Abstract
Kinnow (Citrus reticulata) peels consist of innumerable oil glands that are easily separable and very valuable due to their nutrition. Citrus essential oils are rich source of antioxidants and polyphenols and possess antimicrobial activity and thus have a wide range of applications in the food, pharmaceutical, and cosmetic industries. Henceforth, the present study has been focused on the extraction of essential oils by supercritical fluid extraction (SFE) from kinnow peels and their characterization. Statistical optimization technique using response surface methodology (RSM) was applied to obtain the optimum value of process parameters (i.e., temperature, pressure, and time) to maximize the yield, antioxidant activity, and total phenolic content (TPC) of essential oil extract. Maximum extraction yield (1.57%), antioxidant activity (79.94% DPPH reduction), and TPC (41.22 mg GAE/g extract) of essential oil extract were obtained at 43 °C, 297 bar, and 120 min. The essential oil extract obtained using SFE was characterized using color, FTIR, and GC–MS analyses which confirmed the desirable color and presence of functional compounds. The essential oil extract obtained can be purified further and used by food or pharmaceutical companies for the development of novel functional foods or nutraceuticals.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
Kinnow fruit is a hybrid of Citrus nobilis (King) and Citrus deliciosa (Willow leaf) mandarins. Kinnows are largely grown in India as well as Pakistan and exported all over the world. Major producing states of kinnow in India are Punjab, Haryana, Himachal Pradesh, Rajasthan, Uttar Pradesh, and Jammu [1]. Kinnow production in Punjab (India) alone was 1.12 MT/annum (in 2018–2019) which is 75% of total production in India [2]. After the extraction of juice from Kinnow, a huge amount of by-products such as peels, pulp, seed, and pomace are produced. As the anatomy of kinnow represents 44% peels, it may be estimated that 0.49 MT of peel waste is generated during kinnow processing [3]. Due to high sugar and moisture content, kinnow waste management has been a challenging task for the citrus-processing industries. Kinnow peels have been identified to be rich in health beneficial compounds such as polyphenols, antioxidants [4, 5], and carotenoids [6]. Numerous oil glands are present in kinnow peels, which can be separated easily, as they are loosely bound to the skin [3]. The bioactive compounds present in kinnow peels hold tremendous potential to meet the nutritional demands of the consumers and hence are gaining the interest of functional food manufacturers.
Essential oils of kinnow peels are valuable due to their strong antioxidant, anti-inflammatory, and antimicrobial properties and are classified as generally recognized as safe (GRAS) by USFDA [7]. Essential oils have been extensively used for their application as food preservatives [8] and are also preferred as a food additive [9]. Essential oils have been extracted from fruit peels by both conventional and non-conventional techniques. Conventional methods include Soxhlet extraction, hydro-distillation, and maceration. Non-conventional methods include solvent-free microwave-assisted extraction [10], ultrasound-assisted extraction [11], and supercritical fluid extraction [12]. Due to the benefits of non-conventional techniques such as higher extraction yield, shorter times, lesser/no solvent, and environment-friendly, the present research focuses on an exploration of the supercritical fluid extraction technique (SFE) for separation of essential oils from kinnow peels.
Supercritical fluid extraction is a green extraction technique and requires less time, lower temperature, and lower quantity of non-toxic solvents for extraction [13]. Usually, carbon dioxide (CO2) is used for supercritical fluid extraction due to its unique properties such as low critical temperature (31.1 °C) and low critical pressure (73.8 bar). CO2 is available in high purity at a reasonably low cost and can be easily removed from the extract [14]. Supercritical fluids have many benefits such as non-flammability, non-toxicity, low to moderate operating temperature and pressure, and the inability to leave residual chemicals [15, 16]. Studies on conventional and green techniques such as ultrasonication [4, 11], microwave [5, 10], and supercritical fluid [12, 15] have been carried out for the extraction of bioactive compounds (polyphenols, antioxidants, essential oils) from various citrus peels. In comparison to conventional techniques and other green techniques, supercritical fluid extraction provided a higher yield of solvent-free, better-quality, essential oil extracts that exhibited higher antioxidant potential [13,14,15]. The literature survey revealed that meager studies have been reported on the extraction of essential oils from kinnow peels and their bioactive potential, using SFE. Hence, the present study has been carried out to optimize supercritical fluid extraction of essential oils from kinnow mandarin peels, using response surface methodology.
2 Material and methods
2.1 Materials
Kinnow (mandarin) peels were procured from local juice centers in Longowal, Punjab, India. The collected peels were sun-dried (2–3 days approx.) until the moisture content of 9–10% (d.b.) was attained, and ground into a fine powder (particle size < 0.5 mm). The sieved powder was stored in air-tight and refrigerated conditions.
2.2 Supercritical fluid extraction of kinnow (mandarin) peels
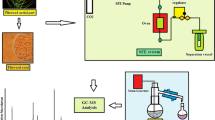
Supercritical fluid extraction (SFE) was carried out using the WATERS-SFE500 System for the extraction of essential oils using the extraction procedure as described by Trabelsi et al. [17] with minor modifications. The extraction system consists of a 500-mL extraction vessel and a separator. The powdered sample (50 g) was loaded into the extraction unit. Pressure (150–300 bar), extractor temperature (40–50 °C), and CO2 flow rate (10 g/min; constant) were controlled by using ChromScope software, as per the design. The static time (90–150 min) was monitored after the desired value of process parameters was achieved. After completion of time and depressurization, the extract was collected in amber-colored vials and stored in a refrigerator for further analysis.
-
a)
Extraction yield
The yield of kinnow peel essential oils was calculated by using the following equation:
$$Yield\;(\mathrm{\%}) = \frac{We}{Ws}\times 100$$where We = weight of extract and Ws = weight of sample (kinnow peels).
-
b)
Antioxidant activity
The in vitro antioxidant capacity using the synthetic radical DPPH is one of the methods mostly used to measure the antioxidant potential of different biological samples. The antioxidant activity of essential oil was calculated using, the method described by Brand-Williams et al. [18] with few modifications. DPPH solution (100 μM) was prepared, and its initial absorbance was measured. Then, 950 μL of the DPPH solution was added to 50 μL of the extract at an 8 mg/mL concentration. The mixture was incubated for 1 h and its absorbance was measured at 517 nm. The antioxidant activity (% inhibition) was calculated using the following equation:
$$\mathrm{\%\;}Inhibition=\frac{Ao-A}{Ao}\mathrm{x}100$$where Ao = absorbance of control sample and A = absorbance of extract.
-
c)
Total phenolic content (TPC)
TPC was determined using the Folin-Ciocalteu method as described by Vrhovsek et al. [19], with some modifications. Twenty microliters of extract at 8 mg/mL of ethanol was taken, and then, 1580 μL of distilled water with 100 μL of Folin-Ciocalteu reagent was added. It was allowed to stand for 8 min, and then, 300 μL of a 20% sodium carbonate solution was added. The mixture was incubated for 45 min in a dark place at room temperature, and the absorbance was read at 765 nm in a spectrophotometer (HACH DR 5000), using a gallic acid (GA) calibration curve. The results were expressed as mg gallic acid equivalents (GAE) per gram extract.
2.3 Experimental design and statistical analysis
Response surface methodology was used for the optimization of process parameters for supercritical fluid extraction of essential oils from kinnow peels. The experimental design matrix was created using central composite rotatable design (CCRD) via Design-Expert software v. 11.1.2.0 (Stat-Ease Inc., Minneapolis, USA), and process parameters chosen for optimization were temperature, pressure, and time. The temperature ranged from 40 to 50 °C, pressure ranged from 150 to 300 bar, and time ranged from 90 to 150 min. The CO2 flow rate was kept constant at 10 g/min, and essential oils extraction yield was considered as the response variable. The best fit quadratic model for ANOVA was used for statistical analysis of experimental data.
2.4 Characterization of essential oil extract
2.4.1 Color of essential oil extract
Hunter Lab color spectrophotometer (GretagMacbeth, color i5, USA) was used to determine the color of various samples. Standardization of instruments was done with a black and white tile each time and the surface L values, a values, and b values of the sample were calculated. L values vary from 0 (darkness) to 100 (lightness) on the hunter scale. The average color values of triplicates have been reported.
2.4.2 FT-IR of essential oil extract
Fourier transform infrared (FT-IR) analysis of extract was determined by using FT-IR spectrometer (Perken Elmer Spectrum, RX-I, USA). The sample was prepared by adding potassium bromide to the dried extract (essential oils) to obtain a solid disc. The FT-IR spectrum was analyzed at the wavenumber range from 4000 to 600 cm−1 [20].
2.4.3 GC–MS analysis of essential oil extract
GC–MS was carried out using GCMS-TQ8050 NX (Shimadzu) equipped with RTX-5MS column (30 m × 0.25 mm I.D.) following the Hudaib et al. [21] method with minor modifications. Helium was used as a carrier gas at a flow rate of 1 mL/min and a split ratio of 1:5. The column temperature was raised from 50 °C (holding time: 2 min) to 300 °C (holding time: 10 min) at a rate of 5 °C/min and the runtime was 62 min. The ion source temperature was 200 °C and the interface temperature was 260 °C. The acquisition mode was Q3 Scan and solvent cut time of 4.5 min. Injector pressure of 250 kPa and temperature of 250 °C. Mass spectra were obtained by electron ionization (EI) at 70 eV, using a spectral range of m/z of 50–800. The major components of the extract were evaluated using the Standard Reference Data Series of the NIST Library—Mass-Spectral Library.
3 Results and discussion
The yield of essential oil extract was calculated as the dry weight of extract obtained after the removal of solvent. The yield, antioxidant activity, and TPC obtained from the extraction were observed in the range of 0.44–1.57%, 25.87–89.74% DPPH reduction, and 11.54–40.66 mg GAE/g extract, respectively, whereas maximum yield (1.57%), maximum antioxidant activity (79.94% DPPH reduction), and TPC (41.22 mg GAE/g extract) were obtained at 44 °C temperature, 244 bar pressure, and time 103 min (Table 1). The interactive effect of different parameters was studied using response surface 3D plots (Fig. 1) of process parameters, i.e., temperature (X1), pressure (X2), and time (X3); the analysis of variance (ANOVA) has revealed that X1, X2, X3, X1X2, X1X3, X2X3, (X1)2, (X2)2, and (X3)2 were significant model terms (Table 2). The model was significant with F value of 69.9, lack of fit (0.1606 p value and 5.52 F value) was non-significant, and R2 of 0.989 was observed.
3.1 Effect of temperature on essential oil extract, antioxidant activity, and TPC
The effect of temperature on yield during SFE of kinnow peels was studied by varying the temperature and keeping the other two variables constant (Fig. 1). From 3D response surface plots, it has been observed that the essential oil yield increased with an increase in temperature up to 45 °C and then declined with a further increase in temperature. The major reason for this pattern is that as the temperature changed the density of supercritical CO2 (SC-CO2), the diffusion rate of the extract in SC-CO2 also changes [22]. It has been observed that the diffusion rate of extract increased with increasing temperature henceforth, resulting in a higher yield of essential oils. On the other hand, when the temperature increased above 45 °C, both the density of SC-CO2 and the solubility of the extract decreased which led to a decrease in the yield of essential oils from the kinnow peels. The combined effect of the two opposing mechanisms which determines the change in yield has been described in previous reports [23, 24]. The two opposing mechanisms of the temperature observed were as follows: an increase in temperature decreased the density as well as solvation power of SC-CO2, and on the contrary, an increase in temperature also increased vapor pressure resulting in increased solubility of SC-CO2 [25]. Similarly, during SFE of tangerine peels, as the temperature increased from 35 to 45 °C (at 20 MPa, 90 min), the extraction yield of essential oils increased from 0.7 to 0.85%, whereas any further increase in temperature led to a decline in essential oils yield [22].
Other than the solubility of essential oils from kinnow peels in supercritical CO2 fluid, vapor pressure should also be considered a key factor for extraction. Higher oil vapor pressure at higher temperatures allows for easier dissipation through sample matrices, while higher SC-CO2 diffusivity and lower surface tension facilitate the transport of target compounds through the matrix and into the solvent, resulting in higher extraction efficiency [26].
The linear effect of temperature was observed on the antioxidant activity of essential oil. As temperature increases, solvent density increases, and solvent diffusion coefficient also increases which further leads to an increase in antioxidant activity of essential oil (Fig. 1b). The temperature may also affect interaction with other independent variables by improving the diffusion rate of analytes [27].
Total phenolic content increased with an increase in temperature, but any further increase beyond the threshold temperature led to a decline in the TPC values, the same can be observed in Fig. 3a. It can be explained by an increase in vapor pressure with the increasing temperature that accelerated the thermal decomposition of the components from the matrix [28]. Alternatively, the rupture of the cell wall increased the mass transfer rate which led to the availability of bioactive components for extraction. An increase in temperature results in the decline of recovery of bioactive components because of the decomposition of volatile compounds and reduction in density of CO2 [29, 30].
3.2 Effect of pressure on essential oil extract yield, antioxidant activity, and TPC
Results indicated that pressure has a significant (p < 0.05) effect on the extraction yield of essential oil extract from kinnow peels. The results revealed that yield increased perpetually until its optimum point of 1.55% at 225 bar, and after that point, a reduction in yield is observed (Fig. 1a). The extraction from Pomelo (Citrus grandis) peels by supercritical fluid method at 70 °C increased extraction yield from 1.48 to 1.69% extract with pressure from 280 to 390 bar [31]. The yield of tangerine peel oil increased from 0.78 to 1.1% essential oil yield with an increase in pressure from 100 to 150 bar and then reduced up to 0.9% essential oil yield after 150 bar at 45 °C for 90 min [22]. The influence of pressure can be attributed to an increase in the specific mass of SC-CO2 with pressure which leads to improved solvation power of SC-CO2. The increase in density with pressure reduces the mean intermolecular distance between the carbon dioxide molecules which turns out as a positive outcome for interaction specifically between the solvent and solute molecules and the solubility for the extract in SC-CO2. When pressure is too high, the diffusion of SC-CO2 and the rate of mass transfer will limit the increase in tangerine peel oil yield [22]. Moreover, kinnow peel grease and wax can be extracted at higher pressures, lowering the quality of essential oils [32].
Antioxidant activity increased at a pressure of 150 to 225 bar and a temperature of 40 to 45℃ (Fig. 1b, c). It can be justified by the correlation between solubility, density, and selectivity of supercritical CO2 (SC-CO2). The pressure augmentation causes an increase in the density of SC-CO2 as well as the solubility of the targeted compounds [33]. Moreover, when the density of SC-CO2 increases, it widely allows components to dissolve in SC-CO2 even the dense molecules such as coumarins and other non-saponifiable components such as phospholipids and phytosterols which leads to an overall increase in antioxidant activity (% DPPH reduction) [33].
Pressure is one of the dominant parameters for the extraction of phenolic compounds. As pressure increased, two prominent trends were observed. As the pressure increased from 150 to 225 bar, the TPC increased slightly, but with further increase in pressure up to 300 bar, a minor decrease in TPC was observed (Fig. 1d, e). Increasing the pressure could increase the fluid density which decreases the distance among the molecules and rupturing effect of pressure thus strength interaction between fluid and matrix [33].
3.3 Effect of time on essential oil extract yield, antioxidant activity, and TPC
The yield of essential oil extract from kinnow peels increased continuously as the time increased from 90 to 120 min; any further increase in temperature led to a slight decline in the yield of essential oil extract (Fig. 1a). A previous study reported that the yield of orange peel essential oil extract yield increased with time (35 to 45 min) at a constant pressure of 265 atm from 0.32 to 0.59% total yield and then slightly decreased with time, i.e., from 45 to 55 min [34], whereas another study indicated that tangerine peel essential oils increased from 0.2 to 1% yield with time from 20 to 180 min at 100 bar pressure [22]. Also stated is that as time increased, yield gradually increased and reached a maximum, and the present study found and validated this rule.
Antioxidant activity is also influenced by time with changes in temperature and pressure [28]. In research conducted by Ndayishimiye and Chun [35], antioxidant activity (% inhibition) was 0.98 ± 0.01 mg/cm3 of extract from citrus (Citrus ichangensis x C. reticulate) peels and seeds by using supercritical fluid extraction at 41 ℃ of temperature and 300 bar of pressure. Franco-Arnedo et al. [15] observed 31.92% antioxidant activity (% inhibition) of essential oil at 120 bar pressure, 50 ℃ temperature, and 5% ethanol, whereas 44.92% antioxidant activity was observed at 220 bar pressure and 50 ℃ temperature, and 5% ethanol from tangerine (Citrus reticulata var. Arryana) peel extract. Extraction time showed an interactive effect with the temperature and pressure, and it affected the TPC of essential oil extract significantly.
3.4 Numerical optimization
Independent variables (temperature, pressure, and time) were in range, and the extraction yield of essential oil extract was set to maximum in the numerical optimization option of the Design-Expert software. The maximum extract yield of 1.57% was predicted at 43 °C temperature, 297 bar pressure, and 120 min time with the desirability of 1.00 in the solutions. Experiments in triplicate had been conducted at obtained optimized values of process parameters for validation of predicted maximum oils extraction yield. The maximum oil extraction yield of 1.56 ± 0.02% was achieved which has been in close agreement with the predicted one. All the results reported in the present study were from the previously reported studies. Minor deviations observed in the study may be attributed to different fruit harvesting locations, fruit varieties, climate, soil, fertilizers, different varieties, cultivars, environmental factors, water supply during fruit ripening, maturation stage, and effect of extraction parameters that influence the essential oil yield significantly [36].
3.5 Characterization
3.5.1 Color of essential oil extract
The consumer acceptability of any food material is judged by its color value. Color values are indicated by L* (lightness), a* (redness), and b* (yellowness) values. L* values signify 0 as black, 50 as mid-gray, and 100 as white color. The positive a* value signifies a red color and the negative value a green color. The positive b* value denotes a yellow color, and the negative value denotes blue color. Kinnow peels were taken as the standard, and the color values for L*, a*, and b* were 68.53 ± 0.51, 14.49 ± 0.22, and 42.11 ± 0.36, respectively, and the color values of the essential oil extract were 54.78 ± 0.19, 19.06 ± 0.15, and 31.45 ± 0.39, respectively. The lightness and b values of the essential oil extract were lower, whereas the redness value was higher than the kinnow peels.
3.5.2 FT-IR of essential oil extract
FT-IR was used to examine the functional groups observed in essential oil components extracted by the supercritical fluid extraction technique (Fig. 2). Major peaks of functional groups observed in SFE extract were at 3318 cm−1 (O–H which represented alcohol), 2922 cm−1 (N–H stretching represented amine salt and O–H stretching of carboxylic acid), 2852 cm−1 (C-H stretching represented alkane group), and 1260 cm−1 (C-O stretching represented aromatic ester functional group). Vibrational peaks at 2900 cm−1, 1700 cm−1, and 1100 cm−1 showed C-H, C = O, and C-O bonds, respectively, which are stretching of terpenoid compounds [37]. In the present study, absorption peaks near 2922 cm−1, 1728 cm−1, and 1148 cm−1 depicted the presence of terpenoid compounds. The peak at 1738 cm−1 represented ester carbonyl functional group of triglycerides [38]; phenols, tocopherols, and sterols were also detected within 3007–722 cm−1 [39]. The vibrational peak at 887 cm−1 (C = H disubstituted double bond) represented the presence of limonene [20].
3.5.3 GC–MS analysis of essential oil extract
Important components that contribute to the characteristic aroma of citrus essential oils are aldehydes and esters [40]. GC–MS analysis showed 164 peaks which were identified on basis of retention time, height, and area of the compounds (Fig. 3). The major compounds such as ascorbic acid, tocopherol, limonene-1,2-diol, decanal, linoleic acid, squalene, and oleic acid (Table 3) were observed in the essential oil extract. These compounds were also reported in the previous studies [41, 42].
4 Conclusions
SFE technique was found to be an effective method for the extraction of essential oils from kinnow peels. Optimization of process parameters helped to achieve the optimal conditions to obtain maximum extraction yield. Optimized parameters for maximum extraction were 43 °C temperature, 297 bar pressure, and 120 min time which resulted experimentally in a maximum extraction yield of 1.57%, maximum DPPH activity of 79.94% reduction, and total phenolic content (TPC) of 41.22 mg GAE/g extract. Characterization studies using color, FT-IR, and GC–MS revealed that extracted essential oils sustain all vital components in the essential oils from kinnow peels. Color values of extract indicated the presence of colored components (carotenoid) at lower concentrations, whereas FT-IR and GC–MS analyses confirmed the presence of important essential oil components, phenolic antioxidants, and flavor compounds in the essential oil extract. This extract can be purified further to obtain pure essential oil components from the extract.
References
Purewal SS, Sandhu KS (2020) Nutritional profile and health benefits of kinnow: an updated review. Int J Fruit Sci 20:S1385–S1405. https://doi.org/10.1080/15538362.2020.1792390
Statement showing the district wise area, Av. Yield & Production of various Fruit crops for the year 2018–19 in the Punjab State., 2018. State Department of Horticulture, Govt. of India.
Rafiq S, Kaul R, Sofi SA, Bashir N, Nazir F, Ahmad Nayik G (2018) Citrus peel as a source of functional ingredient: a review. J Saudi Soc Agric Sci 17:351–358. https://doi.org/10.1016/j.jssas.2016.07.006
Kaur S, Panesar PS, Chopra HK (2021) Standardization of ultrasound-assisted extraction of bioactive compounds from kinnow mandarin peel. Biomass Convers Biorefin. https://doi.org/10.1007/s13399-021-01674-9
Kaur S, Panesar PS, Chopra HK (2022) Exploration of microwaves for biorefining of phenolic antioxidants from Citrus reticulata peels: spectrophotometric and spectroscopic analysis. J Food Process Preserv. https://doi.org/10.1111/jfpp.16395
Wang YC, Chuang YC, Hsu HW (2008) The flavonoid, carotenoid, and pectin content in peels of citrus cultivated in Taiwan. Food Chem 106:277–284. https://doi.org/10.1016/j.foodchem.2007.05.086
Abdel-Aziz MM, Emam TM, Elsherbiny EA (2019) Effects of mandarin (Citrus reticulata) peel essential oil as a natural antibiofilm agent against Aspergillus niger in onion bulbs. Postharvest Biol Technol 156:110959. https://doi.org/10.1016/j.postharvbio.2019.110959
Bakkali F, Averbeck S, Averbeck D, Idaomar M (2008) Biological effects of essential oils - a review. Food Chem Toxicol 46:446–475. https://doi.org/10.1016/j.fct.2007.09.106
Parsaeimehr M, Basti AA, Radmehr B, Misaghi A, Abbasifar A, Karim G, Rokni N, Motlagh MS, Gandomi H, Noori N, Khanjari A (2010) Effect of Zataria multiflora Boiss. Essential Oil, Nisin, and Their combination on the production of enterotoxin C and α-Hemolysin by Staphylococcus aureus. Foodborne Pathog Dis 7:299–305. https://doi.org/10.1089/fpd.2009.0416
Golmakani M-T, Moayyedi M (2015) Comparison of heat and mass transfer of different microwave-assisted extraction methods of essential oil from Citrus limon (Lisbon variety) peel. Food Sci Nutr 3:506–518 https://doi.org/10.1002/fsn3.240
Darjazi BB (2011) A comparison of volatile components of flower of page mandarin obtained by ultrasound-assisted extraction and hydrodistillation. J Med Plants Res 5:2839–2847
Brunner G (2005) Supercritical fluids: technology and application to food processing. J Food Eng 67:21–33. https://doi.org/10.1016/j.jfoodeng.2004.05.060
Kaur S, Panesar PS, Chopra HK (2021) Citrus processing by-products: an overlooked repository of bioactive compounds. Crit Rev Food SciNutr 1–20. https://doi.org/10.1080/10408398.2021.1943647
Sodeifian G, Saadati Ardestani N, Sajadian SA, Ghorbandoost S (2016) Application of supercritical carbon dioxide to extract essential oil from Cleome coluteoides Boiss: experimental, response surface and grey wolf optimization methodology. J Supercrit Fluids 114:55–63. https://doi.org/10.1016/j.supflu.2016.04.006
Franco-Arnedo G, Buelvas-Puello LM, Miranda-Lasprilla D, Martínez-Correa HA, Parada-Alfonso F (2020) Obtaining antioxidant extracts from tangerine (C. reticulata var. Arrayana) peels by modified supercritical CO2 and their use as protective agent against the lipid oxidation of a mayonnaise. J Supercrit Fluids 165:104957. https://doi.org/10.1016/j.supflu.2020.104957
Sharma K, Mahato N, Cho MH, Lee YR (2017) Converting citrus wastes into value-added products: economic and environmentally friendly approaches. Nutrition 34:29–46. https://doi.org/10.1016/j.nut.2016.09.006
Trabelsi D, Aydi A, Zibetti AW, della Porta G, Scognamiglio M, Cricchio V, Langa E, Abderrabba M, Mainar AM (2016) Supercritical extraction from Citrus aurantium amara peels using CO2 with ethanol as co-solvent. J Supercrit Fluids 117:33–39. https://doi.org/10.1016/j.supflu.2016.07.003
Brand-Williams W, Cuvelier ME, Berset C (1995) Use of a free radical method to evaluate antioxidant activity. LWT Food Sci Technol 28:25–30. https://doi.org/10.1016/S0023-6438(95)80008-5
Vrhovsek U, Mattivi F, Waterhouse AL (2001) Analysis of red wine phenolics: Comparison of HPLC and spectrophotometric methods. Vitis 40:87–91
Akolade JO, Nasir-Naeem KO, Swanepoel A, Yusuf AA, Balogun M, Labuschagne P (2020) CO2-assisted production of polyethylene glycol / lauric acid microparticles for extended release of Citrus aurantifolia essential oil. J CO2 Util 38:375–384. https://doi.org/10.1016/j.jcou.2020.02.014
Hudaib M, Speroni E, DiPietra AM, Cavrini V (2002) GC/MS evaluation of thyme (Thymus vulgaris L.) oil composition and variations during the vegetative cycle. J Pharm Biomed Anal 29(4):691–700
Xiong K, Chen Y (2020) Supercritical carbon dioxide extraction of essential oil from tangerine peel: experimental optimization and kinetics modelling. Chem Eng Res Des 164:412–423. https://doi.org/10.1016/j.cherd.2020.09.032
Herzi N, Camy S, Bouajila J, Destrac P, Romdhane M, Condoret JS (2013) Supercritical CO2 extraction of Tetraclinis articulata: chemical composition, antioxidant activity and mathematical modeling. J Supercrit Fluids 82:72–82. https://doi.org/10.1016/j.supflu.2013.06.007
Santos KA, Klein EJ, da Silva C, da Silva EA, Cardozo-Filho L (2019) Extraction of vetiver (Chrysopogon zizanioides) root oil by supercritical CO2, pressurized-liquid, and ultrasound-assisted methods and modeling of supercritical extraction kinetics. J Supercrit Fluids 150:30–39. https://doi.org/10.1016/j.supflu.2019.04.005
Wang H, Liu Y, Wei S, Yan Z (2012) Application of response surface methodology to optimise supercritical carbon dioxide extraction of essential oil from Cyperus rotundus Linn. Food Chem 132:582–587. https://doi.org/10.1016/j.foodchem.2011.10.075
Suetsugu T, Tanaka M, Iwai H, Matsubara T, Kawamoto Y, Saito C, Sasaki Y, Hoshino M, Quitain AT, Sasaki M, Sakamoto J, Goto M (2013) Supercritical CO2 extraction of essential oil from Kabosu (Citrus sphaerocarpa Tanaka) peel. Flavour 2:1–8. https://doi.org/10.1186/2044-7248-2-18
Genena AK, Hense H, Smânia Junior A, de Souza SM (2008) Rosemary (Rosmarinus officinalis): a study of the composition, antioxidant and antimicrobial activities of extracts obtained with supercritical carbon dioxide. Ciênc Tecnol Aliment 28:463–469. https://doi.org/10.1590/S0101-20612008000200030
Sheibani A, Ghaziaskar HS (2009) Pressurized fluid extraction for quantitative recovery of aflatoxins B1 and B2 from pistachio. Food Control 20:124–128. https://doi.org/10.1016/j.foodcont.2008.03.001
Cacace JE, Mazza G (2003) Mass transfer process during extraction of phenolic compounds from milled berries. J Food Eng 59:379–389. https://doi.org/10.1016/S0260-8774(02)00497-1
Maran JP, Priya B, Manikandan S (2014) Modeling and optimization of supercritical fluid extraction of anthocyanin and phenolic compounds from Syzygium cumini fruit pulp. J Food Sci Technol 51:1938–1946. https://doi.org/10.1007/s13197-013-1237-y
He JZ, Shao P, Liu JH, Ru QM (2012) Supercritical carbon dioxide extraction of flavonoids from pomelo (Citrus grandis (L.) osbeck) peel and their antioxidant activity. Int J Mol Sci 13:13065–13078. https://doi.org/10.3390/ijms131013065
Palsikowski PA, Besen LM, Santos KA, da Silva C, da Silva EA (2019) Supercritical CO2 oil extraction from Bauhinia forficata link subsp. pruinosa leaves: composition, antioxidant activity and mathematical modeling. J Supercrit Fluids 153:104588. https://doi.org/10.1016/j.supflu.2019.104588
Uwineza PA, Waśkiewicz A (2020) Recent advances in supercritical fluid extraction of natural bioactive compounds from natural plant materials. Molecules 25(17):3847
Ghadiri K, Raofie F, Qomi M, Davoodi A (2021) Response surface methodology for optimization of supercritical fluid extraction of orange peel essential oil. Pharm Biomed Res 6:303–312. https://doi.org/10.18502/pbr.v6i4.5117
Ndayishimiye J, Chun BS (2017) Optimization of carotenoids and antioxidant activity of oils obtained from a co-extraction of citrus (Yuzu ichandrin) by-products using supercritical carbon dioxide. Biomass Bioenerg 106:1–7. https://doi.org/10.1016/j.biombioe.2017.08.014
Bourgou S, Rahali FZ, Ourghemmi I, Tounsi MS (2012) Changes of peel essential oil composition of four Tunisian citrus during fruit maturation. Sci World J 2012:528593. https://doi.org/10.1100/2012/528593
Cebi N, Taylan O, Abusurrah M, Sagdic O (2021) Detection of orange essential oil, isopropyl myristate, and benzyl alcohol in lemon essential oil by FTIR spectroscopy combined with chemometrics. Foods 10(27):10. https://doi.org/10.3390/foods10010027
Rohman A, Man YBC (2010) Fourier transform infrared (FTIR) spectroscopy for analysis of extra virgin olive oil adulterated with palm oil. Food Res Int 43:886–892. https://doi.org/10.1016/j.foodres.2009.12.006
Valasi L, Arvanitaki D, Mitropoulou A, Georgiadou M, Pappas CS (2020) Study of the quality parameters and the antioxidant capacity for the FTIR-chemometric differentiation of pistacia vera oils. Molecules 25:1614. https://doi.org/10.3390/molecules25071614
Yamanishi T, Kobayashi A, Mikumo Y, Nakasone Y, Kita M, Hattori S (1968) Composition of peel oil from Citrus unshu. Agricultural and Biological Chemistry 32:593–598. https://doi.org/10.1080/00021369.1968.10859106
Cheong MW, Loke XQ, Liu SQ, Pramudya K, Curran P, Yu B (2011) Characterization of volatile compounds and aroma profiles of malaysian pomelo (Citrus grandis (L.) osbeck) blossom and peel. J Essent Oil Res 23:34–44. https://doi.org/10.1080/10412905.2011.9700445
González-Mas MC, Rambla JL, Alamar MC, Gutiérrez A, Granell A (2011) Comparative analysis of the volatile fraction of fruit juice from different citrus species. PLoS ONE 6:e22016. https://doi.org/10.1371/journal.pone.0022016
Acknowledgements
The authors would like to acknowledge the financial support provided by the Council of Scientific and Industrial Research (CSIR) under research scheme 38(1491)/19/EMR-II. The authors are also thankful for the infrastructural support provided by Sant Longowal Institute of Engineering and Technology, Longowal, Sangrur, Punjab, India, to carry out the research work.
Author information
Authors and Affiliations
Contributions
Shreya Rajput: Investigation, formal analysis, data curation, writing (original draft preparation), writing (reviewing and editing).
Samandeep Kaur: Investigation, formal analysis, data curation, writing (original draft preparation), writing (reviewing and editing).
Paramjit S. Panesar: Conceptualization, resources, supervision, funding acquisition, project administration, reviewing, and editing.
Avinash Thakur: Resources, supervision, reviewing, and editing.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Rajput, S., Kaur, S., Panesar, P.S. et al. Supercritical fluid extraction of essential oils from Citrus reticulata peels: optimization and characterization studies. Biomass Conv. Bioref. 13, 14605–14614 (2023). https://doi.org/10.1007/s13399-022-02807-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13399-022-02807-4