Abstract
Although coffee has been reported as a major contributor to antioxidants in the diet, there are limited studies assessing how brewing methods and types of coffee affect antioxidant capacity of coffee consumers. This study was aimed to investigate the impact of coffee preparation on total phenolic content in brewed coffee extracts and their contribution to antioxidant status. We examined how the different brewing methods affect total phenolic content and antioxidant capacity in brewed coffee extracts, then assessed the antioxidant status of coffee consumers after drinking coffee. Results showed that consuming capsule versus French press coffee provides a higher total phenolic content and drinking capsule coffee leads to greater antioxidant status as shown by the higher total antioxidant capacity in urine. Further studies can expand on this by using a broader range of brewing methods to evaluate the contribution of brewing methods of coffee to antioxidant status.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Antioxidants in the diet play an important role in the prevention of chronic diseases such as cardiovascular disease (CVD), type 2 diabetes, and certain cancers. Antioxidants are substances that work to delay or prevent the oxidation of oxidizable substrates such as proteins, lipids, carbohydrates, and DNA (Halliwell, 1997). The ability of antioxidants to reduce the amount of reactive oxygen species (ROS) in the body makes them important to maintaining physiological health. Excess ROS is harmful, and thus antioxidants protect the body from the destructive effects of free radicals, maintaining optimal reduction–oxidation (redox) balance (Marques et al., 2014; Seifried et al., 2007). Oxidative stress has been shown to be related to CVD (Garcia et al., 2017), cancer (Sosa et al., 2012), and other chronic diseases (Christen 2000; Maritim et al., 2003). Antioxidant molecules can hinder oxidative processes by delaying or preventing the oxidative stress reducing the risk of these diseases.
Coffee has a high content of phenolic acids such as such as caffeic acid and chlorogenic acid, making it one of the major contributors to antioxidants in the diet (Lee et al., 2019). Coffee is among the most widely consumed beverages worldwide (Samoggia and Riedel, 2019) and has been reported to account for approximately 40% of total phenolic intake daily (Grosso et al., 2014). Coffee continues to be widely consumed by Americans (Loftfield et al., 2016); for this reason, it is important to investigate its potential health benefits. Due to its widespread consumption, coffee is the greatest contributor to redox-active compounds in the diet, contributing more than 60% of total dietary antioxidants (Bohn et al., 2013). Studies investigated the impacts of coffee consumption on different aspects of health such as breast (Ganmaa et al., 2008; Pathy et al., 2010), prostate (Cao et al., 2014; Liu et al., 2015; Wilson et al., 2011), colorectal (Schmit et al., 2016), and liver cancers (Larsson and Wolk, 2007; Loftfield et al., 2020; Sang et al., 2013). However, there has also been controversy over the impact of coffee consumption on coffee and its effects on blood pressure and CVD (Chrysant, 2017; Rodríguez-Artalejo and López-García, 2018). Coffee variety and brewing methods may affect the phenolic content in brewed coffee extracts (Olechno et al., 2020) and thus differentially contribute to redox balance. The inconsistency of these findings can thus be in part a result of the various types of brewing methods since they may contribute to the antioxidant concentrations in coffee extracts. Mena et al. (2021) found that after coffee consumption mammalian and microbial metabolism occurs with phenolic catabolites appearing mainly as phase II metabolites. When these compounds are ingested, they can be rapidly and partially absorbed and excreted allowing for detection in plasma and urine. This study also found that the bioavailability of coffee phenolic acids was reduced with increased consumption due to the saturation effect.
With the increasing popularity of coffee, it is important to investigate how various coffee brewing methods affect the antioxidant capacity of coffee extracts and contribute to increasing the body’s antioxidant status which ultimately helps with reducing the risk of CVD. However, there are limited studies assessing how the variety of brewing methods and types of coffee affect antioxidant capacity. Preparation methods may lead to differences in phenolic content of brewed coffee, indicating the significance of this study because it will contribute to the limited information on coffee preparation methods. The purpose of this study was to investigate the impact of coffee preparation on total phenolic content in brewed coffee extracts and their contribution to urinary total antioxidant capacity.
Materials and methods
Preparation of coffee extracts
For preparation of the coffee extracts for measuring total phenolics and total antioxidant capacity, a single-serve capsule coffee brewing machine with plastic water reservoir and a stainless-steel French press coffee maker and coffee grinder were used to brew six brands of coffee. The capsule coffee machine and French press were washed thoroughly with distilled deionized water (DDW) before and in between each extraction. Capsule coffee solutions were obtained three times for a total of 540 mL using the brewing instructions for making a small cup of coffee described in the brewing machine manual. For the intervention study, capsule coffee was prepared from a single coffee brand. First, the brewing machine was run three times using only HPLC-grade water to rinse any potential residue. Three capsules were then brewed sequentially using HPLC-grade water, and the resulting coffee brew was mixed (producing approximately 540 mL of coffee). French press coffee was brewed using stainless steel equipment (French press, coffee bean grinder and water kettle) and a whole bean version of the same brand of coffee. First, the coffee grinder was filled with beans and ground for 14 s. Then 27 g of ground coffee (equivalent to the amount of coffee contained in three capsules) was placed in the French press along with 600 mL of hot HPLC-grade water and brewed for 4 min producing approximately 540 mL coffee. Coffee was immediately served in a ceramic mug.
Human study design and procedure
A 15-day randomized crossover trial was conducted with 30 healthy participants who were randomized into one of two groups: capsule or French press (n = 15 each group). Randomization was followed by a 7-day run-in period in which they were instructed not to consume coffee. Then on the day of intervention, participants were provided breakfast (Table 1). Participants were then instructed to finished assigned coffee at the testing site. For the following 6 h after finishing the coffee, participants were allowed to leave the testing site but were permitted to only consume a provided lunch. For the 18 h following this, participants were free to consume food and beverages that adhered to the protocol, including: (1) avoid consuming coffee and soy-based products (e.g., tofu, edamame, soy milk) and (2) avoid using paper/plastic silverware (e.g., cups, plates, utensils), touching paper receipts, and using sunscreen (Sakaki et al., 2022). Participants then repeated the intervention after a 6-day washout period (Fig. 1).
Randomized cross-over intervention design. Participants were assessed for eligibility then randomized into the capsule coffee of French press group. After the 7-day run-in period, participants consumed their assigned coffee and provided urine samples. Following a 6-day washout period, participants then consumed the other coffee and provided urine samples
Enrollment criteria consisted of being between 19 and 55 years old and a self-reported coffee consumer. Exclusion criteria for participation included: (1) individuals with a history of heart diseases, cancer, liver disease, endocrine disease, or kidney dysfunction; (2) having allergic reactions of adverse effects to ingestion or cessation of 540 mL of coffee; (3) current smokers; (4) having a job requiring contact with thermal paper receipts; (5) having a job with a food packaging or plastic manufacturing company; and (6) women taking estrogen therapy or estrogen birth control pills within the last 3 months. The study protocol was approved by the University of Connecticut Institutional Review Board (H18-017).
Urine collection
Three urine samples were collected from each participant, for each coffee type. A spot urine sample was collected immediately prior to consumption of coffee (baseline). A second sample was collected for 6 h post coffee consumption (6H), and a third was collected for 24 h post coffee consumption (hours 6–24; 24H).
Measurements of total phenolic contents
Total phenolic contents were measured from the 6 varieties of capsule coffee and the matching French press coffee by performing a total phenolic assay (Lee et al., 2019). This process included creating a gallic acid standard solution at 10, 30, 60 and 100 mg/L for the standard curve. Using 96 well plates, after the addition of DDW and each sample, the addition of Folin & Ciocalteau’s phenol reagent was timed followed by the addition of a 7% (w/v) Na2CO3 solution. After standing at room temperature for 90 min, the absorbance was measured at 750 nm using the microplate reader (BioTek Synergy 2, BioTek, Winooski, VT, United States). This process was additionally repeated for each of the baseline, 6H, and 24H urine samples from capsule coffee and French press groups. Total phenolic content was expressed as mg gallic acid equivalents (GAE)/L of coffee extract.
Measurement of total antioxidant capacity (TAC) with 1,1-diphenyl-2-picrylhydrazyl (DPPH) assay
The TAC in coffee extracts was first measured using a DPPH scavenging assay. This includes creating the DPPH radical solution and adjusting for its absorbance to 0.650 ± 0.020 at 517 nm. Vitamin C (ascorbic acid) was used as an antioxidant standard to create the standard curve. Using 96 well plates, the extracts of six coffee varieties and standard solution were added to the DPPH solution. After standing in the dark for 30 min, the solutions were measured to find the decrease in absorbance at 517 nm measured by the spectrophotometer. DPPH was used as one method to analyze TAC of coffee extracts because the radical chromogens of this method can only be solubilized in organic media (Kim et al., 2002). Antioxidant capacity was expressed as mg Vitamin C equivalents (VCE)/100 mL of coffee extract.
Measurement of TAC with 2,2ʹ-azino-bis-3-ethylbenzthiazoline-6-sulfonic acid (ABTS) assay
TAC in coffee extracts was also measured using the ABTS radical chromogen (Lee et al., 2017). For this assay, a PBS solution was first prepared to be combined with AAPH (2,2ʹ-azobis-(2-amidinopropane) HCl) and ABTS to create the radical solution. After heating for 30 min, the radical solution was filtered and adjusted for absorbance at 734 nm using PBS. Each sample was then mixed with the radical solution, and absorbance was measured at 734 nm after 10 min. The antioxidant capacity was then calculated by using the reduction in absorbance. This process was repeated for each of the urine samples in duplicate. ABTS was used for both coffee extracts and urine because ABTS radical chromogens can be dissolved in both aqueous and organic phases (Kim et al., 2002). This allows for measurement of TAC of any food samples in which compounds are lipophilic or aqueous (Re et al., 1999). Urine TAC was measured using only ABTS rather than DPPH as urine is largely a carrier of hydrophilic products. Antioxidant capacity measured in both coffee extracts and urine samples was expressed as mg VCE/100 mL of sample.
Statistical analysis
To summarize the total phenolic content and total antioxidant capacity of coffee extracts and urine samples, the mean values and standard deviation are displayed. Correlation and statistical significance were determined using Pearson correlation between capsule coffee and French press extracts and urine collected after capsule coffee and French press consumption. Urine was analyzed using two-tailed, paired t tests tests and coffee extracts were analyzed using two-tailed, independent t tests. Statistical significance was set at P < 0.05.
Results and discussion
Demographic characteristics of participants
The demographic characteristics of all 30 participants based on different variables are shown in Table 2. The majority of participants were female. Caucasian was the most common race/ethnicity follow by Asian-American/Pacific Islander, both encompassing nearly 90% of the racial makeup. The participants were generally young adults and consumed slightly over 1 cup of coffee daily habitually.
Total phenolic content and total antioxidant capacities of coffee extracts
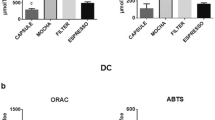
The mean total phenolic content of coffee extracts from six different brands brewed using a capsule coffee machine and French press are shown in Fig. 2. Capsule coffee had higher total phenolic content across all coffee extracts. This trend is also shown in Table 3: the mean total phenolic content of capsule coffee samples is significantly higher than that of French press (P < 0.01). Data from the DPPH assay suggests there is a significant difference between capsule coffee and French press samples (P < 0.01); however, there was no significant difference between brewing methods in the ABTS assay (Fig. 2 and Table 3).
Total phenolic content and total antioxidant capacities of urine samples
Urine samples were analyzed at baseline, 6H, and 24H to analyze TAC and total phenolic content over the course of one day. The results of the total phenolic and TAC assays for urine are displayed in Table 4. In the ABTS assay, 6H urinary TAC after capsule coffee consumption was not significantly higher than after French press consumption (P = 0.06). However, the 24H TAC of capsule coffee was significantly higher than French press (P < 0.01). Both TAC and total phenolic 6H and 24H values did not exceed the mean baseline values. Urine values were adjusted using urinary creatinine levels; however, this did not show any changes in significance with the exception of the total phenolic content 24H comparison between capsule coffee and French press (P < 0.01).
The main objective of this study was to investigate how the brewing methods of capsule coffee and French press contribute to total phenolic content and TAC of coffee extracts and human urine. Coffee consumption contributes greatly to phenolic contents and antioxidant uptake in the diet. However, the variability in brewing methods has not been thoroughly investigated to determine their impacts on the body’s total phenolic and antioxidant status. Of the coffee brands investigated in this study, capsule coffee extracts had significantly higher total phenolic content and total antioxidant capacity than French press coffee extracts.
The difference in antioxidant capacities may be due to the difference in brewing times between the methods which play a role in the extraction of antioxidants (Ludwig et al., 2012), while the difference in total phenolic content may be due to chemical changes that occur during roasting and the roasting intensity (Sacchetti et al., 2009). Differences in total phenolic content may also be due to the limited variety of coffee products and brewing methods investigated, unlike a previous study that measured various other forms, such as cans and plastic bottles, to calculate the approximate daily intake of total phenolics (Grosso et al., 2014). Brewing time and coffee origin are also sources that could lead to differences in values of total phenolics and TAC; however, coffee bean roasting may have a more significant impact on levels of antioxidants and phenolics (Górecki and Hallmann, 2020). A discrepancy in values observed between the DPPH and ABTS assays for coffee extract has been shown a previous study in which the DPPH assay underestimated antioxidant capacity by approximately 33.8% compared with the ABTS assay (Kim et al., 2002). This can be explained by the fact that phenolic compounds on the free radicals react differently between the aqueous phase and organic phase (Kim et al., 2002).
An unexpected result was that urine samples did not display a significant difference between the baseline, 6H, and 24H total phenolic content for both capsule coffee and French press urine samples. This may be a result of the food and beverages consumed because participants were not required to consume or finish the breakfast provided and the difference in food consumed while not on the research site contributing to a difference in TAC. An investigation of specific antioxidants and phenolic compounds in the future may be useful to determine which compounds are specifically contributing to the total calculations.
This study has found that the contribution of total phenolics and antioxidants to the body is greater with the consumption of capsule coffee versus French press. Natural antioxidants, such as phenolic compounds, and roasting-induced antioxidants such as melanoidins contribute to the antioxidant capacity of brewed coffee extracts (Ludwig et al., 2012). This study differs from previous literature based on the investigation of different brews of coffee from different regions of the world using a wider variety of brewing methods and investigating caffeine content. A previous study, demonstrating the difference in total phenolic content and antioxidant capacity of filter brew and espresso coffee, found that coffee with higher caffeine content is thought to account for the higher antioxidant capacity and total phenolic content for both espresso and filter coffee brews (Ludwig et al., 2012). It has been proposed that caffeine acts as an antioxidant compound against lipid peroxidation induced by ROS (Lee, 2000). Another previous study found that the coffee brewed from different regions in the world have a significant impact on the antioxidant potential with cappuccino having the highest antioxidant capacity followed by Turkish coffee and instant coffee (Wolska et al., 2017). These results differ from this study based on the methods: the thermal treatment of coffee beans transforms the biological and chemical properties of coffee beans leading to increased antioxidant activity (Castillo et al., 2002; Vicente et al., 2014). This trend is also seen in this study through the differences in total phenolic content and antioxidant capacity among the different brands of coffee analyzed as well as the brewing methods.
The results of this study should be interpreted with attention to some limitations. First, this study did not test the effect of caffeination and roast methods on TAC. Second, this study only considers two methods of coffee brewing, when there are numerous more methods. However, the study was designed to specifically use a brewing method with minimal plastic material (stainless steel French press) as a control against capsule coffee. Further investigations with various brewing and roasting methods along with variations in caffeine content will prove beneficial. Third, the diet was not strictly controlled for participants during the 24H urine collection. Breakfast and lunch were provided to participants to minimize endocrine-disrupting chemical contamination (primarily through limiting plastic materials); however, participants were not required to finish each meal, leading to possible discrepancy of results among participants. There are, however, notable strengths. First, the crossover design of the study limits the possible variation of confounding variables due to the metabolism of individuals as each participant was their own control. Second, the collection of three urine samples after coffee consumption allowed for analysis of TAC and total phenolic content for the totality of a day. Finally, we assessed two brewing methods that have not been widely assessed contributing to the data on the effects of different brewing methods on coffee.
In conclusion, the data suggest that capsule coffee provides a higher concentration of total phenolic and antioxidant capacity. In line with this, drinking capsule coffee increased the body’s antioxidant status more than French press and was the superior choice in this case. This may impact coffee drinkers’ future choices on brewing methods. The major implication of this study is that although there are a variety of other brewing options, capsule coffee has shown to provide a greater concentration of antioxidants than French press coffee. Further studies can expand on this by using a broader range of brewing methods and providing more strict diet recommendations to evaluate the contribution of brewing methods of coffee to antioxidant status.
References
Bohn SK, Blomhoff R, Paur I. Coffee and cancer risk, epidemiological evidence, and molecular mechanisms. Molecular Nutrition Food Research. 58: 915-930 (2013)
Cao S, Liu L, Yin X, Wang Y, Liu J, Lu Z: Coffee consumption and risk of prostate cancer: A meta-analysis of prospective cohort studies. Carcinogenesis. 35: 256-261 (2014)
Castillo MD, Ames JM, Gordon MH. Effect of roasting on the antioxidant activity of coffee brews. Journal of Agricultural and Food Chemistry. 50: 3698-3703 (2002)
Christen Y. Oxidative stress and Alzheimer disease. The American Journal of Clinical Nutrition. 71: 621S-629S (2000)
Chrysant SG. The impact of coffee consumption on blood pressure, cardiovascular disease and diabetes mellitus. Expert Review of Cardiovascular Therapy. 15: 151-156 (2017)
Ganmaa D, Willett WC, Li TY, Feskanich D, van Dam RM, Lopez-Garcia E, Hunter DJ, Holmes MD. Coffee, tea, caffeine, and risk of breast cancer: A 22-year follow-up. International Journal of Cancer. 122: 2071-2076 (2008)
Garcia KC, Llana-Cornejo D, Husi H. CVD and oxidative stress. Journal of Clinical Medicine. 6: 22 (2017)
Górecki M, Hallmann E. The antioxidant content of coffee and its in vitro activity as an effect of its production method and roasting and brewing time. Antioxidants. 9: 308 (2020)
Grosso G, Stepaniak U, Topor-Madry R, Szafraniec K, Pajak A. Estimated dietary intake and major food sources of polyphenols in the Polish arm of the HAPIEE study. Nutrition. 30: 1398-1403 (2014)
Halliwell B. Antioxidants: The basics – what they are and how to evaluate them. Advances in Pharmacology. 38: 3-20 (1997)
Kim DO, Lee KW, Lee HJ, Lee CY. Vitamin C equivalent antioxidant capacity (VCEAC) of phenolic phytochemicals. Journal of Agricultural and Food Chemistry. 50: 3713-3717 (2002)
Larsson SC, Wolk A. Coffee consumption and risk of liver cancer: A meta-analysis. Gastroenterology. 132: 1740-1745 (2007)
Lee SG, Wang T, Vance TM, Hubert P, Kim DO, Koo SI, Chun OK. Validation of analytical methods for plasma total antioxidant capacity by comparing with urinary 8-isoprostane level. Journal of Microbiology and Biotechnology. 27: 388-394 (2017)
Lee BH, Nam TG, Kim SY, Chun OK, Kim D-O. Estimated daily per capita intakes of phenolics and antioxidants from coffee in the Korean diet. Food Science and Biotechnology. 28: 269-279 (2019)
Lee C. Antioxidant ability of caffeine and its metabolites based on the study of oxygen radical absorbing capacity and inhibition of LDL peroxidation. Clinica Chimica Acta. 295: 141-154 (2000)
Liu H, Hu GH, Wang XC, Huang TB, Xu L, Lai P, Guo ZF, Xu YF. Coffee consumption and prostate cancer risk: A meta-analysis of cohort studies. Nutrition and Cancer. 67: 392-400 (2015)
Loftfield E, Freedman N, Dodd K, Vogtmann E, Xiao Q, Sinha R, Graubard B. Coffee drinking is widespread in the United States, but unusual intake varies by key demographic and lifestyle factors. The Journal of Nutrition. 146: 1762-1768 (2016)
Loftfield E, Rothwell JA, Sinha R, Keski-Rahkonen P, Robinot N, Albanes D, Weinstein SJ, Derkach A, Sampson J, Scalbert A, Freedman. Prospective investigation of serum metabolites, coffee drinking, liver cancer incidence, and liver disease mortality. Journal of the National Cancer Institute. 112: 286-294 (2020)
Ludwig IA, Sanchez L, Caemmerer B, Lothar KW, Peña PD, Cid C. Extraction of coffee antioxidants: Impact of brewing time and method. Food Research International. 48: 57-64 (2012)
Marques SS, Magalhães LM, Tóth IV, Segundo MA. Insights on antioxidant assays for biological samples based on the reduction of copper complexes—the importance of analytical conditions. International Journal of Molecular Sciences. 15: 11387-11402 (2014)
Maritim AC, Sanders RA, Watkins III JB. Diabetes, oxidative stress, and antioxidants: A review. Journal of Biochemical and Molecular Toxicology. 17: 24-38 (2003)
Mena P, Bresciani L, Tassotti M, Rosi A, Martinit D, Antonini M, Dei Cas A, Bonadonna R, Brighenti F, Del Rio D. Effect of different patterns of consumption of coffee and a cocoa-based product containing coffee on the nutrikinetics and urinary excretion of phenolic compounds. The American Journal of Clinical Nutrition. 114: 2107-2118 (2021)
Olechno E, Puścion-Jakubik A, Markiewicz-Żukowska R, Socha K. Impact of brewing methods on total phenolic content (TPC) in various types of coffee. Molecules. 25: 5274 (2020)
Pathy NB, Peeters P, van Gils C, Beulens JWJ, van der Graaf Y, Bueno-de-Mesquita B, Bulgiba A, Uiterwaal. Coffee and tea intake and risk of breast cancer. Epidemiology. 121: 461-467 (2010)
Re R, Pellegrini N, Proteggente A, Pannala A, Yang M, Rice-Evans C. Antioxidant activity applying and improved ABTS radical cation decolorization assay. Free Radical Biology and Medicine. 26: 1231-1237 (1999)
Rodríguez-Artalejo F, López-García E. Coffee consumption and cardiovascular disease: A condensed review of epidemiological evidence and mechanisms. Journal of Agricultural and Food Chemistry. 66: 5257-5263 (2018)
Sacchetti G, Mattia CD, Pittia P, Mastrocola D. Effect of roasting degree, equivalent thermal effect and coffee type on the radical scavenging activity of coffee brews and their phenolic fraction. Journal of Food Engineering. 90: 74-80 (2009)
Sakaki JR, Provatas AA, Perkins C, Chun OK. Urinary excretion of estrogenic chemicals following consumption of capsule coffee and French press coffee: A crossover study. Toxicology Reports. 9: 728-734 (2022)
Samoggia A, Riedel B. Consumers’ perceptions of coffee health benefits and motives for coffee consumption and purchasing. Nutrients. 11: 653 (2019)
Sang LX, Chang B, Li XH, Jiang M. Consumption of coffee associated with reduced risk of liver cancer: A meta-analysis. BMC Gastroenterology. 13 (2013)
Schmit SL, Rennert HS, Rennert G, Gruber SB. Coffee Consumption and the risk of colorectal cancer. Cancer Epidemiology, Biomarkers and Prevention. 25: 634-639 (2016)
Seifred HE, Anderson DE, Fisher EI, Milner JA. A review of the interaction among dietary antioxidants and reactive oxygen species. The Journal of Nutritional Biochemistry. 18: 567-579 (2007)
Sosa V, Moliné T, Somoza R, Paciucci R, Kondoh H, Lleonart ME. Oxidative stress and cancer: An overview. Aging Research Reviews. 12: 376-390
Vicente SJV, Queiroz YS, Gotlieb SL, Torres EA. Stability of phenolic compounds and antioxidant capacity of regular and decaffeinated coffees. Brazilian Archives of Biology and Technology. 57: 110-118 (2014)
Wilson KM, Kasperzyk JL, Rider JR, Kenfield S, van Dam RM, Stampfer MJ, Giovannucci E, Mucci LA. Coffee consumption and prostate cancer risk and progression in the health professional’s follow-up study. Journal of the National Cancer Institute. 103: 876-884 (2011)
Wolska J, Janda K, Jakubczyk K, Syzmkowiak M, Chlubek D, Gutowska I. Levels of antioxidant activity of arabica, robusta, and green coffee beans in according to their brewing methods. Biological Trace Element Research. 179: 327–333 (2017)
Acknowledgements
This work was supported by the National Institute of Environmental Health Sciences (Grant Number 1R03ES027983-01A1) to Dr. Ock K Chun. It was also supported by the University of Connecticut McNair Scholars Program and Summer Undergraduate Research Fund to Briana Nosal.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflicts of interest.
Informed consent
All procedures followed were in accordance with the ethical standards of the responsible committee on human experimentation (institutional and national) and with the Helsinki Declaration of 1975, as revised in 2008. Informed consent was obtained from all patients for being included in the study.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Nosal, B.M., Sakaki, J.R., Kim, DO. et al. Impact of coffee preparation on total phenolic content in brewed coffee extracts and their contribution to the body’s antioxidant status. Food Sci Biotechnol 31, 1081–1088 (2022). https://doi.org/10.1007/s10068-022-01100-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10068-022-01100-4