Abstract
We compared the effects of medium light roast (MLR) and medium roast (MR) paper-filtered coffee on antioxidant capacity and lipid peroxidation in healthy volunteers. In a randomized crossover study, 20 volunteers consumed 482 ± 61 ml/day of MLR or MR for four weeks. Plasma total antioxidant status (TAS), oxygen radical absorbance capacity (ORAC), oxidized LDL and 8-epi-prostaglandin F2α, erythrocyte superoxide dismutase (SOD), glutathione peroxidase (GPx), and catalase (CAT) activity were measured at baseline and after the interventions. MLR had higher chlorogenic acids—(CGA; 334 mg/150 mL) and less caffeine (231 mg/150 ml) than MR had (210 and 244 mg/150 ml, respectively). MLR also had fewer Maillard reaction products (MRP) than MR had. Compared with baseline, subjects had an increase of 21 and 26 % in TAS, 13 and 13 % in CAT, 52 and 75 % in SOD, and 62 and 49 % in GPx after MLR and MR consumption (P < 0.001), respectively. ORAC increased after MLR (P = 0.004). No significant alteration in lipid peroxidation biomarkers was observed. Both coffees had antioxidant effects. Although MLR contained more CGA, there were similar antioxidant effects between the treatments. MRP may have contributed as an antioxidant. These effects may be important in protecting biological systems and reducing the risk of diseases related to oxidative stress.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Coffee is widely consumed worldwide and contributes to the total antioxidant ingestion in several countries [1]. The roasting process leads to intense changes in coffee’s chemical composition, which includes thermal degradation of natural chlorogenic acids (CGA) and formation of the Maillard reaction products (MRP) with antioxidant activity [2]. A previous study reported that the antioxidant activity of coffee is more dependent on the roasting techniques than on the brewing methods and the source of the coffee beans [3]. Some recent studies [4–6] have reported the effect of different coffee roasts on in vitro antioxidative. However, so far it has not been determined which roasting degree creates the greatest antioxidant capacity.
Human epidemiological studies have indicated that moderate consumption of coffee is associated with a lower incidence of heart disease, type 2 diabetes, hypertension, and other chronic diseases related to reactive oxygen species (ROS) [7]. In addition, in vitro cell studies and animal experiments have consistently shown that antioxidants in coffee may reduce low-density lipoprotein cholesterol (LDL-c) susceptibility to oxidation and ROS scavenging [6, 8–10]. Gordon & Wishart [9] found that CGA increased LDL-c resistance to oxidation in a concentration-dependent manner. The authors indicate that a small concentration of CGA could bind to LDL-c and contribute to the inhibition of oxidation. Furthermore, an in vitro study [10] showed that MRPs were incorporated into LDL and protected them against oxidation.
We raised the hypothesis that the effects of antioxidants in coffee differ among the different degrees of roasting. We tested whether paper-filtered coffees with different degrees of roasting (medium light and medium) have different antioxidant properties by comparing their effects on antioxidant activity and lipid peroxidation biomarkers in healthy volunteers.
Materials and Methods
Subjects
Healthy volunteers were recruited and provided informed consent. The study was approved by the Institutional Review Board of the School of Public Health, University of São Paulo (São Paulo, Brazil) and registered on Australian New Zealand Clinical Trials Registry (ACTRN12609001064291).
Potential participants were screened for good health by medical history questionnaire, physical examination, and standardized blood tests, including a complete blood cell test with leukocyte differential count, clinical chemistry panel, and lipid profile. Eligibility criteria were: age 20–65 years, healthy, plasma cholesterol <240 mg/dl, blood glucose <100 mg/dl, non-smoker or former smoker (more than two years), alcohol consumption <1 time/week, absence of chronic illnesses, no use of any medication or supplements with antioxidative or lipid-lowering properties.
Two subjects dropped out during the washout period. One could not attend the meetings, and one consumed coffee. Twenty healthy subjects (14 women) were evaluated.
Study Design
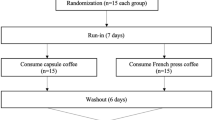
This randomized, crossover, clinical trial lasted eight weeks. After a 1-week run-in, subjects consumed medium light roast (MLR) or medium roast (MR) paper-filtered coffee for four weeks and then switched to the other roast for an additional four weeks (Figure 1. Online Resource 1). A computer-generated list of random numbers was used for allocation of the participants.
Subjects were asked to make no other changes to their diets or lifestyle during the study. We asked about their physical activity in the baseline interview and after each four weeks intervention period. A 3-day food diary (two days during the week and one on the weekend) was collected before baseline and during each intervention period to control for possible confounding factors and to verify compliance with the dietary instructions. Nutrient intake was calculated using the computer-based nutrient calculation program Avanutri 4.0.
Weight, height, abdominal circumference, and body fat were measured after the run-in period and after each intervention. Body mass index was calculated as weight (kg) divided by height (m2). Body fat percentage was measured by bipolar impedance on a portable electronic scale (Plenna, São Paulo, Brazil).
At the end of each intervention period, venous blood samples were taken after a 12 h overnight fast and centrifuged for plasma separation. For lipid peroxidation assays, 10 μl of BHT (butylated hydroxytoluene) in 5 mg/ml ethanol was added per 1 ml of plasma. Erythrocyte sediment was washed four times with 9 g/l NaCl with centrifugation between washes. Plasma and erythrocyte samples were stored at −80 °C until analyses.
Coffee Samples and Brew Preparations
Two commercially available blends (80 % Coffea arabica L. cv. Bourbon and 20 % C. canephora cv. Robusta) of caffeinated, roasted and ground coffee were used in the study. Both coffees were cultivated in the same geographic region. They were vacuum packed in 500 g aluminized bags, and were provided by the same manufacturer. Packages were kept at 4 °C (refrigerator) in the dark to preserve coffee antioxidants during the analyses. A study conducted in our laboratory showed that there was not significant loss of phenolic compounds in commercial coffee samples stored at 4 °C for up to two months (unpublished data). Roasting degree classification was done according to “Roast Color Classification System” (Agtron/SCAA, Reno, NV, 1995).
Coffee was distributed to participants in 500 g packages at the beginning of the each intervention. Subjects were instructed to prepare the brew in the household coffee maker filtering the coffee (15 g per one 150 ml cup) through the paper filters, and consume the total daily amount in three or four separate cups without a fixed schedule.
Determination of Antioxidants in MLR and MR Coffee Brews
All analyses were performed immediately after coffee preparation. We prepared the coffee beverages as instructed to the subjects. Phenolic compounds and caffeine were determined by high performance liquid chromatography (HPLC; Agilent Technologies, USA) with diode-array detector (DAD) and mass spectrometer [11]. Total phenolic content was also estimated using the Folin-Ciocalteau method [12]. A 5-caffeoylquinic acid (5-CQA) calibration curve was used. The MRP content was estimated by the browning indices at 420 nm [13]. Total antioxidant capacity (TAC) was measured by DPPH (1,1-diphenyl-2-picryl-hydrazyl-hydrate) scavenging capacity [14] and oxygen radical absorbance capacity (ORAC) [15] assays.
Antioxidant Characterization in Subjects
Plasma TAC was determined using a commercial kit (total antioxidant status—TAS, Randox Labs, Crumlin, UK) and by ORAC [15].
To express the enzyme activities as units per gram hemoglobin (U/g Hb), Hb concentration was measured by the Drabkin method [16]. Superoxide dismutase (SOD) activity was determined with the Ransod SD 125 kit (Randox Labs, Crumlin, UK), glutathione peroxidase (GPx) activity with the Ransel RS 504 kit (Randox Labs, Crumlin, UK) and catalase (CAT) activity according to Aebi [17].
Plasma Lipid Peroxidation Biomarkers
Oxidized LDL (oxLDL) analysis was performed according to the manufacturer’s protocol using Oxidized LDL ELISA kit (Mercodia AB, Uppsala, Sweden). Total (free and esterified) F2-isoprostanes were isolated using an F2-isoprostane affinity column (Cayman Chemical, Ann Arbor, MI, USA). The 8-epi-prostaglandin F2α (8-epi-PGF2α) concentration was measured according to the manufacturer’s protocol by a specific enzyme immunoassay (EIA) kit (Cayman Chemical, Ann Arbor, MI, USA).
Statistical Analysis
Data are reported as mean ± standard deviation. Antioxidant content and TAC of the two types of roasted coffees were compared by t-test for independent samples. Differences in human variables were analyzed by repeated-measures analysis of variance for comparisons of MLR coffee intake with MR and of each roast with the baseline. Evaluation of the two groups’ mean profiles was carried out to measure the effects of the MLR and MR order of consumption and the potential carryover effect. Statistical analyses were performed using SPSS 18.0 (SPSS Inc., Chicago, IL, USA). A two-tailed P < 0.05 was considered significant.
Results and Discussion
Subjects
Twenty habitual coffee drinkers (49 ± 9 years, range 37–63) were evaluated. Most subjects were female (70 %), overweight (76 %), and sedentary (physical activity less than 3 h/week) (75 %). Their baseline characteristics are summarized in Table 1 (Online Resource 2).
After the run-in period, participants were randomly assigned to one of the two interventions (MLR or MR). During the first 4-week intervention period, 45 % of the participants (55.6 % women) ingested MLR coffee and 55 % (81.8 % women) MR coffee. In the next 4-week period, they switched to the opposite roasted coffee. Coffee consumption was 482 ± 61 ml throughout the study.
Self-reported diets showed that none of the subjects consumed a significant quantity of polyphenol-rich foods other than coffee during the study, and the nutritional intake was similar before and after each intervention period in all subjects (P > 0.05). In addition, we did not find any significant differences in physical activity and body composition throughout the study (data not shown).
Antioxidant Characterization of Coffee Brews
Five CGA (four caffeoylquinic acids—CQA isomers and one feruloylquinic acid—FQA isomer) and two caffeoylquinic lactones (CQL) were identified in the MLR and MR coffees. Caffeic, ferulic, and p-coumaric acids were not identified in free form. Total phenolic content, CGA, caffeine, and MRP in MLR and MR coffees are presented in Table 1. A 150 ml cup of coffee prepared with either MLR or MR contained 334 and 210 mg of total CGA, respectively. According to the t-test for independent samples, CGA were statistically lower and MRP was higher (P < 0.001) with the increased degree of roasting, confirming the influence of the roasting process on the chemical composition of coffee beans. Similar results were obtained in another study [18].
Caffeine consumption was 231 mg/cup of MLR and 244 mg/cup of MR (P = 0.003). Although caffeine’s antioxidant properties have been described, it is unlikely that this compound was primarily responsible for the antioxidant activity, because MR had no significant difference in ORAC compared with baseline. Another study showed that caffeine had no antioxidant activity at concentrations up to 570 times than those found in our study [19]. A recent study compared the ORAC values of decaffeinated and regular coffees and concluded that caffeine had little or no direct antioxidant activity [8].
Comparing the antioxidant activity of the two brewed coffees, we observed that there were no significant differences in DPPH scavenging activity and ORAC between the MLR and MR coffees (data not shown). Possibly, MRP balanced the thermal loss of naturally occurring phenolic compounds. Consistent with our results, Bakuradze et al. [18] did not observe a significant difference in ORAC between the MLR and MR extracts.
Antioxidant Activity and Lipid Peroxidation in the Subjects
Table 2 shows the results of antioxidant activity and lipid peroxidation. Compared with baseline, there was a 21 % increase in TAS and 12 % in ORAC after MLR coffee intake. After consumption of MR, we observed a 26 % increase in TAS and a 12 % increase in ORAC.
Erythrocyte antioxidant enzyme activity increased significantly after consumption of both types of coffee. In MLR and MR, SOD activity increased 52 and 75 %, and GPx activity 62 and 49 %, respectively. CAT activity increased 13 % after both intervention periods. We did not observe a significant difference in antioxidant effects between the two intervention periods. Although about 30 % of melanoidins (the most abundant compounds of Maillard reaction) are absorbed in the human body [10, 20], these compounds may have contributed as antioxidants, which explains the similar antioxidant effects between the two intervention periods.
We observed a significant increase in plasma TAS and in erythrocyte antioxidant enzyme activities after both MLR and MR intake. We also observed a significant increase in ORAC after MLR intake. Plasma TAC is the sum of endogenous and exogenous antioxidants, as well as the synergistic effects that occur among them [21]. Because there were no changes in dietary composition during the study, we suggest that coffee antioxidants may have contributed to these effects. Other studies [19, 22–24] support our findings that coffee antioxidants contribute to the antioxidant effect in animals and humans. Our data suggest that the antioxidant effect of coffee may be important in protecting biological systems against oxidants and, consequently, reducing the risk of chronic diseases related to ROS.
Antioxidant enzymes are the first line of defense against oxidative stress. The intracellular antioxidant enzyme activity protects cells against superoxide radical anions and peroxides [24].
There are some hypotheses for the increase in antioxidant enzyme activity. An increase in SOD activity may be caused by an increased formation of superoxide radical anions, whereas an increase in GPx and CAT activity may be due to high concentrations of peroxides. The increased intracellular ROS production activates the Nrf2/antioxidant response elements (ARE) pathway, which regulates the expression of genes encoding for antioxidant enzymes. This hypothesis needs further investigation since TAC concentration increased and lipid peroxidation biomarkers did not change. The ingested CGA may regenerate the endogenous α-tocopherol, and consequently decrease the ROS concentration and restore the antioxidant enzyme concentration; coffee GCA and N-methyl pyridinium, a compound formed from trigonelline during the roasting process, may induce antioxidant enzymes in erythrocytes, because they are a strong inducer of ARE-dependent phase II gene expression in vitro [4, 22, 25]; coffee cafestol and kahweol, as well as ROS, are related to the activation of Nrf2 [25].
Although both MLR and MR coffee had increased TAC and antioxidant enzyme activities, there were no significant changes in plasmatic concentration of 8-epi-PGF2α and oxLDL. Possibly, the intervention time and/or the amount of coffee consumed were not sufficient to promote changes in these parameters. A study that compared the consumption of 0, 450, and 900 ml of filtered coffee for three weeks in healthy subjects also did not showed significant changes in plasma 8-epi-PGF2α concentration [23]. Kempf et al. [26] verified a decrease in these concentrations after a daily intake of 1,200 ml of filtered coffee for one month, twice the amount consumed in our study. A decrease in the 8-epi-PGF2α concentration was also observed by Hoelzl et al. [27], albeit when healthy subjects ingested 800 ml/day of instant coffee, containing around 300 mg CGA, for five days. However 8-epi-PGF2α concentration was measured from urine, where it is greater than in plasma. Relative to oxLDL, recent studies evaluated the consumption of 800 ml/day of instant [27] and filtered coffee [25] and also did not observe a significant change in this biomarker.
Lipid peroxidation is the primary oxidation process that contributes to cardiovascular disease. Therefore, we focused on lipid peroxidation biomarkers.
Only one study has compared the effects of two roasts of coffee on antioxidant activity and lipid peroxidation in healthy subjects [22]. However, the degree of roasting was not the same, and the lipid peroxidation biomarker (malonaldehyde) evaluated was less specific than the one used in our study [28, 29].
Our study has two limitations. First, it comprised a small sample size, which may be explained by its considerable participation period (9 weeks) and the large coffee intake. Finally, the absence of a washout period between the interventions was a potential limitation. However, the statistical analysis showed that there was no carryover effect.
Both coffee roasts showed antioxidant effects in the subjects and did not change lipid peroxidation biomarkers. Although MLR contained more CGAs, there were similar antioxidant effects between the 2 treatments. Because coffee is a much-consumed beverage, the study of its antioxidant effects is of great relevance. More clinical trials comparing different coffee roasts are needed to assess which are the compounds that contribute to its antioxidant effects.
Abbreviations
- BHT:
-
Butylated hydroxytoluene
- CAT:
-
Catalase
- CGA:
-
Chlorogenic acids
- CQA:
-
Caffeoylquinic acids
- CQL:
-
Caffeoylquinic lactone
- DPPH:
-
1,1-diphenyl-2-picrylhydrazyl-hydrate
- FQA:
-
Feruloylquinic acids
- GPx:
-
Glutathione peroxidase
- LDL:
-
Low density lipoprotein
- MLR:
-
Medium light roast
- MR:
-
Medium roast
- MRPs:
-
Maillard reaction products
- 8-epi-PGF2α:
-
8-epi-prostaglandin F2α
- ORAC:
-
Oxygen radical absorbance capacity
- oxLDL:
-
Oxidized low density lipoprotein
- ROS:
-
Reactive oxygen species
- SOD:
-
Superoxide dismutase
- TAS:
-
Total antioxidant status
References
Ranheim T, Halvorsen B (2005) Coffee consumption and human health—beneficial or detrimental?—Mechanisms for effects of coffee consumption on different risk factors for cardiovascular disease and type 2 diabetes mellitus. Mol Nutr Food Res 49:274–284
Verzelloni E, Tagliazucchi D, Del Rio D, Calani L, Conte A (2011) Antiglycative and antioxidative properties of coffee fractions. Food Chem 24:1430–1435
Sacchetti G, Di Mattia C, Pittia P, Mastrocola D (2009) Effect of roasting degree, equivalent thermal effect and coffee type on the radical scavenging activity of coffee brews and their phenolic fraction. J Food Engin 90:74–80
Boettler U, Volz N, Pahlke G, Teller N, Kotyczka C, Somoza V, Stiebitz H, Bytof G, Lantz I, Lang R (2011) Coffees rich in chlorogenic acid or N methylpyridinium induce chemopreventive phase II enzymes via the Nrf2/ARE pathway in vitro and in vivo. Mol Nutr Food Res 55:798–802
Hecimovic I, Belscak-Cvitanovic A, Horzic D, Komes D (2011) Comparative study of polyphenols and caffeine in different coffee varieties affected by the degree of roasting. Food Chem 129:991–1000
Duarte SMS, Abreu CMP, Menezes HC, Santos MH, Gouvêa CMCP (2005) Effect of processing and roasting on the antioxidant activity of coffee brews. Ciênc Tecnol Aliment 25:387–393
Bonita JS, Mandarano M, Shuta D, Vinson J (2007) Coffee and cardiovascular disease: In vitro, cellular, animal, and human studies. Pharmacol Res 55:187–198
Chu YF, Brown PH, Lyle BJ, Chen Y, Black RM, Williams CE, Lin YC, Hsu CW, Cheng IH (2009) Roasted coffees high in lipophilic antioxidants and chlorogenic acid lactones are more neuroprotective than green coffees. J Agric Food Chem 57:9801–9808
Gordon MH, Wishart K (2010) Effects of chlorogenic acid and bovine serum albumin on the oxidative stability of low density lipoproteins in vitro. J Agric Food Chem 58:5828–5833
Gómez-Ruiz J, Ames J, Leake D (2008) Antioxidant activity and protective effects of green and dark coffee components against human low density lipoprotein oxidation. Eur Food Res Technol 227:1017–1024
Jaiswal R, Patras MA, Eravuchira PJ, Kuhnert N (2010) Profile and characterization of the chlorogenic acids in green robusta coffee beans by LC-MSn: Identification of seven new classes of compounds. J Agric Food Chem 58:8722–8737
Singleton VL, Rossi JA Jr (1965) Colorimetry of total phenolics with phosphomolybdic-phosphotungstic acid reagents. Am J Enol Vitic 16:144–158
Yen WJ, Wang BS, Chang LW, Pin-Der D (2005) Antioxidant properties of roasted coffee residues. J Agric Food Chem 53:2658–2663
Brand-Williams W, Cuvelier ME, Berset C (1995) Use of a free radical method to evaluate antioxidant activity. Lebensm Wiss Technol 28:25–30
Ou B, Hampsch-Woodill M, Prior RL (2001) Development and validation of an improved oxygen radical absorbance capacity assay using fluorescein as the fluorescent probe. J Agric Food Chem 49:4619–4626
Bauer JD, Ackermann PG, Toro G (1974) Clinical laboratories methods. Mosby Company, London
Aebi H (1984) Catalase in vitro. Methods Enzimol 105:121–126
Bakuradze T, Lang R, Hofmann T, Stiebitz H, Bytof G, Lantz I, Baum M, Eisenbrand G, Janzowski C (2010) Antioxidant effectiveness of coffee extracts and selected constituents in cell free systems and human colon cell lines. Mol Nutr Food Res 54:1734–1743
Natella F, Nardini M, Giannetti I, Dattilo C, Scaccini C (2002) Coffee drinking influences plasma antioxidant capacity in humans. J Agric Food Chem 50:6211–6216
Morales FJ, Somoza V, Fogliano V (2010) Physiological relevance of dietary melanoidins. Amino Acids 42:1097–1109
Perez-Jimenez J, Serrano J, Tabernero M, Arranz S, Diaz-Rubio ME, Garcia-Diz L, Goñi I, Saura-Calixto F (2009) Bioavailability of phenolic antioxidants associated with dietary fiber: Plasma antioxidant capacity after acute and long-term intake in humans. Plant Foods Hum Nutr 64:102–107
Kotyczka C, Boettler U, Lang R, Stiebitz H, Bytof G, Lantz I, Hofmann T, Marko D, Somoza V (2011) Dark roast coffee is more effective than light roast coffee in reducing body weight, and in restoring red blood cell vitamin E and glutathione concentrations in healthy volunteers. Mol Nutr Food Res 55:1582–1586
Mursu J, Voutilainen S, Nurmi T, Alfthan G, Virtanen JK, Rissanen TH, Happonen P, Nyyssonen K, Kaikkonen J, Salonen R, Salonen JT (2005) The effects of coffee consumption on lipid peroxidation and plasma total homocysteine concentrations: A clinical trial. Free Radic Biol Med 38:527–534
Viana ALM, Fonseca MDM, Meireles ELJ, Duarte SMS, Rodrigues MR, Paula FBA (2012) Effects of the consumption of caffeinated and decaffeinated instant coffee beverages on oxidative stress induced by strenuous exercise in rats. Plant Foods Hum Nutr 67:82–87
Misik M, Hoelzl C, Wagner KH, Cavin C, Moser B, Kundi M, Simic T, Elbling L, Kager N, Ferk F, Ehrlich V, Nersesyan A, Dusinska M, Schilter B, Knasmuller S (2010) Impact of paper filtered coffee on oxidative DNA-damage: Results of a clinical trial. Mutat Res Fundam Mol Mech Mutagen 692:42–48
Kempf K, Herder C, Erlund I, Kolb H, Martin S, Carstensen M, Koenig W, Sundvall J, Bidel S, Kuha S, Tuomilehto J (2010) Effects of coffee consumption on subclinical inflammation and other risk factors for type 2 diabetes: A clinical trial. Am J Clin Nutr 91:950–957
Hoelzl C, Knasmuller S, Wagner KH, Elbling L, Huber W, Kager N, Ferk F, Ehrlich V, Nersesyan A, Neubauer O, Desmarchelier A, Marin-Kuan M, Delatour T, Verguet C, Bezencon C, Besson A, Grathwohl D, Simic T, Kundi M, Schilter B, Cavin C (2010) A clinical instant coffee with high chlorogenic acid levels protects humans against oxidative damage of macromolecules. Mol Nutr Food Res 54:1722–1733
Roberts LJ, Morrow JD (2000) Measurement of F2-isoprostanes as an index of oxidative stress in vivo. Free Radic Biol Med 28:505–513
Gutteridge JMC, Halliwell B (1990) The measurement and mechanism of lipid peroxidation in biological systems. Trends Biochem Sci 15:129–135
Acknowledgments
Authors thank the volunteers for their participation, FAPESP for financial support (grant 2009/05792-7) and a PhD fellowship to TAFC (Process 2008/10933-6), and Melitta do Brasil for providing the roasted ground coffees and paper filters.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Corrêa, T.A.F., Monteiro, M.P., Mendes, T.M.N. et al. Medium Light and Medium Roast Paper-Filtered Coffee Increased Antioxidant Capacity in Healthy Volunteers: Results of a Randomized Trial. Plant Foods Hum Nutr 67, 277–282 (2012). https://doi.org/10.1007/s11130-012-0297-x
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11130-012-0297-x