Abstract
Well-known health-protective phytochemicals from muscadine grape and kale were stably complexed with food grade protein (soy or hemp protein isolates) to create biofortified food ingredients for use in a variety of convenient, portable food formulations. The bioactive (anti-inflammatory) potential, sensory attributes and proximates of the prepared formulations were evaluated in this study. Anti-inflammatory properties of the protein-phytoactive ingredient particles were contributed by the polyphenolic content (muscadine-protein) or the combination of polyphenol, carotenoid, and glucosinolate content (kale-protein aggregates). Phytoactive compounds from the fortified matrices suppressed at least two biomarkers of inflammation; most notable with the expression of chronic pro-inflammatory genes IL-6 and Mcp1. Sensory analysis suggested both sweet and savory functional food applications for the biofortified ingredients. Proximate analyses determined that fortification of the soy protein isolate (SPI) with muscadine or kale bioactives resulted in elevated dietary fibers, total carbohydrates, and free sugars, but did not increase calories/100 g dry matrix compared to unfortified SPI. Overall protein content in the aggregate matrices was about 37 % less (muscadine-SPI, kale-SPI and kale- HP50) or 17.6 % less (muscadine-HP50) on a weight basis, likely due to solubility of some proteins during preparation and partial displacement of some protein mass by the fruit and vegetable phytoactive constituents.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The benefits associated with regular dietary consumption of phytochemically-rich fruits and vegetables, which contribute to reduced chronic inflammation and can provide anti-infective and other immunoprotective benefits [1, 2], are widely accepted. A recent study in Britain demonstrated a strong inverse association between consumption of fruits and vegetables (up to seven servings/day), and human mortality, with the most robust protection from vegetable intake [3]. In particular, athletes, outdoorsmen, soldiers or others who routinely experience strenuous physical exertion and mental stress may experience compromised immune system responses, which warrants consumption of immunoprotective phytoactive compounds, and high levels of protein in routine foods [4–8].
Recently we described the development of a series of functional ingredient aggregates, which captured and concentrated the phytoactive natural compounds from fruits and vegetables and stably bound them to edible proteins, for incorporation into portable food product applications including combat rations, camping, or lunchboxes [9]. Muscadine grapes and kale leaves were selected as key examples of phytochemically-rich fruit and vegetable sources, respectively, due to their inherently high concentrations of immunoprotective, health-beneficial phytochemicals. Muscadine grape (Vitis rotundifolia var. Noble), native to the southeastern United States, features a unique anthocyanin chemistry comprised of 3,5-O-diglucosides (as opposed to the more common 3-O-glucosides) of delphinidin, cyanidin, petunidin and peonidin, as well as malvidin in a non-acylated form. Muscadine grapes also differ from standard table or wine grapes (V. vinifera) in that they are distinguished by high ellagic acid, gallic acid, and glycosidic flavonoid concentrations, and high overall polyphenol content [10]. A combination of seed/skin extracts from Ison (purple) variety have shown significant topical anti-inflammatory properties on mouse ear inflammation, attributed to the direct actions of the polyphenolic compounds and their indirect actions through modulation of gene transcription in various cell types [11]. Kale (Brassica oleracea L. var. acephala), a leafy green-to-red/purple-colored vegetable, contains a complex mixture of health related phytochemicals including carotenoids, phenolic compounds and glucosinolates [12–14]. The glucosinolates consist of a β-thioglucose moiety, a sulfonated oxime moiety, and a variable side chain derived from an amino acid [14].
The rational for the work presented here was to test the ability of fruit and vegetable phytoactive compounds (polyphenolics, carotenoids and glucosinolates), after complexing into shelf-stable food grade protein matrices, to retain bioactivity and utility as food ingredients. Biofortified matrices, created with muscadine grape or kale phytoactives bound to edible proteins were tested for their ability to inhibit biomarkers of acute and chronic inflammation in the macrophage-based quantitative, reverse transcription polymerase chain reaction (q-RT-PCR) system optimized to screen extracts and bioactive components for anti-inflammatory activity [15]. Sensory analysis was conducted to determine the potential for using the chimeric ingredients in functional food formulations, and samples were subjected to proximate (nutritional) analysis to verify utility as functional ingredients.
Materials and Methods
Ingredients
Muscadine (var. Noble) juice concentrate (Muscadine Products Corp., Wray, GA) and pomace (The Muscadine Group, LLC, Pine Level, NC) and fresh harvested curly kale from a North Carolina grower were used as sources of phytoactive chemicals. Edible proteins included: SPI (90 % protein, Archer Daniels Midland Company; Decatur, IL), HP50 (Hemp Pro 50, 50 % protein, Manitoba Harvest Hemp Foods & Oils; Manitoba, Canada).
Preparation of Phytoactive-protein Matrices
SPI or HP50 were mixed with diluted muscadine juice concentrate (1:1, v/v) at a 100 g/L ratio for 15 min, to allow sorption of the medium-polarity polyphenolic constituents to the proteins, centrifuged, and the pelleted polyphenol-protein complex was freeze-dried to create the functional food biofortified matrices (muscadine-SPI and muscadine-HP50). The biofortified matrices were ground into fine powders (flours) and stored at −20 °C. The supernatant (containing sugars, pectin, and water from the juice) was discarded [9, 16]. Because raw kale contains myrosinase enzyme, which will cause, after cell disruption, autolytic breakdown of the health-protective glucosinolates [14], it was necessary to deactivate this enzyme prior to juicing. Fresh kale was placed into Ziploc® microwaveable steam bags, and microwaved for 2 min at 90 % power (1.21 KW) before juicing. Protein matrices (SPI or HP50) were added to the kale juice (100 g/L), lyophilized and ground to a fine powder before storing at −20 °C. This co-drying process captured and stabilized both the medium polarity polyphenolic and glucosinolate phytoactives and the low polarity carotenoids from the juice into the protein-rich matrices.
A sample (0.5 g × 3 replicates) from each unfortified protein source (SPI or HP50) or biofortified matrix was extracted with 1 % acetic acid in 80 % methanol (8 mL, three times), and total phenolics was assayed by Folin Ciocalteu method [17]. A sample (2 mL) from each extract was evaporated to dryness and stored at −20 °C for the anti-inflammatory assay.
Anti-inflammatory Bioactivity Analysis
Macrophage Cell Culture
The mouse macrophage cell line RAW 264.7 (ATCC TIB-71, obtained from American Type Culture Collection; Livingstone, MT) was maintained in Dulbecco’s modified Eagle’s medium (DMEM, Life Technologies, NY), supplemented with 100 IU/mL penicillin/100 μg/mL streptomycin (Fisher) and 10 % fetal bovine serum (Life Technologies) at a density not exceeding 5 × 105 cells/mL and maintained at 37 °C in a humidified incubator with 5 % CO2. Matrices extracts for cell culture use were prepared in DMSO as 1,000× stocks and stored at −20 °C until use.
Cell Viability Assay and Dose Range Determination Studies
RAW 264.7 cells were seeded in a 96 well plate for the viability assay. Cell viability was measured after 24 h of exposure by the MTT [3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl-tetrazolium bromide] assay in triplicate and quantified spectrophotometrically at 550 nm using a microplate reader SynergyH1 (BioTek). The concentrations of test reagents that showed no changes in cell viability compared with that vehicle were selected for further studies.
Anti-inflammatory In vitro Assay
Cells were seeded in 24-well plates (5 × 105 cells/well) 24 h prior to treatment. The cells were pre-treated for 1 h with muscadine-SPI, muscadine-HP50, kale-SPI, or kale-HP50 extracts at 50 μg/mL. Cells were then elicited with LPS at 1 μg/mL for an additional 6 h. For every experiment, one positive control (dexamethasone at 10 μM) and one negative control (vehicle) were included. Three biological replicates were used in all the experiments performed. Assays were performed on three different cell passages for both the treatments and the controls.
Total RNA Extraction, Purification, and cDNA Synthesis
The total RNA was isolated from RAW macrophages using TRIzol reagent (Life Technologies) following the manufacturer’s instructions. RNA was quantified using the SynergyH1/Take 3 spectrophotometer (BioTek, Winooski, VT). The cDNAs were synthesized using 2 μg of RNA for each sample using commercially available high-capacity cDNA Reverse Transcription kit (Life Technologies), following the manufacturer’s protocol on an ABI GeneAMP 9700 (Life Technologies).
Quantitative PCR Analysis
The resulting cDNA was amplified by real-time quantitative PCR using SYBR green PCR Master Mix (Life Technologies). To avoid interference due to genomic DNA contamination, only intron-overlapping primers were selected using the Primer Express version 2.0 software (Applied Biosystems, Foster City, CA) as follows: ß-actin, forward primer: 5′-AAC CGT GAA AAG ATG ACC CAG AT-3΄, reverse primer: 5΄-CAC AGC CTG GAT GGC TAC GT-3΄; COX2, forward primer: 5΄-TGG TGC CTG GTC TGA TGA TG-3΄, reverse primer: 5΄-GTG GTA ACC GCT CAG GTG TTG-3΄; iNOS, forward primer: 5΄-CCC TCC TGA TCT TGT GTT GGA-3΄, reverse primer: 5΄-TCA ACC CGA GCT CCT GGA A-3΄; IL6, forward primer: 5′-TAG TCC TTC CTA CCC CAA TTT CC-3′, reverse primer: 5′-TTG GTC CTT AGC CAC TCC TTC-3′; and IL1ß, forward primer: 5΄-CAA CCA ACA AGT GAT ATT CTC CAT G-3΄, reverse primer: 5΄-GAT CCA CAC TCT CCA GCT GCA-3΄ and Mcp1, forward primer: 5΄- CTT CTG GGC CTG CTG TTC A-3΄, reverse primer: 5΄- GCA GCC TAC TCA TTG GGA TCA-3΄. Quantitative PCR (qPCR) amplifications were performed on an ABI 7500 Fast real time PCR (Life Technologies) using 1 cycle at 50 °C for 2 min and 1 cycle of 95 °C for 10 min, followed by 40 cycles of 15 s at 95 °C and 1 min at 60 °C. The dissociation curve was completed with 1 cycle of 1 min at 95 °C, 30 s at 55 °C, and 30 s at 95 °C. mRNA expression was analyzed using the ΔΔCT method and normalized with respect to the expression of the β-actin housekeeping genes using 7500 Fast System SDS Software v1.3.0 (Life Technologies). A value of less than 1 indicates transcriptional down-regulation (inhibition of gene expression) compared with LPS, which shows maximum genetic induction. Values higher than 1 imply overexpression of the particular gene in excess of LPS stimulation. Amplification of specific transcripts was further confirmed by obtaining melting curve profiles.
Nutritional and Sensory Analysis
Nutritional (promixate) analysis of selected ingredient matrices was performed by Medallion Labs (Minneapolis, MN). Total calories (by calculation), calories from fat (by calculation), carbohydrates (by calculation), moisture (AOAC: 945.43, 934.01), ash (AOAC: 923.03), dietary fiber (AOAC: 991.43), total sugars (AOAC: 977.20), protein (by Dumas, AOAC: 992.15), and total fat content (gravimetric, AOAC: 948.15) were quantified in biofortified matrices as well as in unfortified protein sources [18]. Sensory analysis of selected ingredients was performed by Sensory Spectrum Discovery Center (Kannapolis, NC). Trained evaluation panels assigned a “Degree of Difference” score (0 to 10) for each sample as compared to the untreated edible proteins. Qualitative information about distinguishing features and differences observed between treated and untreated proteins included appearance, flavor, and texture. Observations in differences do not indicate the degree of likeability of the treated versus the untreated material, or vice versa.
Statistical Analysis
Statistical analyses for testing the bioactivity of phytoactives sorbed onto muscadine and kale fortified matrices (HP50 and SPI) were performed using Prism 4.0 (GraphPad Software, San Diego, CA). Data were analyzed by one-way ANOVA with treatment as a factor. Post hoc analyses of differences between individual experimental groups were made using the Dunnett’s multiple comparison tests at P < 0.05. Values are reported as means ± SEM.
Results and Discussion
Bioactivity of Phytoactives Bound to Matrices
Cell viability assays showed that none of the fortified matrices were cytotoxic to RAW 264.7 macrophages at 50 μg/mL. We investigated the ability of the fortified matrices, compared to unfortified protein materials, to modulate gene expression profiles for acute and chronic inflammation in the LPS-stimulated macrophages. For each assay, a negative control (vehicle alone), an induction control (1 μg/mL LPS), and a positive control (1 μg/mL LPS and 10 μM dexamethasone) were used to establish a reference baseline, maximum induction of marker genes, and assay effectiveness, respectively. The use of LPS as a systemic inducer of inflammatory response is warranted by its dual role in inducting both acute inflammatory responses as well as low-level of chronic inflammation. Dexametasone, used as positive control for assay effectiveness, significantly suppressed expression of all markers (Cox-2 0.44 ± 0.01; iNOS 0.29 ± 0.1; IL-1β 0.31 ± 0.1; IL-6 0.46 ± 0.1; MCP1 0.26 ± 0.2). All fortified matrices suppressed at least two biomarkers of inflammation (Fig. 1). Monocyte chemotactic protein (MCP1) expression was strongly affected by all treatments. Muscadine and kale fortified matrices were most effective at suppressing the expression of chronic pro-inflammatory genes, IL-6 and MCP1. They showed no significant effect for attenuation of the LPS-induced Cox-2, iNOS or IL-1β expression (Fig. 1), except for the muscadine-SPI matrix, which showed significant inhibition of IL-1β expression. Unfortified edible proteins had no significant influence on suppressing any of the tested biomarkers (Fig. 2), indicating that the bioactivity is linked to the muscadine and kale phytoactive components complexed in the protein matrices. Total phenolics, measured by Folin Ciocalteu method, in muscadine-SPI was significantly higher (13.4 mg/g matrix) compared to muscadine-HP50, kale-SPI and kale HP50 (10.5, 8.7, 8.0 mg/g matrix, respectively). The higher anti-inflammatory activity of muscadine-SPI matrix can be attributed to higher total phenolic content of this ingredient. The anti-inflammatory activity of fruit and vegetable phytoactives has been previously described [19, 20]. These results demonstrate that the fortified ingredient matrices, at reasonable dietary levels, contained phytoactives that suppressed activation of key pro-inflammatory genes triggered by an inflammatory stimulus. However, the signaling cascades that mediate the effects of fortified matrices on proinflammatory factors production have not been characterized. The experiments demonstrated that binding process does not alter the anti-inflammatory activity of phytochemicals, but stabilized them in a dry concentrated form. These results support our previous findings indicating that the binding process does not affect the biological activity of phytochemicals [16].
Effects of muscadine and kale-fortified matrices on pro-inflammatory gene expression associated with acute and chronic inflammatory responses. Macrophages were pretreated with 50 μg/mL of muscadine-SPI (a) or muscadine-HP50 (b) or kale-SPI (c) or kale-HP50 (d) extracts and inflammatory response was induced with 1 μg/mL LPS for 6 h. Fold changes in gene expression are reported as the mean ± SEM relative to LPS controls. *differences at P < 0.05 were considered significant
Effects of unfortified protein matrices on pro-inflammatory gene expression associated with acute and chronic inflammatory responses. Macrophages were pretreated with 50 μg/mL of SPI (a) or HP50 (b) extracts and inflammatory response was induced with 1 μg/mL LPS for 6 h. Fold changes in gene expression are reported as the mean ± SEM relative to LPS controls. No significant differences at P < 0.05 were observed
Nutritional Analysis of Matrices
The nutritional analyses of the muscadine and kale fortified SPI and HP50 matrices in comparison to the unfortified/blank protein rich carriers are presented in Table 1. Fortification of the SPI matrices with muscadine or kale bioactives did not raise the estimated calories/100 g dry matrix (366 and 376 cal/100 g for muscadine and kale, respectively compared to 384 cal in unfortified SPI). Total carbohydrates, including dietary fibers and free sugars, were elevated in the fortified matrices with no increase in calories. Protein content decreased in the fortified matrices by about 37 % (muscadine-SPI, kale-SPI and kale-HP50), and 17.6 % (muscadine-HP50), compared to their untreated edible proteins. The changes in proximate analysis for some muscadine fortified ingredients can be attributed to solubility of some of the protein isolates in the discarded supernatant after centrifugation with the juice [16]. Protein decrease in the kale fortified proteins is due to the increase in weight (due to addition of the vegetable compounds) of the final product by the co-drying technique which led to dilution of proteins per 100 g dry wt. There was a small decrease in total fat for all fortified matrices.
Sensory Analysis of Matrices
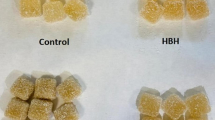
Sensory analysis of the fortified and unfortified SPI matrices for muscadine and kale was performed by Sensory Spectrum (www.sensoryspectrum.com) where trained evaluation panels assigned a “Degree of Difference” (DOD) score (0 to 10) for each sample. Qualitative information about distinguishing features and differences observed between the fortified and unfortified proteins included: appearance, flavor, and texture (Table 2). The muscadine-SPI matrix was characterized by purple-red coloration due to the anthocyanin content, whereas SPI is pale beige. Kale-SPI had a bright green hue due to chlorophyll incorporation into the matrix. Professional panelists provided descriptive analyses and evaluations of suitability of the ingredients for particular food formulations (Table 2). Panel evaluators noted that the fortified muscadine-SPI matrix: 1) effectively masked soy overtones, 2) had a pleasant flavor, 3) would be well suited to food formulations where grape and muscadine notes were desired, and 4) would be favorably accepted by consumers. Similarly, kale-SPI was ranked as having a very high DOD score from SPI, and was suggested for food applications where green and green pea notes would be suitable. Panelists suggested that unfortified SPI protein formed clumps in the mouth, whereas fortified muscadine and kale SPI matrices acquired a creamy consistency in the mouth. While the panelists evaluated the chimeric ingredients (dry granular format), the ingredients are ultimately intended for incorporation into alternative food formats (e.g. bars, spreads, etc.) prior to human consumption.
Conclusions
Fortified matrices efficiently sorbed target phytoactives from kale and muscadine, and demonstrated nutritional and sensory attributes amenable to formulation in nutritive food formats, while delivering anti-inflammatory benefits expected from kale or muscadine consumption. Results of anti-inflammatory bioassays demonstrated that muscadine and kale fortified matrices, at reasonable dietary levels, contained phytoactives that suppressed the activation of key pro-inflammatory genes triggered by an inflammatory stimulus. Matrices were most effective at suppressing the expression of chronic pro-inflammatory genes, IL-6 and MCP1.
Abbreviations
- HP50:
-
Hemp protein
- SPI:
-
Soy protein isolate
References
Cassidy A, Mukamal KJ, Liu L, Franz M, Eliassen H, Rimm E (2013) High anthocyanin intake is associated with a reduced risk of myocardial infarction in young and middle-aged women. Circulation 127:188–196
De Pascual-Teresa S, Moreno DA, Garcia-Viguera C (2010) Flavanols and anthocyanins in cardiovascular health: a review of current evidence. Int J Mol Sci 11:1679–1703
Oyebode O, Gordon-Dseagu V, Walker A, Mindell J (2014) Fruit and vegetable consumption and all-cause, cancer and CVD mortality: analysis of Health Survey for England data. J Epidemiol Community Health 0:1–7
Nieman DC, Gillitt ND, Knab AM, Shanely RA, Pappan KL, Jin F, Lila MA (2013) Influence of a polyphenol-enriched protein powder on exercise-induced inflammation and oxidative stress in athletes: a randomized trial using a metabolomics approach. PLoS ONE 8:e72215
Racicot K, Anderson DJ, Davis BB (2012) Chapter 11. Performance-optimizing ration components. In: Barrett AH, Cardello AV (eds) Military food engineering and ration technology. DEStech Publications, Inc, Lancaster, pp 275–295
Lila MA, Cheng D (2012) Comprehensive strategies for evaluating the adaptogenic properties of phytochemicals. In: Carkeet C, Grann K, Randolf R, Venzon D, Izzy S (eds) Phytochemicals: health promotion and therapeutic potential. Taylor and Francis/CRC Press, Florida, pp 95–112
Esposito D, Rathinasabapathy T, Schmidt B, Shakarjian MP, Komarnytsky S, Raskin I (2013) Acceleration of cutaneous wound healing by brassinosteroids. Wound Repair Regen 21:688–696
Nieman DC, Stear AJ, Castell LM, Burke LM (2012) A-Z of nutritional supplements: dietary supplements, sports nutrition foods and ergogenic aids for health and performance. Br J Sports Med 44:1202–1205
Roopchand DE, Grace MH, Kuhn P, Cheng DM, Plundrich N, Poulev A, Howell A, Fridlender B, Lila MA, Raskin I (2012) Efficient sorption of polyphenols in soybean flour enables natural fortification of foods. Food Chem 131:1193–1200
Pastrana-Bonilla E, Akoh CC, Sellappan S, Krewer G (2003) Phenolic content and antioxidant capacity of muscadine grapes. J Agric Food Chem 51:5497–5503
Greenspan P, Bauer JD, Pollock SH, Gangemi JD, Mayer EP, Ghaffar A, Hargrove JL, Hartle DK (2005) Antiinflammatory properties of the muscadine grape (Vitis rotundifolia). J Agric Food Chem 53:8481–8484
Azevedo CH, Rodriguez-Amaya DB (2005) Carotenoid composition of kale as influenced by maturity, season and minimal processing. J Sci Food Agric 85:591–597
Ferioli F, Giambanelli E, D’Antuono LF, Costa HS, Albuquerque TG, Silva AS, Hayran O, Kocaoglu B (2013) Comparison of leafy kale populations from Italy, Portugal, and Turkey for their bioactive compound content: phenolics, glucosinolates, carotenoids, and chlorophylls. J Sci Food Agric 93:3478–3489
Velasco P, Cartea MA, González C, Vilar M, Ordáz A (2007) Factors affecting the glucosinolate content of kale (Brassica oleracea acephala group). J Agric Food Chem 55:955–962
Esposito D, Chen A, Grace M, Komarnytsky S, Lila M (2014) Inhibitory effects of wild blueberry anthocyanins and other flavonoids on biomarkers of acute and chronic inflammation in vitro. J Agric Food Chem 62:7022–7028
Grace MH, Guzman I, Roopchand DE, Moskal K, Cheng DM, Pogrebnyak N, Raskin I, Howell A, Lila MA (2013) Stable binding of alternative protein-enriched food matrices with concentrated cranberry bioflavonoids for functional food applications. J Agric Food Chem 61:6856–6864
Singleton VL, Orthofer R, Lamuela-Raventós RM (1999) Analysis of total phenols and other oxidation substrates and antioxidants by means of Folin-Ciocalteu reagent. Methods Enzymol 299:152–178
AOAC Official Methods of Analysis of the Association of Official Chemists. 17th edition. Edited by William Horwitz. Maryland, USA (2000):pp 20877–2417
Mueller M, Hobiger S, Jungbauer A (2010) Anti-inflammatory activity of extracts from fruits, herbs and spices. Food Chem 122:987–996
Grace M, Esposito D, Dunlap K, Lila M (2014) Comparative analysis of phenolic content and profile, antioxidant capacity, and anti-inflammatory bioactivity in wild Alaskan and commercial Vaccinium berries. J Agric Food Chem 62:4007–4017
Conflicts of Interest
The authors declare no conflicts of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Grace, M.H., Yousef, G.G., Esposito, D. et al. Bioactive Capacity, Sensory Properties, and Nutritional Analysis of a Shelf Stable Protein-rich Functional Ingredient with Concentrated Fruit and Vegetable Phytoactives. Plant Foods Hum Nutr 69, 372–378 (2014). https://doi.org/10.1007/s11130-014-0444-7
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11130-014-0444-7