Abstract
Arbutus unedo, Prunus spinosa, Rosa micrantha and Rosa canina are good sources of phenolic compounds, including anthocyanins. These compounds have potent antioxidant properties, which have been related to anticancer activity. Herein, the in vitro antioxidant and antitumor properties of enriched phenolic extracts (non-anthocyanin phenolic compounds enriched extract- PE and anthocyanins enriched extract- AE) of the mentioned wild fruits were evaluated and compared. PE gave higher bioactive properties than the corresponding AE. It was observed a high capacity of A. unedo phenolic extract to inhibit lipid peroxidation in animal brain homogenates (EC50 = 7.21 μg/mL), as also a high antitumor potential against NCI-H460 human cell line (non-small lung cancer; GI50 = 37.68 μg/mL), which could be related to the presence of galloyl derivatives (exclusively found in this species). The bioactivity of the studied wild fruits proved to be more related to the phenolic compounds profile than to the amounts present in each extract, and could be considered in the design of new formulations of dietary supplements or functional foods.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Phenolic compounds are common constituents of fruits and vegetables that are considered an important class of antioxidant natural substances [1–3]. In fact, the interest of plant phenolic extracts derives from the evidence of their potent antioxidant activity and their wide range of pharmacologic properties including anticancer activity [4]. However, the considerable diversity of their structures affects their biological properties such as bioavailability, antioxidant activity, specific interactions with cell receptors and enzymes [5].
The antioxidant properties are conferred to phenolic compounds by hydroxyl groups attached to aromatic rings and they can act as reducing agents, hydrogen donators, singlet oxygen quenchers, superoxide radical scavengers and even as metal chelators [6]. They also activate antioxidant enzymes, reduce α-tocopherol radicals (tocopheroxyls), inhibit oxidases, mitigate nitrosative stress, and increase levels of uric acid and low molecular weight compounds [6]. For many years, phenolic compounds have been intensely studied for their antitumor, proapoptotic and antiangiogenic effects and, in recent years, the usage of these compounds has increased considerably [4]. Anthocyanins, from the flavonoids family, are found mainly in berries and have high antioxidant activity, which plays a vital role in the prevention of neuronal and cardiovascular illnesses, diabetes and cancer, among others [7].
As previously demonstrated by our research group, species such as Arbutus unedo L., Prunus spinosa L., Rosa micrantha Borrer ex Sm. and Rosa canina L. are good sources of phenolic compounds, including anthocyanins [8]. The fruits of A. unedo are used in folk medicine as antiseptics, diuretics and laxatives [9]. P. spinosa fruits have also been used as astringent, diuretic and purgative. R. canina fruits possess prophylactic and therapeutic activities for inflammatory disorders such as arthritis, rheumatism, gout, colds and gastrointestinal disorders [10, 11].
The antioxidant properties of extracts of A. unedo, P. spinosa, R. micrantha and R. canina fruits were previously reported by different authors [12–15], but nothing is known regarding different fractions of the mentioned extracts. Fujji et al. [16] studied the effects of an aqueous extract of R. canina hips on mouse melanoma cells, and demonstrated that proanthocyanidins contributed greatly to its melanogenesis-inhibiting effect on those cells. Tumbas et al. [17] reported that the flavonoids fraction from R. canina tea showed high antioxidant activity towards 2,2-diphenyl-1-picrylhydrazyl (DPPH), as also antiproliferative activity in three human tumor cell lines (HeLa, MCF-7 and HT-29; IC50 values 80.63, 248.03 and 363.95 mg/L, respectively).
Despite the mentioned studies reporting antioxidant properties of fruits of the four species, as far as we know, this is the first study regarding antitumor effects of A. unedo, R. micrantha and P. spinosa. Moreover, the available reports on antioxidant properties refer to crude and not purified/enriched extracts, and no conclusions could be taken about the contributions of different phenolic fractions to the bioactivity of those fruits. Therefore, in the present work, the in vitro antioxidant and antitumor properties of enriched phenolic extracts (non-anthocyanin phenolic compounds enriched extract and anthocyanins enriched extract) of A. unedo, P. spinosa, R. micrantha and R. canina wild fruits were evaluated and compared in order to clarify anthocyanins contribution for bioactivity and the advantageous of using purified/enriched instead of crude phenolic extracts.
Materials and Methods
Standards and Reagents
2,2-Diphenyl-1-picrylhydrazyl (DPPH) was obtained from Alfa Aesar (Ward Hill, MA, USA). Foetal bovine serum (FBS), L-glutamine, Hank’s balanced salt solution (HBSS), trypsin-EDTA (ethylenediaminetetraacetic acid), penicillin/streptomycin solution (100 U/mL and 100 mg/mL, respectively), RPMI-1640 and DMEM media were from Hyclone (Logan, USA). Acetic acid, ellipticine, sulforhodamine B (SRB), trypan blue, trichloroacetic acid (TCA) and Tris were from Sigma Chemical Co. (Saint Louis, USA). Water was treated in a Milli-Q water purification system (TGI Pure Water Systems, USA).
Samples
The fruits of Arbutus unedo L. (strawberry-tree) from Ericaceae, and the Rosaceae species Prunus spinosa L. (blackthorn), Rosa canina sl. (dog rose) and Rosa micrantha Borrer ex Sm. (similar to eglantine rose) were gathered in the Natural Park of Montesinho territory, in Trás-os-Montes, Northeastern Portugal. Strawberry-tree berries were collected fully ripened in November 2008; well matured blackthorn and dog rose hips were gathered in late September 2008. R. micrantha overripe hips, that is fleshy and soft dark red fruits, were collected in late autumn 2009. Morphological key characters from the Flora Iberica [18] were used for plant identification. The fruits with seeds were lyophilized (Ly-8-FM-ULE, Snijders, Holland) and stored in the deep-freezer at −20 °C for subsequent analysis.
Samples Preparation
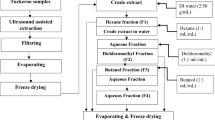
Non-anthocyanin Phenolic Compounds Enriched Extract (PE)
Each sample (1 g) was extracted with 30 mL of methanol:water 80:20 (v/v) at room temperature, 150 rpm, for 1 h. The extract was filtered through Whatman no 4 paper. The residue was then re-extracted twice with additional 30 mL portions of methanol:water 80:20 (v/v). The combined extracts were evaporated at 35 °C (rotary evaporator Büchi R-210) to remove methanol. For purification, the extract solution was deposited onto a C-18 SepPak® Vac 3 cc cartridge (Phenomenex), previously activated with methanol followed by water; sugars and more polar substances were removed by passing through 10 mL of water and phenolic compounds were further eluted with 5 mL of methanol. The methanolic extract obtained (designated by phenolic extract) was concentrated under vacuum and stored at 4 °C for further use.
Anthocyanins Enriched Extract (AE)
Each sample (1 g) was extracted with 30 mL of methanol containing 0.5 % TFA, and filtered through a Whatman no 4 paper. The residue was then re-extracted twice with additional 30 mL portions of 0.5 % TFA in methanol. The combined extracts were evaporated at 35 °C to remove the methanol, and re-dissolved in water. For purification, the extract solution was deposited onto a C-18 SepPak® Vac 3 cc cartridge (Phenomenex), previously activated with methanol followed by water; sugars and more polar substances were removed by passing through 10 mL of water and anthocyanin pigments were further eluted with 5 mL of methanol:water (80:20, v/v) containing 0.1 % TFA. The methanolic extract (designated by anthocyanins extract) was concentrated under vacuum, lyophilized and stored at 4 °C for further use.
Evaluation of Bioactivity
The extracts were re-dissolved in water at a final concentration 10 mg/mL and 8 mg/mL for antioxidant and antitumor activity evaluation, respectively. The final solutions were further diluted in water to different concentrations to be submitted to distinct bioactivity evaluation in vitro assays (1,000–4 μg/mL and 400–25 μg/mL for antioxidant and antitumor assays, respectively). The results were expressed in i) EC50 (extract concentration providing 50 % of antioxidant activity or 0.5 of absorbance in the reducing power assay) values for antioxidant activity or ii) GI50 (extract concentration that inhibited 50 % of the net cell growth) values for antitumor activity. Water was used as negative control, and trolox and ellipticine were used as positive controls in antioxidant and antitumor activity evaluation assays, respectively.
Antioxidant Activity Assays
To evaluate the antioxidant activity the following assays were used: DPPH radical-scavenging activity assay; reducing power assay; inhibition of β-carotene bleaching assay; and lipid peroxidation inhibition by thiobarbituric acid reactive substances (TBARS) assay [14, 19].
DPPH radical-scavenging activity was evaluated by using a ELX800 microplate Reader (Bio-Tek Instruments, Inc; Winooski, USA), and calculated as a percentage of DPPH discolouration using the formula: [(ADPPH − AS)/ADPPH] × 100, where AS is the absorbance of the solution containing the extract at 515 nm, and ADPPH is the absorbance of the DPPH solution. Reducing power was evaluated by the capacity to convert Fe3+ into Fe2+, measuring the absorbance at 690 nm in the microplate Reader mentioned above. Inhibition of β-carotene bleaching was evaluated though the β-carotene/linoleate assay; the neutralization of linoleate free radicals avoids β-carotene bleaching, which is measured by the formula: β ‐ carotene absorbance after 2 h of assay/initial absorbance) × 100. Lipid peroxidation inhibition in porcine (Sus scrofa) brain homogenates was evaluated by the decreasing in TBARS; the color intensity of the malondialdehyde-thiobarbituric acid (MDA-TBA) was measured by its absorbance at 532 nm; the inhibition ratio (%) was calculated using the following formula: [(A − B)/A] × 100%, where A and B were the absorbance of the control and the extract solution, respectively.
Antitumor Activity
Five human tumor cell lines were used: MCF-7 (breast adenocarcinoma), NCI-H460 (non-small cell lung cancer), HCT-15 (colon carcinoma), HeLa (cervical carcinoma) and HepG2 (hepatocellular carcinoma). Cells were routinely maintained as adherent cell cultures in RPMI-1640 medium containing 10 % heat-inactivated FBS (MCF-7, NCI-H460 and HCT-15) and 2 mM glutamine or in DMEM supplemented with 10 % FBS, 2 mM glutamine, 100 U/mL penicillin and 100 mg/mL streptomycin (HeLa and HepG2 cells), at 37 °C, in a humidified air incubator containing 5 % CO2. Each cell line was plated at an appropriate density (7.5 × 103 cells/well for MCF-7, NCI-H460 and HCT-15 or 1.0 × 104 cells/well for HeLa and HepG2) in 96-well plates and allowed to attach for 24 h. Cells were then treated for 48 h with various extract concentrations. Following this incubation period, the adherent cells were fixed by adding cold 10 % trichloroacetic acid (TCA, 100 μL) and incubated for 60 min at 4 °C. Plates were then washed with deionized water and dried; sulforhodamine B solution (0.1 % in 1 % acetic acid, 100 μL) was then added to each plate well and incubated for 30 min at room temperature. Unbound SRB was removed by washing with 1 % acetic acid. Plates were air-dried, the bound SRB was solubilized with 10 mM Tris (200 μL) and the absorbance was measured at 540 nm in the microplate reader mentioned above [20].
Hepatotoxicity
A cell culture was prepared from a freshly harvested porcine liver obtained from a local slaughter house, and it was designed as PLP2. Briefly, the liver tissues were rinsed in Hank’s balanced salt solution containing 100 U/mL penicillin, 100 μg/mL streptomycin and divided into 1 × 1 mm3 explants. Some of these explants were placed in 25 cm2 tissue flasks in DMEM medium supplemented with 10 % fetal bovine serum, 2 mM nonessential amino acids and 100 U/mL penicillin, 100 mg/mL streptomycin and incubated at 37 °C with a humidified atmosphere containing 5 % CO2. The medium was changed every 2 days. Cultivation of the cells was continued with direct monitoring every 2 to 3 days using a phase contrast microscope. Before confluence was reached, cells were subcultured and plated in 96-well plates at a density of 1.0 × 104 cells/well, and cultivated in DMEM medium with 10 % FBS, 100 U/mL penicillin and 100 μg/mL streptomycin [20].
Statistical Analysis
All the assays were carried out in triplicate in three different extracts, and the results are expressed as mean values ± standard deviation (SD). The statistical differences represented by letters were obtained through one-way analysis of variance (ANOVA) followed by Tukey’s honestly significant difference post hoc test with α = 0.05. These treatments were carried out using SPSS v. 18.0 program.
Results and Discussion
The results of antioxidant activity, determined by free radicals scavenging activity, reducing power and inhibition of lipid peroxidation in brain cell homogenates, are shown in Table 1. The studied extracts were chemically characterized in a previous work of our research group [8]. Herein, two different enriched phenolic extracts were prepared, in order to evaluate and compare their bioactivity: a non-anthocyanin phenolic compounds enriched extract (PE; with phenolic acids, flavones/ols, flavan-3-ols and galloyl derivatives) and a separate anthocyanins enriched extract (AE).
Regarding PE of the studied wild fruits, A. unedo presented the highest antioxidant activity in all the in vitro assays, which could be related to the presence of galloyl derivatives (exclusively in A. unedo PE) and/or to the presence of higher levels of flavan-3-ols. The second one with the highest antioxidant effects was P. spinosa, in which the main contributors seemed to be phenolic acids (exclusive in P. spinosa PE) and flavones/ols, present in this PE in higher amounts. The studied Rosa species revealed the lowest antioxidant activity, presenting similar phenolic compounds profile (flavan-3-ols and flavones/ols); the higher levels of these compounds found in R. micrantha comparatively to R. canina, might explain the higher antioxidant activity observed in the first case (Table 1, Fig. 1).
Concentrations of phenolic compounds present in the wild fruits, determined by HPLC-DAD-MS/ESI according to reference [8]
Concerning AE, a pro-oxidant effect of anthocyanins seemed to occur, since the samples with the highest amounts revealed the lowest antioxidant activity (Table 1, Fig. 1). For that reason, P. spinosa gave the lowest antioxidant activity (in β-carotene-bleaching inhibition assay it was not possible to determine EC50 value due, in our opinion, to pro-oxidant effects of anthocyanins), while R. canina showed the highest antioxidant effects.
PE gave higher antioxidant properties than the corresponding AE, and according to their chemical characterization, those properties seem to be related to galloyl derivatives, flavan-3-ols, phenolic acids and flavones/ols. In general, PE and AE presented higher antioxidant activity than the methanolic extracts (crude extracts) of the same fruits previously studied by us [14, 15]. It seems that purified/enriched extracts (such as the cases herein presented) are more suitable than crude extracts, in which antagonistic effects between the compounds present could be observed, conducting to a decrease in the antioxidant activity. The only exception was for the β-carotene bleaching inhibition assay (higher capacity in crude extracts); in this case, other molecules rather than the ones previously mentioned are probably involved and might bring synergistic effects.
The antitumor potential was tested in human tumor cell lines (breast, lung, colon, cervical and hepatocellular carcinomas), and the hepatotoxicity was evaluated using a porcine liver primary cell culture. All the extracts inhibited the growth of tumor cell lines, except R. canina PE and AE, P. spinosa AE and R. micrantha AE for MCF-7 (breast carcinoma). A. unedo, followed by P. spinosa, PE gave the best antitumor inhibition (Table 2), which could be correlated as mentioned above for antioxidant activity (similar behaviour), to the phenolic groups present in each of the wild fruits (Fig. 1), i.e., exclusive presence of galloyl derivatives and the highest levels of flavan-3-ols for A. unedo PE, and exclusive presence of phenolic acids and the highest levels of flavones/ols for P. spinosa PE. Regarding AE, samples with the highest amounts of anthocyanins (P. spinosa and A. unedo) revealed the highest antitumor effects, except in the case of MCF-7 that was not inhibited by P. spinosa AE. None of the samples showed toxicity for non-tumor liver primary culture.
As far as we know, this is the first study regarding antitumor effects of A. unedo, R. micrantha and P. spinosa wild fruits. In the case of R. canina, the antitumor effects of an aqueous extract from its hips were studied in mouse melanoma cells [16], and similarly to the herein studied PE, the higher contributors are proanthocyanidins (flavan-3-ols). Otherwise, the flavonoids fraction from R. canina tea showed higher antiproliferative activity in HeLa cell line (IC50 = 80.63 μg/mL; [17]) than the one observed in the present study for PE (GI50 = 253.03 μg/mL); contrarily to the observed result (no activity up to 400 μg/mL), those authors reported effects against MCF-7 cell line (IC50 = 248.03 μg/mL).
Overall, the bioactivity of the studied wild fruits proved to be more related to phenolic compounds profile than to the amounts present in each extract, being PE more bioactive than AE. It should be highlighted the high capacity of A. unedo PE to inhibit lipid peroxidation in animal brain homogenates (EC50 = 7.21 μg/mL), as also its antitumor potential against NCI-H460 human cell line (non-small lung cancer; GI50 = 37.68 μg/mL). Regarding chemical characterization of the mentioned sample, the presence of galloyl derivatives exclusively in A. unedo wild fruits could be related to its higher bioactivity. Further studies are needed in order to confirm the specific role of these compounds in antioxidant and antitumor effects. Due to the observed bioactive properties, the mentioned species could be considered in the design of new formulations of dietary supplements or functional foods.
Abbreviations
- DPPH:
-
2,2-Diphenyl-1-picrylhydrazyl
- FBS:
-
Foetal bovine serum
- HBSS:
-
Hank’s balanced salt solution
- SRB:
-
Sulforhodamine B
- TBARS:
-
Thiobarbituric acid reactive substances
- TCA:
-
Trichloroacetic acid
References
Rice-Evans CA, Miller NJ, Paganga G (1997) Antioxidant properties of phenolic compounds. Review. Trends Plant Sci 2:152–159
Yao LH, Jiang YM, Shi J, Tomás-Barberán FA, Datta N, Singanusong R, Chen SS (2004) Flavonoids in food and their health benefits. Plant Foods Hum Nutr 59:113–122
Szajdek A, Borowska EJ (2008) Bioactive compounds and health-promoting properties of berry fruits: a review. Plant Foods Hum Nutr 63:147–156
Carocho M, Ferreira ICFR (2013) The role of phenolic compounds in the fight against cancer- a review. Anti-Cancer Ag Med Chem 13:1236–1258
Scalbert A, Williamson G (2000) Dietary intake and bioavailability of polyphenols. J Nutr 130:2073–2085
Carocho M, Ferreira ICFR (2013) A review on antioxidants, prooxidants and related controversy: natural and synthetic compounds, screening and analysis methodologies and future perspectives. Food Chem Toxicol 51:15–25
Kong JM, Chia LS, Goh NK, Chia TF, Brouillard R (2003) Analysis and biological activities of anthocyanins. Phytochemistry 64:923–933
Guimarães R, Barros L, Dueñas M, Carvalho AM, Queiroz MJRP, Santos- Buelga C, Ferreira ICFR (2013) Characterization of phenolic compounds in wild fruits from Northeastern Portugal. Food Chem 141:3721–3730
Bnouham M, Merhfour FZ, Legssyer A, Mekhfi H, Maallem S, Ziyyat A (2007) Antihyperglycemic activity of Arbutus unedo, Ammoides pusilla and Thymelaea hirsuta. Pharmazie 62:630–632
Carvalho AM (2010) Plantas y sabiduría popular del Parque Natural de Montesinho. Un estudio etnobotánico en Portugal. Biblioteca de Ciencias 35. Madrid: Consejo Superior de Investigaciones Científicas
Orhan DD, Hartevioğlu A, Küpeli E, Yesilalada E (2007) In vivo anti-inflammatory and antinociceptive activity of the crude extract and fractions from Rosa canina L. fruits. J Ethnopharmacol 112:394–400
Fortalezas S, Tavares L, Pimpão R, Tyagi M, Pontes V, Alves PM, McDougall G, Stewart D, Ferreira RB, Santos CN (2010) Antioxidant properties and neuroprotective capacity of strawberry tree fruits (Arbutus unedo). Nutrients 2:214–229
Mendes L, Freitas V, Baptista P, Carvalho M (2011) Comparative antihemolytic and radical scavenging activities of strawberry tree (Arbutus unedo L.) leaf and fruit. Food Chem Toxicol 49:2285–2291
Barros L, Carvalho AM, Morais JS, Ferreira ICFR (2010) Strawberry-tree, blackthorn and rose fruits: detailed characterization in nutrients and phytochemicals with antioxidant properties. Food Chem 120:247–254
Guimarães R, Barros L, Carvalho AM, Ferreira ICFR (2010) Studies on chemical constituents and bioactivity of Rosa micrantha: an alternative antioxidants source for food, pharmaceutical, or cosmetic applications. J Agric Food Chem 58:6277–6284
Fujii T, Ikeda K, Saito M (2011) Inhibitory effect of rose hip (Rosa canina L.) on melanogenesis in mouse melanoma cells and on pigmentation in brown guinea pigs. Biosci Biotechnol Biochem 75:489–495
Tumbas VT, Čanadanović-Brunet JM, Četojević-Simin DD, Ćetković GS, Dilas SM, Gille L (2012) Effect of rosehip (Rosa canina L.) phytochemicals on stable free radicals and human cancer cells. J Sci Food Agric 92:1273–1281
Castroviejo S et al (2004) Flora Iberica. Plantas vasculares de la Península Ibérica e Islas Baleares, vol IV. CSIC, Real Jardín Botánico
Dias MI, Barros L, Sousa MJ, Ferreira ICFR (2011) Comparative study of lipophilic and hydrophilic antioxidants from in vivo and in vitro grown Coriandrum sativum. Plant Foods Hum Nutr 66:181–186
Guimarães R, Barros L, Dueñas M, Calhelha RC, Carvalho AM, Santos Buelga C, Queiroz MJRP, Ferreira ICFR (2013) Nutrients, phytochemicals and bioactivity of wild Roman chamomile: a comparison between the herb and its preparations. Food Chem 136:718–725
Acknowledgments
Foundation for Science and Technology (FCT, Portugal) for PEst-OE/AGR/UI0690/2011, PEst-C/QUI/UI0686/2011, BD/78307/2011 (R. Guimarães), BPD/68344/2010 (R. Calhelha) and researcher contract of L. Barros.
Conflict of interest
The authors declare that they have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Guimarães, R., Barros, L., Calhelha, R.C. et al. Bioactivity of Different Enriched Phenolic Extracts of Wild Fruits from Northeastern Portugal: A Comparative Study. Plant Foods Hum Nutr 69, 37–42 (2014). https://doi.org/10.1007/s11130-013-0394-5
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11130-013-0394-5