Abstract
Several studies support the health-promoting benefits of lupins, particularly lupin proteins. It has been demonstrated that Lupinus albus gamma conglutin (Cγ) protein lowered blood glucose levels; thus, Cγ showed promise as a new anti-diabetic compound for type 2 diabetes (T2D) treatment. The aim of this study was to evaluate the effect of Cγ on Ins-1 gene expression and on pancreatic insulin content in streptozotocin-mediated diabetic rats. Cγ was isolated from Lupinus albus seeds. Its identification was confirmed with polyacrylamide gel electrophoresis under native and denaturing conditions. We used streptozotocin (STZ) to induce T2D on the 5th day of life of newborn male Wistar rats (n5-STZ). After 20 weeks post-induction, these animals (glycemia > 200 mg/dL) were randomly assigned to three groups that received the following one-week treatments: vehicle, 0.90 % w/v NaCl (n5 STZ-Ctrl); glibenclamide, 10 mg/kg (n5 STZ-Glib); or Cγ, 120 mg/kg (n5 STZ-Cγ). Glucose and insulin levels were measured before and after treatment. Ins-1 gene expression was quantified using real time polymerase chain reaction and the pancreatic insulin content was evaluated with immunohistochemistry. Post-treatment, the n5 STZ-Cγ and n5 STZ-Glib groups showed reductions in glucose, increments in serum insulin, and increases in Ins-1 gene expression and beta cell insulin content compared to the n5 STZ-Ctrl group. The results showed that Cγ had beneficial effects on Ins-1 gene expression and pancreatic insulin content. These biological effects of Cγ strengthen its promising potential as a nutraceutical and/or new agent for controlling hyperglycemia.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
In recent years, the beneficial effects of plant extracts and compounds on human health have been scientifically validated and published [1–3]. Several beneficial properties have been reported for lupins, a legume consumed for centuries in the Mediterranean and Andean regions, due to its high protein content [4]. This genus comprises more than 200 species, and it is distributed in Europe, Africa, America, and Australia. Lupins are cultivated for animal and human nutrition. Recently, proteins from some species of the Lupinus genus have acquired importance, due to their abilities to lower blood pressure, glycemia, and cholesterolemia, among other effects [5–7]. Scientific interest has focused on proteins found in the seeds of Lupinus albus (a widely cultivated, domesticated species) that exert hypoglycemic effects in vivo and in vitro. These effects were attributed to the protein, gamma conglutin (Cγ), found in the globulin fraction of L. albus seeds [7–9]. Cγ is a homo-tetramer of the 7S glycoprotein, which constitutes about 4–5 % of total plant protein. The Cγ monomer has a relative mass of about 50 kDa, and each monomer is composed of two subunits of 17 and 29 kDa, linked by disulfide bonds [10]. Cγ is resistant to proteolytic enzymes at pH greater than 4 [11]. It has been reported that Cγ is capable of interacting with insulin [7]; moreover, it stimulates the activation of protein kinase pathways, and increases translocation of glucotransporter-4 (Glut-4) [12]. In addition, this protein augments glucose uptake in HepG2 cells, and potentiates insulin and metformin effects [9]. These findings ratified the therapeutic potential of Cγ for controlling hyperglycemia; thus, Cγ may be considered for use in a multi-therapy approach for managing type 2 diabetes (T2D). Although Cγ has been evaluated in several metabolic contexts, no study has investigated its effects on insulin expression or pancreatic insulin levels in a chronic experimental T2D model. In a previous study, we characterized the basal levels of these parameters in an animal model of chronic stage T2D (n5-STZ). This animal model was produced by treating neonatal rats with streptozotocin (STZ) on day 5 after birth; this causes the rats to develop T2D in adulthood [13]. Here, we aimed to quantify Ins-1 gene expression and insulin protein expression in pancreatic beta cells after Cγ treatment in n5-STZ adult rats.
Materials and Methods
Animals
Wistar rats were obtained from the Bioterium of the University of Guadalajara. The experimental animals were maintained in standard laboratory conditions (24 ± 2 °C ambient temperature, 55.0 ± 5 % humidity, and a 12-h light–dark cycle) with ad libitum access to a standard rodent diet (Purina LabDiet® 5001) and water. The local ethics committee approved this protocol, and all animal procedures were conducted in accordance with the production, care, and use of laboratory animals established in the Mexican Official Standard (NOM-062-ZOO-1999).
Plant Material
Dry, certified L. albus seeds were kindly donated by E. van Santen from the College of Agriculture, Auburn University, Alabama, USA.
Isolation of Cγ and Characterization by PAGE
Lupin seeds were dehulled, ground to flour, and defatted with hexane. The proteins were extracted as described previously by Martínez-Ayala and Paredes-López [14]. Briefly, the albumin fraction was removed in two steps; in step 1, the defatted flour was added to double-distilled water (ddH2O), and stirred for 2 h at 4 °C. The solution was centrifuged, and the supernatant (albumin fraction) was discarded. In step 2, the pellet was resuspended in ddH2O, and step 1 was repeated. Then, the total globulin fraction (pellet) was resuspended in 10 % NaCl (pH 7), and stirred for 12 h at 4 °C. The solution was then centrifuged (30 min, 8,000 rpm at 4 °C), and the supernatant was recovered. The globulins contained in the supernatant were precipitated out with ammonium sulfate until reach 85 % saturation. After centrifugation, the pellet was dissolved in phosphate buffer (0.1 M, pH 6.8), and then dialyzed against 0.2 M acetate buffer (pH 4.8) for 18 h. The solution was then centrifuged; the resulting pellet contained the alpha conglutin (Cα), and the supernatant contained β- and γ-conglutins (Cβ and Cγ, respectively). To separate Cβ and Cγ, the supernatant was dialyzed against distilled water at 4 °C for 48 h. After centrifugation, the supernatant, which contained Cγ, was lyophilized (Freeze Drying 4.5, LABCONCO) at −50 °C, 0.036 mbar, for 8 h. Then, the Cγ fraction was analyzed by SDS-PAGE under denaturing and non-denaturing conditions on a 12 % SDS-polyacrylamide gel, according to Schägger and von Jagow [15]. Briefly, two aliquots of protein (2 μg each) were mixed in Laemmli Sample Buffer, one with and one without 1 % ß-mercaptoethanol. The proteins were separated by electrophoresis with the mini-gel kit, Protean®Tetra cell (Bio-Rad, Milan, Italy). After electrophoresis, gels were stained with Coomassie brilliant blue G-250 (BioRad, Milan, Italy), and the relative molecular masses of denatured and native Cγ were determined by comparison to a protein ladder (BenchMark TM Prestained protein ladder, Invitrogen).
Experimental Induction of T2D
A group of male Wistar rat pups (9–11 g body weight) were separated from their mothers at 5 days after birth. After an 8-h fast, the animals were injected intraperitoneally with a fresh solution of STZ (150 mg/kg; Sigma, St. Louis, MO, USA) diluted in citrate buffer (10 mmol/L sodium citrate, pH 4.5), as previously described by Takada et al. [16]. After weaning (day 21), the animals were placed in standard cages, five rats per cage. To ascertain the T2D induction, weight gain and glucose levels were monitored periodically after STZ treatment.
Experimental Groups
At 20 weeks post-induction, glucose levels were measured in adult n5-STZ rats to verify the diabetic stage (glycemia > 200 mg/dL). Then, the diabetic animals were divided randomly into three groups. The control group (n5 STZ-Ctrl, n = 5) received only vehicle (normal saline solution, 0.90 % w/v NaCl). The standard treatment group (n5 STZ-Glib, n = 5) was treated with glibenclamide (10 mg/kg, dissolved in normal saline), as previously described by Pareek et al. [17]. The test treatment group (n5 STZ-Cγ, n = 5) was treated with Cγ (120 mg/kg dissolved in normal saline), as previously described by Magni et al. [7]. For one week, treatments were administrated orally, once per day.
Determination of Glucose and Insulin Levels
The animals were anesthetized, and blood was collected from the retro-orbital plexus at the beginning of the treatments (pre-treatment values) and before they were sacrificed (post-treatment values). Serum was separated by centrifugation at 6,000 × g for 15 min at 4 °C and stored at −70 °C until use for glucose and insulin determinations. Serum glucose levels were determined with the glucose oxidase-peroxidase method (BioSystems, Spain) and quantified in a semi-quantitative spectrophotometer (BTS-330, BioSystems, Spain). Serum insulin concentration was quantified with an enzyme-linked immunosorbent assay (ELISA) kit with rat insulin antibody (DRG® International, Marburg, Germany), according to the manufacturer’s instructions.
Immunohistochemistry for Determining Beta Cell Insulin Content
To determine the insulin protein content in pancreatic beta cells, the animals were anesthetized, and a laparotomy was performed to excise a fragment of pancreatic tissue. The resected tissue was washed with normal saline solution, fixed immediately with 4 % paraformaldehyde in 1X phosphate buffered saline (PBS), and embedded in paraffin. Paraffin-embedded samples were cut (4 μm) and sections were incubated overnight at 4 °C with a rabbit monoclonal antibody against rat insulin (Cell Signaling Technology, Danvers, MA). Detection was performed with a secondary antibody provided in the Mouse/Rabbit ImmunoDetector HRP/DAB Detection System (BIO SB, USA). Subsequently, the sections were counterstained with hematoxylin-eosin. For negative controls, the primary antibodies were replaced with 1X PBS. The percentage of beta cells that stained positive for insulin was determined with LeicaQwin (Leica, France) and Motic Images Plus 2.0 (Motic China Group Co. Ltd., China) software.
RNA Extraction, Reverse-Transcription, and Quantification of Ins-1 Gene Expression
To quantify Ins-1 gene expression, total RNA was isolated from pancreatic tissue with the RNeasy® Protect Mini Kit (QIAGEN, USA). RNA (2 μg) was reverse-transcribed into cDNA with the Transcriptor First Strand cDNA Synthesis kit (Roche, Germany), according the manufacturer’s instructions. Then, Ins-1 gene expression was determined by real-time PCR with a LightCycler® FastStart DNA MasterPLUS SYBR Green I Kit (Roche, Germany). The Rps 18 housekeeping gene served as an internal control. All reactions were performed in triplicate with a 2.0 LightCycler® (Roche, Germany), under the following cycling conditions: Ins-1 gene: 95 °C for 10 min and 40 cycles at 95 °C for 10 s, 65 °C for 10 s, and 72 °C for 8 s; Rps 18 gene: 95 °C for 10 min and 45 cycles at 95 °C for 10 s, 61 °C for 10 s, and 72 °C for 6 s. The primer sequences were: Ins-1 Forward 5′-CCATCAGCAAGCAGGTCAT-3′, reverse 5′-TGTGTAGAAGAAACCACGTTCC-3′; Rps 18 Forward 5′-CATGTGGTGTTGAGGAAAGCAG-3′, reverse 5′-GGGATCTTGTATTGTCGTGGGT-3′. cDNA was replaced with sterile water in negative controls. The threshold (Ct) values obtained for the target gene were normalized against Rps 18 Ct values. Relative quantification of PCR products was determined with the 2−∆∆Ct method. A melting curve analysis was performed to confirm that a single amplicon was amplified for each analyzed gene [18, 19].
Statistical Analysis
Values are expressed as the mean ± standard error of the mean (S.E.M.) for serum glucose and insulin concentrations. For Ins-1 gene expression, the mean was expressed in relative light units (R.L.U.) ± the S.E.M. The number of beta cells that stained positive for insulin was expressed as a percentage. Differences among groups for Ins-1 gene expression and beta cell insulin positivity were assessed with the Mann–Whitney test, and changes were compared among groups with the Wilcoxon test. The paired t-test was used to compare differences between pre- and post-treatment concentrations of serum glucose and insulin within each experimental group (intra-group differences). Data analysis was performed with PASW statistical software version 18 (Chicago, IL, USA).
Results
Characterization of Cγ by SDS-PAGE
Figure 1 shows the SDS-PAGE profiles of the Cγ employed in the n5 STZ-Cγ treatment group. Under non-reducing conditions, Cγ appears as one band of about 50 kDa (Fig. 1a, lanes 1, 2). As expected, under reducing conditions, two major bands appeared of about 17 and 29 kDa; these bands corresponded to the two Cγ sub-units (Fig. 1b, lanes 3, 4).
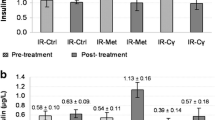
Cγ Treatment Reduced Glucose Levels and Increased Insulin Levels
Both the n5 STZ-Glib and n5 STZ-Cγ groups showed reductions (post-treatment vs. pre-treatment values) in glucose levels. However, this reduction was statistically significant only in the n5 STZ-Cγ group (P < 0.05). On the other hand, insulin levels were increased with both treatments (Table 1). To compare the changes in glucose and insulin levels among groups, we calculated the percentage change from pre-treatment values. The pre-treatment values were taken as 100 %. The n5 STZ-Cγ group showed a significant 17 % reduction (P < 0.05) and the STZ-Glib group showed a 12 % reduction in serum glucose after treatment. On the other hand, serum insulin levels were increased by 63 % in the STZ-Cγ group and 143 % in the STZ-Glib group.
Pancreatic Insulin Content was Augmented by Cγ and Glibenclamide
Pancreatic insulin content was evaluated with immunohistochemistry. Insulin-positive beta cells were more abundant in n5 STZ-Cγ and n5 STZ-Glib samples compared to untreated diabetic control samples (Fig. 2a-c). The assay was validated by the absence of cross-reactivity (negative control; Fig. 2d). An image analysis of these tissues indicated that the percentage increase in insulin-positive cells was higher in the treated groups (n5 STZ-Cγ 36.45 ± 4.84 % and n5 STZ-Glib 42.21 ± 3.38 %) than the control group (30.59 ± 2.99 %). These results were consistent with our findings for serum insulin levels in the n5 STZ- Cγ and n5 STZ-Glib groups.
Photomicrographs of islets of Langerhans showing positive insulin beta cells (400×). (a) Insulin-positive beta cells were less abundant in the islets of Langerhan’s from the diabetic control animals than in the treated animals. (b) n5 STZ-Glib and (c) n5 STZ-Cγ samples showed increases in insulin-positive beta cells with treatment. (d) Negative control sample. Arrows indicate insulin-positive cells in islets of Langerhan’s
Cγ and Glibenclamide Increased Ins-1 Gene Expression in Diabetic Rats
Relative expression of the Ins-1 gene was quantified with real-time PCR, and the results are expressed in relative light units (R.L.U.). Melting point analysis was performed to verify the amplification of specific DNA sequences (data not shown). Ins-1 gene expression was 0.77 fold higher in n5 STZ-Cγ than in n5 STZ-Ctrl rats. Similarly, Ins-1 gene expression was 2.84 fold higher in the n5 STZ-Glib than in n5 STZ-Ctrl rats (Fig. 3).
Discussion
Legume seeds, particularly soybean and lupin seeds, are important in animal and human nutrition, due to their high protein content [10]. In addition, some soybean and lupin proteins exert beneficial health effects. It has been shown that soybean proteins lowered total serum cholesterol, low-density lipoprotein (LDL) cholesterol, and triglycerides [20]. Likewise, lupin proteins were reported to have beneficial biological activities; for example they reduced lipemia, glycemia, and blood pressure [5–7]. Lupin seeds contain two major classes of proteins: albumins and globulins (α-, β-, δ-, and γ-conglutins) [10]. In recent years, the Cγ protein has attracted scientific interest, due to its hypoglycemic effects demonstrated in vitro and in vivo [7]. In addition, a Cγ-enriched protein fraction exerted a glucose-lowering effect in healthy subjects [8].
Due to the therapeutic potential of Cγ for T2D, in the present study, we evaluated the effect of Cγ on Ins-1 gene expression and insulin content in pancreatic beta cells of diabetic animals. In this study, we selected the n5-STZ model of T2D, because these rats develop chronic hyperglycemia (serum glucose greater than 200 mg/dL), polydipsia, and polyuria, among other characteristics [16]. Additionally, these diabetic rats exhibit reduced Ins-1 gene expression and reduced insulin immunoreactivity in beta cells [13].
The results of this study showed that Cγ treatment significantly reduced serum glucose levels in diabetic rats, confirming the hypoglycemic effects previously reported for Cγ in healthy, normo- and hyperglycemic rats [7, 9]. On the other hand, Cγ increased serum insulin levels compared to pre-treatment values in diabetic animals.
It was previously shown that Cγ protein was resistant to the proteolytic activity of some intestinal and exogenous enzymes [11]. This property facilitates its oral administration. Furthermore, Capraro and colleagues [21] demonstrated that Cγ could transit, in an intact form, from the apical to the basolateral membranes of Caco-2 cells, an in vitro model that mimicked the human intestinal epithelium. Similar to glucagon-like peptide-1 (GLP-1), which binds to its pancreatic receptor and stimulates insulin exocytosis [22], we hypothesized that, upon absorption, Cγ may stimulate pancreatic insulin secretion in a similar manner.
On the other hand, it has been shown that STZ caused selective damage to pancreatic beta cells [23]. Accordingly, the beta cell insulin content was diminished in the untreated diabetic control rats; however, in the Cγ and glibenclamide treatment groups, this pancreatic insulin content was augmented.
In this study, we also demonstrated that Cγ increased Ins-1 gene expression. This finding was consistent with a previous in vivo study that reported a significant increase in insulin mRNA expression in rats fed raw soybeans compared to rats fed a normal diet [24]. A possible explanation for the Cγ effect on Ins-1 gene expression might involve the c-Jun N-terminal protein kinase (JNK) pathway. In chronic hyperglycemia (reported for the n5-STZ model), the JNK pathway is active, and insulin gene expression is reduced [25]. Consequently, in our experimental model of diabetes, Cγ treatment might increase insulin gene expression by inhibiting the JNK pathway. Another hypothesis related to insulin gene expression involves a Cγ effect on the insulin signaling pathway, as suggested by Terruzzi et al. [12]. They showed in vitro that Cγ might be involved in gene transcription, protein synthesis, and muscle metabolism through activation of kinases involved in the insulin signaling pathway. Moreover, it has been reported that Cγ can be internalized into HepG2 cells. Once in the cytoplasm, it is phosphorylated on multiple residues, which probably initiates activation of the signaling pathways previously mentioned [26].
In addition, a potential effect of the Cγ-mediated increases in Ins-1 gene expression and beta cell insulin content may be related to the effect of insulin on hepatic gluconeogenesis. Insulin reduces the expression of glucose-6-phosphatase, a key regulator of gluconeogenesis [27]. Our results were consistent with results from a parallel study conducted within our scientific group, where Cγ treatment decreased the expression of glucose-6-phosphatase (paper in preparation).
Moreover, it is important to recall that the hypoglycemic effect of lupins was attributed to a variety of proteins, including Cγ. In addition, the quinolizidine alkaloids (QAs), which also stimulated insulin secretion in vitro, are found mainly in wild lupin species; these QAs include lupanine, 13-α-OH lupanine, and 17-oxolupanine [28]. Furthermore, consumption of cooked Lupinus mutabilis seeds reduced blood glucose levels in patients with diabetes [29]. Lupinus mutabilis is a semi-domesticated American lupin species, which contains proteins and QAs. Also, it has been hypothesized that integrating L. mutabilis seeds into the diet could provide beneficial health effects and may represent a feasible alternative for treating chronic hyperglycemic diseases [30].
In conclusion, our data showed that Cγ of Lupinus albus seeds increased Ins-1 gene expression and pancreatic insulin content. Thus, Cγ constitutes a beneficial hypoglycemic agent, and its incorporation in a therapeutic strategy for T2D management may provide added benefit. To that end, Cγ should be tested for its therapeutic potential in subgroups of patients with well-characterized T2D. Furthermore, future scientific study is required to elucidate the complete mechanism of action of Cγ to provide a basis for continued investigation in this field.
Abbreviations
- Cγ:
-
gamma conglutin
- n5 STZ:
-
neonatal streptozotocin induced rat model
- STZ:
-
streptozotocin
- T2D:
-
type 2 diabetes
References
Yeh GY, Eisenberg DM, Kaptchuck TJ, Phillips RS (2003) Systematic review of herbs and dietary supplements for glycemic control in diabetes. Diabetes Care 26(4):1277–1294
Perez-Gutierrez RM, Muñiz-Ramirez A, Gomez YG, Ramírez EB (2010) Antihyperglycemic, antihyperlipidemic and antiglycation effects of Byrsonima crassifolia fruit and seed in normal and streptozotocin-induced diabetic rats. Plant Foods Hum Nutr 65(4):350–357
Fadzelly AB, Asmah R, Fauziah O (2006) Effects of Strobilanthes crispus tea aqueous extracts on glucose and lipid profile in normal and streptozotocin-induced hyperglycemic rats. Plant Foods Hum Nutr 61(1):7–12
Cerletti P, Duranti M, Restani P (1983) Properties of lupine proteins relevant to their nutritional performance. Plant Foods Hum Nutr 32(2):145–154
Sirtori CR, Lovati MR, Manzoni C, Castiglioni S, Duranti M, Magni C, Morandi S, D’Agostina A, Arnoldi A (2004) Proteins of white lupin seed, a naturally isoflavone-poor legume, reduce cholesterolemia in rats and increase LDL receptor activity in HepG2 cells. J Nutr 134(1):18–23
Lee YP, Mori TA, Puddey IB, Sipsas S, Ackland TR, Beilin LJ, Hodgson JM (2009) Effects of lupin kernel flour-enriched bread on blood pressure: a controlled intervention study. Am J Clin Nutr 89(3):766–772
Magni C, Sessa F, Accardo E, Vanoni M, Morazzoni P, Scarafoni A, Duranti M (2004) Conglutin-γ, a lupin seed protein, binds insulin in vitro and reduces plasma glucose levels of hyperglycemic rats. J Nutr Biochem 15(11):646–650
Bertoglio JC, Calvo MA, Hancke JL, Burgos RA, Riva A, Morazzoni P, Ponzone C, Magni C, Duranti M (2011) Hypoglycemic effect of lupin seed γ-conglutin in experimental animals and healthy human subjects. Fitoterapia 82(7):933–938
Lovati MR, Manzoni C, Castiglioni S, Parolari A, Magni C, Duranti M (2012) Lupin seed γ-conglutin lowers blood glucose in hyperglycaemic rats and increases glucose consumption of HepG2 cells. Br J Nutr 107(1):67–73
Duranti M, Consonni A, Magni C, Sessa F, Scarafoni A (2008) The major proteins of lupin seed: characterisation and molecular properties for use as functional and nutraceutical ingredients. Trends Food Sci Tech 19:624–633
Capraro J, Chiara M, Alessio S, Duranti M (2009) Susceptibility of lupin gamma-conglutin, the plasma glucose-lowering protein of lupin seeds, to proteolytic enzymes. J Agric Food Chem 57:8612–8616
Terruzzi I, Senesi P, Magni C, Montesano A, Scarafoni A, Luzi L, Duranti M (2011) Insulin-mimetic action of conglutin-γ, a lupin seed protein, in mouse myoblasts. Nutr Metab Cardiovasc Dis 21(3):197–205
Vargas-Guerrero B, García-López PM, González-Santiago AE, Domínguez-Rosales JA, Gurrola-Díaz CM (2013) Reduction of Ins-1 gene expression and tissue insulin levels in n5-STZ rats. Biol Res 46(3):281–288
Martínez-Ayala AL, Paredes-López O (2001) Molecular characterization of the β-conglutin of lupin seeds. J Food Biochem 25(1):15–31
Schägger H, von Jagow G (1987) Tricine-sodium dodecyl sulfate-polyacrilamide gel electrophoresis for the separation of proteins in the range from 1 to 100 kDa. Anal Biochem 166(2):368–379
Takada J, Machado MA, Peres SB, Brito LC, Borges-Silva CN, Costa CE, Fonseca-Alaniz MH, Andreotti S, Lima FB (2007) Neonatal streptozotocin-induced diabetes mellitus: a model of insulin resistance associated with loss of adipose mass. Metabolism 56(7):977–984
Pareek H, Sharma S, Khajja BS, Jain K, Jain GC (2009) Evaluation of hypoglycemic and anti-hyperglycemic potential of Tridax procumbens (Linn.). BMC Complement Altern Med 29(9):48. doi:10.1186/1472–6882–9–48
Livak KJ, Schmittgen TD (2001) Analysis of relative gene expression data using real-time quantitative PCR and the 2(−delta delta C(T)) method. Methods 25(4):402–408
Pfaffl MW (2001) A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Res 29(9):e45
Anderson JW, Johnstone BM, Cook-Newell ME (1995) Meta-analysis of the effects of soy protein intake on serum lipids. N Engl J Med 333(5):276–282
Capraro J, Clemente A, Rubio LA, Magni C, Scarafoni A, Duranti M (2011) Assessment of the lupin seed glucose-lowering protein intestinal absorption by using in vitro and ex vivo models. Food Chem 125(4):1279–1283
Buteau J, Roduit R, Susini S, Prentki M (1999) Glucagon-like peptide-1 promotes DNA synthesis, activates phosphatidylinositol 3-kinase and increases transcription factor pancreatic and duodenal homeobox gene 1 (PDX-1) DNA binding activity in beta (INS-1)-cells. Diabetologia 42(7):856–864
Pisarev VB, Snigur GL, Spasov AA, Samokhina MP, Bulanov AE (2009) Mechanisms of toxic effect of streptozotocin on β-cells in the islets of langerhans. Bull Exp Biol Med 148(6):937–939
Lee SH, Park IS (2000) Effects of soybean diet on the beta cells in the streptozotocin treated rats for induction of diabetes. Diabetes Res Clin Pract 47(1):1–13
Kaneto H, Xu G, Fujii N, Kim S, Bonner-Weir S, Weir GC (2002) Involvement of c-Jun N-terminal kinase in oxidative stress-mediated suppression of insulin gene expression. J Biol Chem 277(33):30010–30018
Capraro J, Chiara M, Faoro F, Maffi D, Scarafoni A, Tedeschi G, Maffioli E, Parolari A, Manzoni C, Lovati MR, Duranti M (2013) Internalisation and multiple phosphorylation of γ-conglutin, the lupin seed glycaemia-lowering protein, in HepG2 cells. Biochem Biophys Res Commun 437(4):648–652
Saltiel AR, Kahn CR (2001) Insulin signalling and the regulation of glucose and lipid metabolism. Nature 414(6865):799–806
García-López PM, de la Mora PG, Wysocka W, Maiztegui B, Alzugaray ME, Del Zotto H, Borelli MI (2004) Quinolizidine alkaloids isolated from Lupinus species enhance insulin secretion. Eur J Pharmacol 504(1–2):139–142
Baldeón ME, Castro J, Villacrés E, Narváez L, Fornasini M (2012) Hypoglycemic effect of cooked Lupinus mutabilis and its purified alkaloids in subjects with type-2 diabetes. Nutr Hosp 27(4):1261–1266
Fornasini M, Castro J, Villacrés E, Narváez L, Villamar MP, Baldeón ME (2012) Hypoglycemic effect of Lupinus mutabilis in healthy volunteers and subjects with dysglycemia. Nutr Hosp 27(2):425–433
Acknowledgments
This study was supported by a grant from Consejo Nacional de Ciencia y Tecnología, México (CONACyT, project number 60283) to C.M. Gurrola-Díaz. B. Vargas-Guerrero received a graduate-fellowship from CONACyT (225001). The authors thank Ing. Rogelio Troyo-Sanromán for statistical advice and Dr. van Santen for the lupin seeds.
Conflict of Interest
The authors declare no conflicting interests or financial disclosures.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Vargas-Guerrero, B., García-López, P.M., Martínez-Ayala, A.L. et al. Administration of Lupinus albus Gamma Conglutin (Cγ) to n5 STZ Rats Augmented Ins-1 Gene Expression and Pancreatic Insulin Content. Plant Foods Hum Nutr 69, 241–247 (2014). https://doi.org/10.1007/s11130-014-0424-y
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11130-014-0424-y