland Company; Decatur, IL), hemp protein (HP50, Hemp Pro 50, 50 % protein, Manitoba Harvest Hemp Foods & Oils; Manitoba, Canada), and BiPro whey protein isolate (WPI, 95 % protein, Davisco Foods International, Inc.; Eden Prairie, MN).
Preparation of Phytoactive-protein Matrices
SPI or HP50 were combined with diluted muscadine juice concentrate (1:1, v/v) at a 100 g/L ratio at room temperature to allow sorption of medium-polarity polyphenolic constituents to the edible proteins [12]. After centrifugation, the pelleted polyphenol-protein complex was freeze-dried to create muscadine-SPI and muscadine-HP50 matrices. Matrices were ground into fine powders (flours) and stored at −20 °C. The supernatant (containing sugars, pectin, and water from the juice) was discarded.
The above procedure could not be used to prepare muscadine-WPI due to the relative solubility of whey and consequent loss of protein in the discarded supernatant that would occur during the centrifugation step. Instead, muscadine grape pomace (the ground waste material after juice processing, consisting of skins, seeds, and debris) was extracted in 50 % food-grade ethanol (1:5, w/v) by refluxing at 80 °C for 2 h, centrifuged at 4,000 rpm, and supernatant was filtered to afford the muscadine pomace extract. WPI was added to the hydro-alcoholic extract (100 g/L), ethanol removed by rotary evaporation, and remaining aqueous mixture lyophilized to yield dry powdered muscadine-WPI matrix.
Because raw kale contains myrosinase enzyme, which after cell disruption will cause autolytic breakdown of the health-protective glucosinolates [16], it was necessary to deactivate this enzyme prior to juicing. Fresh kale was placed into Ziploc® microwaveable steam bags and microwaved for 2 min at 90 % power (1.21 KW). This timing was empirically determined to be ideal for deactivation of the myrosinase without compromising the bioactive phytochemical constituents inherent to kale. Approx. 1.0 kg kale was juiced to produce 0.75 L of homogenous kale juice. Protein matrices (SPI, HP50 or WPI) were complexed with kale juice (100 g/L), lyophilized, ground to a fine powder and stored at −20 °C. This co-drying process captured and stabilized both the medium polarity polyphenolic and glucosinolate phytoactives and the low polarity carotenoids from the juice into the protein-rich matrix.
Measurement of Total Phenolics (TP) in the Matrices
TP were extracted from each matrix fortified with muscadine grape or kale phytochemicals by treating samples (0.5 g) with 8 mL of 1 % acetic acid in 80 % aqueous methanol by sonication for 5 min at 55 °C. Samples were centrifuged (10 min) and the supernatant was collected in 25-mL volumetric flasks. The process was repeated on the pellet two more times, and the eluents were pooled together and brought to 25 mL with the extraction solvent. TP were determined with Folin-Ciocalteu reagent [17] and expressed as mg/g gallic acid equivalents (based on a gallic acid external standard curve, with a range of 25–500 μg/ml in 10 % ethanol/water solution).
Estimation of Carotenoid Content in Kale-SPI Matrix
Kale-SPI samples were extracted for carotenoids using procedures described previously [18]. Briefly, 0.2 g samples were incubated with 9 mL of ethanol with 0.01 % butylated hydroxytoluene (BHT), at 40 °C for 10 min, then placed on ice where 3 mL of cold water was added followed by 3 mL hexane (0.01 % BHT), and then centrifuged at 3,000 rpm for 10 min at 10 °C. Supernatant hexane extraction was repeated 3x to collect approx. 9 mL extract, hexane was evaporated, dry residue was re-suspended in 1 mL hexane (0.01 % BHT), filtered using 0.2 μm PTFE syringe filters (Fisher Scientific, Pittsburg, PA) into 2-mL HPLC amber vials, and stored at −80 °C until HPLC analysis. Samples were injected (5 μL) into an Agilent 1260 Infinity system (Agilent Technology Inc., Santa Clara, CA) equipped with diode array detector and autosampler (4 °C). Carotenoids were separated on a RP C30 column 250 × 4.6 mm and 5 μm (YMC America, Inc., Allentown, PA) using 0.05 % ammonium acetate in water (A), and acetonitrile: methanol: dichloromethane: triethylamine (75:20:5: 0.1 v/v, 0.01 % BHT (B). The solvent gradient system was performed as 95, 95, 100, 100, 95, and 95 % of B at 0, 10, 20, 50, 55, and 60 min, respectively, with a constant flow rate (1.8 mL/min) and column temperature (35 °C). Carotenoids were monitored at 450 nm, and DAD spectral data from 250 to 550 nm were stored to examine peak spectrum [19]. Carotenoid concentrations were calculated using the β-carotene standard curve with concentrations at 125, 63, 31, 16, 8, 4 and 2 μg/mL and data were presented as μg/g dry matrix (β-carotene equivalents).
Estimation of Glucosinolate Content in Kale-SPI Matrix
Dry kale-SPI matrix samples were extracted in 50 % aqueous MeOH for glucosinolates, as previously described [20]. The supernatant was poured off into a new glass tube and saved on ice to prevent degradation during extraction procedure. The pellet was extracted twice and 1 mL from supernatant was combined with 150 μL of 0.5 M lead and barium acetate solution, vortexed, and centrifuged for 3 min at 2,000 rpm to allow proteins to precipitate. The supernatant from each 2-mL Eppendorf tube was poured off onto a drained polyprep chromatography column (Bio-Rad, Hercules, CA) containing pre-charged DEAE Sephadex A-25 (Sigma). Once the solution had passed through the column, 3 mL of 0.02 M pyridine acetate was added, followed by 3 mL water. To each polyprep column, 10 units of sulfatase suspended in 500 μL water were added and the columns were capped for 18 h to desulfate glucosinolates. Desulfanated glucosinolates were eluted from the polyprep columns with 3 mL water. Individual compounds were separated using a 1200 HPLC system attached to a 6510 Q-TOF (Agilent Technologies, Santa Clara, CA). The separation of glucosinolates was achieved using LiChrospher 100 RP C18 column, 250 × 4.6 mm × 5 μm (Grace Davison Discovery Science, Deerfield, IL). The mobile phase was composed of solvent A (0.1 % ammonium acetate in H2O with 0.1 % acetonitrile) and solvent B (100 % acetonitrile). The gradient system was 0, 25, 0, and 0 % of solvent B at 0, 32, 34, 36, and 40 min, respectively with a constant flow rate of 1 mL/min. Triplicate samples were analyzed for individual glucosinolate concentrations (μmol/g dry matrix) which were calculated in comparison to certified glucosinolate levels in a standard rapeseed reference material (BCR 367, Commission of the European Community Bureau of References, Brussels, Belgium).
Stability of the Phytochemicals in the Fortified Matrices
Phytochemicals captured in the matrices were gauged over time up to 6 months storage at 4, 20, or 37 °C. Polyphenol stability was measured in muscadine-SPI, muscadine-HP50, kale-SPI, and kale-HP50 matrices, whereas glucosinolate and carotenoid stability was monitored in the kale-SPI matrix. Three random samples of each matrix were prepared and analyzed at each of the following time points: 0, 2, 4, 8, 12, 16, 20, and 24 weeks of storage in the dark at each of the three temperature treatments. Detailed analyses for each phytochemical class were performed as described above.
Statistical Analysis
Statistical analyses for TP, carotenoid, and glucosinolate stability data were performed using SAS software version 9.2 (SAS Institute Inc., Cary, NC, 2009). Data were analyzed by two-way ANOVA using PROC GLM procedures to compare phytochemical concentrations among treatments (4, 20, and 37 °C each at 0 to 24 weeks of storage). The statistical model was yijk = μ + Ti + Sj + Rk + TxSij + ε(ijk); where y = response from the experimental unit, μ = overall mean, T = temperature, S = storage time, R = replication, TxS = temperature x storage time interaction, ε = experimental random error. Treatment mean separations were performed using the least significant difference (LSD) test at P ≤ 0.05 to evaluate temperature and storage effects on concentration of sorbed phytoactives in matrices.
Results and Discussion
Concentration of Phytoactives in Matrices
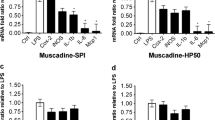
TP concentrations from muscadine captured in SPI, HP50 and WPI were 13.4, 10.5, and 42.3 mg/g DW, respectively (Table 1). TP levels were comparable for the two matrices prepared by complexing the edible proteins with diluted muscadine juice concentrate (muscadine-SPI and muscadine-HP50), however TP levels were nearly 4-fold higher for muscadine-WPI, which was prepared using the alternative method of co-drying muscadine pomace extract with WPI. The TP concentration in the diluted muscadine juice (6,265 mg/L) used to prepare muscadine-SPI and muscadine-HP50 was only slightly lower than the polyphenolic concentration in the pomace extract (~7,000 mg/L) used to prepare muscadine-WPI, however, in the latter method, the extract can be loaded at higher volumes and there are no losses of TP in discarded supernatant. The pelleted method efficiently concentrates and separates phytoactives from the large volume of water and sugars in fruit juices, but the co-drying technique can be used successfully for extracts that are low in or devoid of extraneous sugars, such as the muscadine pomace extract. The co-drying technique is also preferable when the protein matrix has a high tendency to partially dissolve in water, as was true for the WPI.
Similarly, comparable TP concentrations were observed for kale-SPI, kale-HP50, and kale-WPI prepared by the co-drying method: 8.7, 8.0, and, 8.3 mg/g dry weight, respectively. Kale polyphenols included kaempferol, quercetin and hydroxycinnamic acids (p-coumaric, caffeic, ferulic, and sinapic) [21].
Primary carotenoids identified and measured in the kale-SPI matrix were lutein and β-carotene, in addition to neoxanthin, violaxanthin, lutein epoxide, and β-cryptoxanthin. A representative HPLC chromatogram is presented in Fig. 1. Carotenoid compound identification was in agreement with previous publications [22]. Lutein (36.4 μg/g) and β-carotene (25 μg/g) constituted 88 % of the total carotenoids in kale-SPI. As carotenoids are very low polarity compounds, they do not have the strong affinity to proteins as expected for medium polarity polyphenolic compounds or glucosinolates, therefore, the co-drying method was required to sorb these phytoactives to protein matrices. Carotenoids are recognized antioxidant compounds and are linked to prevention of age related diseases, particularly macular degeneration [23].
HPLC chromatogram showing the carotenoid profile in the kale-fortified soy protein isolate matrix (SPI) at the start of the stability experimentation. Compound identification: 1 = neoxanthin, 2 = violaxanthin, 3 = lutein expoxide, 4 = lutein, 5 = chlorophyll b, 6 = chlorophyll a, 7 = β-cryptoxanthin, 8 = all trans β-carotene, 9 = cis β-carotene
A total of eight glucosinolates were identified in kale-SPI matrix (Fig. 2), including glucoiberin (1), progoitrin (2), glucoraphanin (3), sinigrin (4), gluconapin (5), glucobrassicin (6), gluconasturtiin (7), and neoglucobrassicin (8). Glucososinolate compound identification was in agreement with previous publications [16, 20]. Based on the chemical structure of the side chain, glucosinolates are categorized into three classes, aliphatic (compounds 1–5; 5.3 μmol/g matrix), indolyl (compounds 6 and 8) and aromatics (compound 7). The last two classes constituted a minor proportion in kale-SPI (2.2 μmol/g matrix). The major glucosinolates observed in kale-SPI were glucoiberin (1) and sinigrin (4). However, an appreciable amount of glucoraphanin (3) was also observed in kale-SPI matrix (0.23 μmol/g), a compound associated with prevention against several types of cancers [24, 25]. The bioactivity of glucosinolates is attributed to their hydrolysis products even at low concentrations [26], but this can also depend on their profile in plant tissue [27]. In a recent study, as little as 3–5 servings of broccoli per week were associated with a 30 % or 40 % decrease in risk for a number of cancers [28]. In our study, glucosinolates were maintained in their intact form during extraction by deactivating the glucosinolate hydrolysis enzyme (myrosinase), a required enzyme for the release of the health beneficial (but unstable) isothiocyanates. A recent study has shown that human colonic microbiota can perform the hydrolysis of glucosinolates [29].
HPLC chromatogram showing the glucosinolate profile in the kale- fortified soy protein isolate matrix (SPI) at the start of the stability experimentation. Compound identification: 1 = glucoiberin, 2 = progoitrin, 3 = glucoraphanin, 4 = sinigrin, 5 = gluconapin, 6 = glucobrassicin, 7 = gluconasturtiin, 8 = neoglucobrassicin. * = benzyl glucosinolate (internal standard)
Phytoactive Equivalencies
To compare phytoactive equivalents in the matrices to fresh muscadine grapes and kale leaves, concentrations were calculated and converted on a per fresh plant tissue basis. In a recent survey for 10 muscadine varieties [14], TP averaged 21.8, 3.7, and 0.2 mg/g FW in seed, skin, and pulp, respectively. The muscadine-HP50 and muscadine-SPI, which used muscadine juice as the TP source, resulted in 3 times higher concentration of TP in the matrix, as compared to the fruit, whereas muscadine-WPI, which used pomace as the TP source and a co-drying technique, concentrated up to 11 times more TP on a per volume basis (that is, only 7 g of ingredient provided a TP equivalent of a full 75 g serving size of muscadine grape fruits). In a recent large survey of kale varieties, TP, glucosinolates, and carotenoids averaged 3.5, 0.7, and 0.2 mg/g fresh matter, respectively [15]. For the kale-SPI matrices, TP was 4 times concentrated, and glucosinolates were 12 times concentrated compared to the fresh kale leaves. In contrast, carotenoids measured in kale-SPI were available in the matrices at approximately the same levels as in the fresh kale leaf. TP, carotenoids, and glucosinolates available in the recommended serving size of kale leaves (67 g) could be delivered in 16, 67, and 5.6 g of kale-SPI matrices, respectively. These values can depend on many factors including the plant species used, the type of phytoactives, and the type of matrices selected as observed in this study and previous reports [11, 12]. In this study, polyphenolic and glucosinolate components were concentrated in matrices, but carotenoids were not concentrated beyond the levels inherently present in kale leaves.
Stability of Phytoactives Captured in Matrices
Long term stability of the bound phytoactives was evaluated in muscadine-SPI and muscadine-HP50 (TP), and kale-SPI (TP, carotenoids, and glucosinolates). Phytoactive constituents were extracted from the fortified matrices stored at 4, 20 and 37 °C, at time intervals of 0, 2, 4, 8, 12, 16, 20 and 24 weeks.
The stability data (shelf-life) for phytoactives sorbed into matrices of muscadine-SPI and muscadine-HP50 revealed efficient TP stability for up to 6 months of storage at 4, 20, or 37 °C (Table 1). The muscadine TP was comprised of colored pigments (anthocyanins) and colorless flavonoids. While anthocyanins are prone to degradation with time [12], they were protected in the protein matrices and storage did not appear to affect the values for TP in matrices. Similar trends were observed in kale-SPI matrix samples, where comparable concentrations were observed after 24 weeks of storage (Table 1). Carotenoids were significantly less stable over time and subject to degradation especially at higher temperatures (20 and 37 °C) (Table 2). Over 6 months of storage, about 63 % of the total carotenoids remained stable (were not degraded) in the kale-SPI matrix samples stored at 4 °C. At 37 °C, over 88 % of total carotenoids were degraded or could not be detected in the samples. Lutein appeared to be the most stable carotenoid, as its concentration did not decline significantly over 4 months of storage at 4 °C. Carotenoids are known to be susceptible to oxidation and degradation over time particularly after extraction [30] and our results indicate that an antioxidant additive is warranted to preserve carotenoids during storage in the matrix. For glucosinolates, stability data for kale-SPI showed that the aliphatic and indol glucosinolate classes as well as total glucosinolates showed reasonable stability over 6 months of storage at 4 °C (Table 2). A non-significant decline in the total glucosinolates was observed with temperature storage of 20 or 37 °C. After 6 months, glucosinolates were 90, 70, and 70 % of the initial content at 4, 20, and 37 °C, respectively.
Conclusion
Fortified matrices efficiently sorbed target phytoactives from kale and muscadine. While both SPI and HP50 matrices showed comparable phytochemical sorption efficiency, muscadine was a better source of TP, while kale was a resource for carotenoids and glucosinolates. With long term storage, TP and glucosinolates showed no significant degradation, while carotenoids degraded faster, particularly at high storage temperatures. To the best of our knowledge, this is the first report to address the stability of three significant phytoactive classes complexed with edible protein isolates when stored over 6 months at three different temperature regimes.
Abbreviations
- HP50:
-
Hemp protein
- SPI:
-
Soy protein isolate
- WPI:
-
Whey protein isolate
- TP:
-
Total phenolics
References
Cassidy A, Mukamal KJ, Liu L, Franz M, Eliassen H, Rimm E (2013) High anthocyanin intake is associated with a reduced risk of myocardial infarction in young and middle-aged women. Circulation 127:188–196
De Pascual-Teresa S, Moreno DA, Garcia-Viguera C (2010) Flavanols and anthocyanins in cardiovascular health: a review of current evidence. Int J Mol Sci 11:1679–1703
Grace MH, Ribnicky DM, Kuhun P, Poulev A, Logendra S, Yousef GG, Raskin I, Lila MA (2009) Hypoglygemic activity of a novel anthocyanin-rich formulation from lowbush blueberry, Vaccinium angustifolium Aiton. Phytomedicine 16:406–415
Cheng DM, Kutzler LW, Boler DD, Drnevich J, Killefer J, Lila MA (2013) Continuous infusion of 20-hydroxyecdysone increased mass of triceps brachii in C57BL/6 mice. Phytother Res 27:107–111
Nieman DC, Gillitt ND, Knab AM, Shanely RA, Pappan KL, Jin F, Lila MA (2013) Influence of a polyphenol-enriched protein powder on exercise-induced inflammation and oxidative stress in athletes: a randomized trial using a metabolomics approach. PLoS One 8:e72215
Chirumbolo S (2012) Plant phytochemicals as new potential drugs for immune disorders and cancer therapy: really a promising path? J Sci Food Agric 92:1573–1577
Zanchi JA (2012) Chapter 1: An overview of U.S. military field feeding and combat rations. In: Barrett AH, Cardello AV (eds) Military food engineering and ration technology. DEStech Publications Inc, Lancaster, pp 3–35
Racicot K, Anderson DJ, Davis BB (2012) Chapter 11. Performance-optimizing ration components. In: Barrett AH, Cardello AV (eds) Military food engineering and ration technology. DEStech Publications Inc, Lancaster, pp 275–295
Lila MA, Cheng D (2012) Comprehensive strategies for evaluating the adaptogenic properties of phytochemicals. In: Carkeet C, Grann K, Randolf R, Venzon D, Izzy S (eds) Phytochemicals: health promotion and therapeutic potential. Taylor and Francis/CRC Press, Florida, pp 95–112
Nieman DC, Stear AJ, Castell LM, Burke LM (2012) A-Z of nutritional supplements: dietary supplements, sports nutrition foods and ergogenic aids for health and performance. Br J Sports Med 44:1202–1205
Grace MH, Guzman I, Roopchand DE, Moskal K, Cheng DM, Pogrebnyak N, Raskin I, Howell A, Lila MA (2013) Stable binding of alternative protein-enriched food matrices with concentrated cranberry bioflavonoids for functional food applications. J Agric Food Chem 61:6856–6864
Roopchand DE, Grace MH, Kuhn P, Cheng DM, Plundrich N, Poulev A, Howell A, Fridlender B, Lila MA, Raskin I (2012) Efficient sorption of polyphenols in soybean flour enables natural fortification of foods. Food Chem 131:1193–1200
Ribnicky DM, Roopchand DE, Oren A, Grace M, Poulev A, Lila MA, Havenaar R, Raskin I (2014) Effects of a high fat meal matrix and protein complexation on the bioaccessibility of blueberry anthocyanins using the TNO gastrointestinal model (TIM-1). Food Chem 142:349–357
Pastrana-Bonilla E, Akoh CC, Sellappan S, Krewer G (2003) Phenolic content and antioxidant capacity of muscadine grapes. J Agric Food Chem 51:5497–5503
Ferioli F, Giambanelli E, D’Antuono LF, Costa HS, Albuquerque TG, Silva AS, Hayran O, Kocaoglu B (2013) Comparison of leafy kale populations from Italy, Portugal, and Turkey for their bioactive compound content: phenolics, glucosinolates, carotenoids, and chlorophylls. J Sci Food Agric 93:3478–3489
Velasco P, Cartea MA, González C, Vilar M, Ordáz A (2007) Factors affecting the glucosinolate content of kale (Brassica oleracea acephala group). J Agric Food Chem 55:955–962
Singleton VL, Orthofer R, Lamuela-Raventós RM (1999) Analysis of total phenols and other oxidation substrates and antioxidants by means of Folin-Ciocalteu reagent. Methods Enzymol 299:152–178
Kurilich AC, Tsau GJ, Brown A, Howard L, Klein BP, Jeffery EH, Kushad M, Wallig MA, Juvik JA (1999) Carotene, tocopherol, and ascorbate contents in subspecies of Brassica oleracea. J Agric Food Chem 47:1576–1581
Guzman I, Yousef GG, Brown AF (2012) Simultaneous extraction and quantification of carotenoids, chlorophylls, and tocopherols in Brassica vegetables. J Agric Food Chem 60:7238–7244
Brown AF, Yousef GG, Jeffery EH, Klein PB, Wallig MA, Kushad MM, Juvik JA (2002) Glucosinolate profiles in broccoli: variation in levels and implications in breeding for cancer chemoprotection. J Am Soc Hortic Sci 127:807–813
Schmidt S, Zietz M, Schreiner M, Rohn S, Kroh LW, Krumbein A (2010) Identification of complex, naturally occurring flavonoid glycosides in kale (Brassica oleracea var. sabellica) by high-performance liquid chromatography diode-array detection/electrospray ionization multi-stage mass spectrometer. Rapid Commun Mass Spectrom 24:2009–2022
Azevedo CH, Rodriguez-Amaya DB (2005) Carotenoid composition of kale as influenced by maturity, season and minimal processing. J Sci Food Agric 85:591–597
Krinsky NI, Landrum JT, Bone RA (2003) Biologic mechanisms of the protective role of lutein and zeaxanthin in the eye. Annu Rev Nutr 23:171–201
Jain MG, Hislop GT, Howe GR, Ghadirian P (1999) Plant foods, antioxidants, and prostate cancer risk: findings from case-control studies in Canada. Nutr Cancer 34:173–184
Terry P, Wolk A, Persson I, Magnusson C (2001) Brassica vegetables and breast cancer risk. J Am Med Assoc 285:2975–2977
Jeffery EH, Araya M (2009) Physiological effects of broccoli consumption. Phytochem Rev 8:283–298
Lippmann D, Lehmann C, Florian S, Barknowitz G, Haack M, Mewis I, Wiesner M, Schreiner M, Glatt H, Brigelius-Flohe R, Kipp AP (2014) Glucosinolates from pak choi and broccoli induce enzymes and inhibit inflammation and colon cancer differently. Food Funct 5:1073–1081
Jeffery EH, Keck AS (2008) Translating knowledge generated by epidemiological and in vitro studies into dietary cancer prevention. Mol Nutr Food Res 52(Suppl 1):S7–S17
Angelino D, Jeffery E (2014) Glucosinolate hydrolysis and bioavailability of resulting isothiocyanates: focus on glucoraphanin. J Funct Foods 7:67–76
Boon CS, Mcclements DJ, Weiss J, Decker EA (2010) Factors influencing the chemical stability of carotenoids in foods. Crit Rev Food Sci Nutr 50:515–532
Acknowledgments
We are grateful for financial support provided by the Center for Advanced Processing and Packaging Studies (CAPPS), a National Science Foundation-sponsored Industry-University Cooperative Research Center (NSF-I/UCRC), and for the support and advice of Dr. Tom Yang, Senior Food Technologist, Combat Feeding Directorate, US Army Natick Soldier Research, Development, and Engineering Center, who served as the Industry Advisory Board champion for this project. Thanks to David “Buddy” Edwards, who supplied fresh kale for this initiative. Thanks also to Muscadine Products Corp. (Wray, GA), The Muscadine Group, LLC (Pine Level, NC), and Davisco Foods International for donated food materials.
Conflict of interest
The authors declare no conflicts of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Yousef, G.G., Grace, M.H., Medina, J.L.G. et al. Concentrating Immunoprotective Phytoactive Compounds from Fruits and Vegetables into Shelf-stable Protein-rich Ingredients. Plant Foods Hum Nutr 69, 317–324 (2014). https://doi.org/10.1007/s11130-014-0445-6
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11130-014-0445-6