Abstract
Leaves of Ilex paraguariensis are used to prepare a tea known as maté which is a common beverage in several South American countries. The ethanol extract was fractionated to identify the compounds responsible for the anti-adipogenic activity in 3T3-L1 cells. Extracts of both fresh and dried maté leaves were subjected to column chromatography using molecular permeation to obtain the saponin (20 % yields) and the polyphenol extracts (40 % yields) from the fresh and dried leaves. The phenolic content was determined using high-performance liquid chromatography analysis and the Folin-Ciocalteau method. Also, maté extracts (50 μg/ml to 1,000 μg/ml) did not display citotoxicity using MTT. The polyphenol extract from the dried leaves was the most effective (50 μg/ml) in the inhibition of triglyceride accumulation in 3T3-L1 adipocytes, and rutin (100 μg/ml) likely accounted for a large portion of this activity. Additionally, maté extracts had a modulatory effect on the expression of genes related to the adipogenesis as PPARγ2, leptin, TNF-α and C/EBPα.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Ilex paraguariensis is an important South American crop, known as yerba mate or maté, from which leaves and twigs are used to prepare a tea. Maté is one of the most commonly consumed beverages in several South American countries, including Brazil (especially the southern states), Uruguay, Paraguay, and Argentina. The leaves of Ilex species have been extensively studied [1]. In addition to polyphenols such as flavonoids (quercetin and rutin) and phenolic acids (chlorogenic and caffeic acids), maté is rich in caffeine and saponins. The saponins isolated from maté to date are composed of ursolic acid or oleanolic acid as the aglycone and various sugars [1, 2]. Recently, beneficial effects of I. paraguariensis have been demonstrated, including antioxidant activity [1–3], a protective effect against induced DNA damage [4] and anti-obesity effects [5, 6].
Obesity is a complex condition involving social, biological and psychosocial factors. A sedentary lifestyle and a high-calorie diet seem to be the most important factors in the development of obesity [7]. Co-morbidities associated with obesity are serious and include both psychosocial and biological factors involving several metabolic problems [8]. Metabolic syndrome is a set of metabolic and hemodynamic abnormalities often present in obese individuals [9]. Adipose tissue is the largest reservoir of energy in the body. This tissue consists of adipocytes that synthesize, store and mobilize fatty acids in response to changes in physiological demands. These processes are highly regulated by genetic, nutritional, hormonal and paracrine mechanisms [10, 11].
Previous studies have shown that the consumption of maté has an anti-obesity and anti-inflammatory effect in animal models [5, 6]. Thus, the aim of the present study was to evaluate the effect on adipogenesis in vitro of the main bioactive compounds of maté and to characterize which of these compounds is most likely responsible for the anti-obesity activity.
Materials and Methods
All chemicals (analytical grade) were purchased from Fluka Chemie (Switzerland) and Merck (Germany). Caffeic acid, chlorogenic acid, gallic acid, rutin and ursolic acid were purchased from Sigma-Aldrich (USA). Matesaponin 4 was isolated as previously described [12].
Plant Material and Extraction
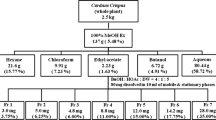
Leaves from I. paraguariensis A. St. Hil. were harvested from a cultivated area, and a voucher specimen (ICN 163413) was deposited at the herbarium of the University. A portion of the material was used fresh, and the residue was dried for 15 days in an air-circulating stove. Hydroethanolic extracts were prepared by maceration using fresh (F) or dried (D) leaves (100 g) in 70 % ethanol (1:10, plant:solvent, m/v). After ethanol elimination, the aqueous phases were separately freeze dried to obtain the ethanol extracts from fresh (FE) and dried (DE) leaves. Both ethanol extracts were subjected to column chromatography using molecular permeation (Sephadex LH-20®) with a water:ethanol gradient as an eluent. These fractions were grouped together according to thin-layer chromatography (TLC) using Si gel GF254 (Sigma-Aldrich®), chloroform:ethanol:acetic acid (100:40:6, v/v) and ethyl acetate:acetone:acetic acid:water (60:20:10:10, v/v) as eluents. The plates were observed under UV254/366 after being sprayed with anisaldehyde sulfuric acid/100 °C to visualize the saponins or with the Natural Reagent A to visualize the phenolic compounds [13]. The saponin extract (20 % yields) was obtained from the fresh (FS) and dried (DS) leaves, and the polyphenol extract (40 % yields) was obtained from the fresh (FP) and dried (DP) leaves.
HPLC Analysis of Chlorogenic Acid and Rutin
High-performance liquid chromatography (HPLC) analysis was conducted according to previously described methods [14] using a Shimadzu® Prominence HPLC system (Kyoto, Japan) coupled to an SPD-20A UV/VIS detector. Additionally, an RP-18 column (CLC-ODS (M) 250 × 4.6 mm i.d., 5-μm particle size) was used coupled to a Waters® pre-column (20 x 3.9 mm i.d., 10-μm particle size). The mobile phase consisted of (A) 2.0 % acetic acid (v/v) and (B) methanol:water (8.5:1.5, v/v). The elution gradient was as follows: 31 % B (0–10 min), 31–56 % B (10–25 min), 56 % B (25–33 min), 56–77 % B (33–45 min), 77–56 % B (45–50 min) and 56–31 % B until 60 min. The flow rate was 0.7 ml/min, and the injection volume was 20 μl. A 340-nm wavelength was used, and the analysis was performed at 23 ± 1 °C. Chlorogenic acid and rutin exhibited retention times of 11.06 and 30.66 min, respectively, when dissolved in methanol:water (50:50, v/v) at 2, 4, 6, 8 or 10 μg/ml. For the chlorogenic acid and rutin calibration curves, the linear equations (n = 5) were y = 140094x – 14056 (r2 = 0.999) and y = 81381x + 2130.9 (r2 = 0.999), respectively. For samples, a solution of each FE and DE sample dissolved in methanol:water (1:1, v/v) was prepared at 0.4 mg/ml. The solutions were filtered through a 0.45-μm membrane (Millipore HVLP), and the data are expressed as means ± SE of triplicate experiments.
Determination of Phenolic Compounds Using the Folin-Ciocalteau Method
FE and DE samples were tested along with the FS, DS, FP and DP samples. Methanol solutions of gallic acid at 15, 20, 25, 50 or 75 μg/ml were prepared as a standard with a linear equation of y = 0.0405x–0.4847 (r2 = 0.994). Samples of 1 mg/ml were prepared. To conduct the assay, 30 μl of each sample was added to 96-well plates followed by 75 μl of Folin-Ciocalteau reagent (2 N), 30 μl Na2CO3 (15 %) and 15 μl distilled water [15]. After 2 h, the absorbance was read at 750 nm; the data are expressed as gallic acid equivalents (GAE) per g on a dry-weight basis (means ± SE of triplicate experiments) (see Supplementary Material).
3T3-L1 Cell Culture
The 3T3-L1 cell line (ATCC) was cultured to confluence in DMEM medium supplemented with 10 % fetal bovine serum and 10 ml/l penicillin/streptomycin at 37 °C in a 5 % CO2 atmosphere. Forty-eight hours after achieving confluence (Day 0), the cells were incubated in differentiation medium (DMEM medium supplemented with 10 % fetal bovine serum, 0.25 μM dexamethasone, 10 μg/ml insulin and 0.5 mM IBMX). After 48 h, the cells were exposed to maturation medium (DMEM medium supplemented with 10 % fetal bovine serum and 5 μg/ml insulin) and cultured for 15 days.
Assessment of 3T3-L1 Differentiation
Differentiation was assessed using oil red O staining. Briefly, the cells were incubated with extracts or isolated compounds (50 μg/ml to 100 mg/ml) during the differentiation period. After 15 days, the cells were washed with PBS, fixed in 4 % paraformaldehyde for 30 min and incubated for 1 h with 1 % oil red O solution (Sigma-Aldrich) [16]. After repeated washings with water, the oil red O was dissolved in 100 % isopropyl alcohol, and the optical density was measured on a microplate spectrophotometer at 540 nm.
Determination of Cell Viability
The MTT (3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide) reduction method described by Mosmann [17] was used, with modifications, to determine cell viability. Briefly, 3T3-L1 cells were used after the period of cell maturation (Day 12), as described above. Extracts were added to the cells at concentrations ranging from 50 μg/ml to 1,000 μg/ml, and reference or isolated compounds were added as follows: ursolic acid at 800, 900 or 1,000 μg/ml; caffeic acid, matesaponin 4 and rutin, all of them at 100, 500 or 1,000 μg/ml. Cells were incubated at 37 °C and 5 % CO2 for 24 h. Subsequently, wells were washed with 100 μl Hank's buffer solution followed by the addition of 10 μl MTT solution (5 mg/ml). After 3 h (37 °C and 5 % CO2), 100 μl of 10 % SDS in 0.01 M HCl was added to lysed the cells, followed by incubation for 18 h (37 °C and 5 % CO2). Absorbance was read on a microplate spectrophotometer at 540 nm. The absorbance of control cells (untreated) was considered as 100 % cell viability (see Supplementary Material).
RNA Extraction and cDNA Synthesis
Cell culturing for RNA extraction was performed as described for 3T3-L1 differentiation. For RNA stabilization and protection, all samples were stored in RNAlater (QIAGEN, Valencia, CA, USA) at −80 °C until the time of RNA extraction using an RNeasy® tissue kit (QIAGEN) according to the manufacturer's instructions. After the extraction, ~100 μg of RNA was used for cDNA synthesis using a High Capacity cDNA Archive Kit (Applied Biosystems, Foster City, CA, USA), as described by Xin et al. [18].
Quantification of Expression by Real-Time PCR
The expression of the PPARγ2, leptin, TNF-α and C/EBPα genes as well as the constitutively expressed β–actin gene was analyzed using real-time PCR (see Supplementary Material). The primers used in this study were designed with the aid of the website http://fokker.wi.mit.edu/primer3/input.htm. The real-time PCR was performed using Platinum® SYBR GREEN® qPCR Supermix UDG (Invitrogen) according to the manufacturer's recommendations. The samples were cycled with a 7300 Real-Time PCR System and analyzed with RQ Study Software (Applied Biosystems). All reactions were performed in triplicate, and the average Ct value was used to assess gene expression. Relative expression was calculated according to previously described methods [19].
Statistical Analysis
Results are expressed as means ± SD; the statistical significance was determined by ANOVA followed by the Dunnett test (*p < 0.05), (**p < 0.01) and (***p < 0.001).
Results and Discussion
Chemical Composition
Extracts of both fresh and dried maté leaves were made in 70 % ethanol to evaluate the influence of the drying process on the chemical and biological activity of the constituent compounds. Both ethanol extracts were further fractionated to determine the active compounds involved in adipogenesis. Because the main maté constituents are phenolic compounds and saponins, polyphenol and saponin extracts were obtained from fresh and dried maté leaves. HPLC revealed that in the ethanol extracts, the chlorogenic acid content (mg/g on a dry-weight basis) in fresh leaves was twice (10.40 ± 0.04) as high as in dried leaves (5.61 ± 0.02), whereas the rutin content (mg/g on a dry-weight basis) was 6.10 ± 0.21 in fresh leaves and 8.51 ± 0.02 in dried leaves. In dried leaves, Silva et al. [14] found a higher concentration of both chlorogenic acid and rutin in 40 % hydroethanolic extract solutions by turbo extraction (2.06 and 0.74 mg/ml, respectively) than by either decoction (1.53 and 0.49 mg/ml, respectively) or infusion (1.75 and 0.55 mg/ml, respectively). Thus, the amount and composition of the chemical compounds obtained may vary according to the type of extraction, solvent and temperature used.
In the phenolic analysis of maté samples, rutin and chlorogenic acid were determined using the Folin-Ciocalteau method, results are expressed as gallic acid equivalents (see Supplementary Material). The phenolic contents of the ethanol extracts of fresh and dried leaves (151.61 mg and 170.91 mg GAE/g, respectively) were significantly different (p < 0.05). Of all maté samples, the polyphenol extract of fresh leaves exhibited higher content of total phenols (250.29 mg GAE/g, p < 0.05), followed by the polyphenol extract and the ethanol extract of dried leaves (180.17 and 170.91 mg GAE/g, respectively). As expected, the saponin extracts of both fresh and dried leaves exhibited lower contents of phenolic compounds (79.42 and 76.34 mg GAE/g, respectively). Moreover, in relation to the reference compounds, the rutin content was 124.72 mg GAE/g, and the chlorogenic acid content was 179.37 mg GAE/g being this latter note significantly different from that of the polyphenol extract of dried leaves (p < 0.05). A previous study [20] reported the phenolic content measured by the Folin-Ciocalteau method in aqueous extracts of Ardisia compressa, I. paraguariensis and Camellia sinensis, demonstrating that I. paraguariensis had a higher phenolic content (94.91 mg GAE/g) than A. compressa (39.07 mg GAE/g). However, C. sinensis had the highest content (148.77 mg GAE/g).
Adipocyte Differentiation and Gene Expression
Adipogenesis, the differentiation of new fat cells, is considered a dynamic process and is a field of intensive research [11]. Several studies of adipogenesis have been performed using the 3T3-L1 cell line [16, 21–23]. Many phytochemicals are potential anti-obesity agents, and understanding the activities of these compounds during adipogenesis is essential to the development of new treatments for obesity [22, 23]. The differentiation of preadipocytes into adipocytes is associated with an increased number of oil red O-positive cells due to lipid accumulation. We demonstrated that incubation with some I. paraguariensis extracts or using isolated compounds reduced the lipid accumulation in a dose-dependent manner (p < 0.05) (Fig. 1). This anti-adipogenic effect was achieved at concentrations that did not affect cell viability according to the MTT assay (see Supplementary Material).
Figure 1 presents data related only to the samples displaying the best inhibition of intracellular lipid accumulation. In this sense, the highest anti-adipogenic effect was detected in the polyphenol extract of dried leaves at 50 μg/ml, followed by the saponin extract of fresh leaves at 100 μg/ml and by the polyphenol extract of fresh leaves at 500 μg/ml (p < 0.05). Among the reference substances, rutin exhibited the highest inhibitory activity (100 μg/ml), followed by chlorogenic acid (300 μg/ml), matesaponin 4 (500 μg/ml) and ursolic acid (800 μg/ml). Caffeic acid did not inhibit intracellular lipid accumulation up to a concentration of 1,000 μg/ml (data not shown). Thus, among the tested samples, phenolic compounds were the most active, and rutin likely accounted for a large portion of the activity detected in the polyphenol extracts. These findings demonstrate that it was possible to obtain a polyphenol-enriched extract exhibiting a potent anti-adipogenic effect.
Adipocyte differentiation depends upon the coordinated regulation of gene expression. Adipogenic transcription factors, including the peroxisome-activated receptor gamma (PPARγ), the linking-element-binding protein 1c, regulated by sterols (SREBP-1c) and the proteins that bind to the amplifier CCAAT (CCAAT/enhancer binding proteins, or C/EBPs), play a key role in the complex transcriptional cascade that occurs during adipogenesis [24, 25]. To evaluate the expression of genes related to adipogenesis, we used the samples that inhibited lipid accumulation in 3T3-L1 cells at the lowest concentrations, including the saponin extract from the fresh leaves (FS), polyphenol extracts from the fresh and dried leaves (FP, DP), chlorogenic acid, rutin, ursolic acid and matesaponin 4. In brief, all assayed samples and standards restrained the expression of the PPARγ2, leptin, TNF-α, and C/EBPα genes, as shown in Table 1 (see also Supplementary Material).
Several studies have shown that PPARγ is the main regulator of adipogenesis and its maintenance is critical to the progression of the final stages of adipocyte differentiation [26]. PPARγ2 is mainly expressed in adipose tissue and promotes adipocyte differentiation and proliferation, resulting in an increase in adiposity [27]. Several studies have demonstrated that the activation of PPARγ results in the expression of several pro-adipogenic genes, including C/EBP-α [28]. Studies have also demonstrated that the expression of C/EBP-α increases the expression of PPARγ [29]. Thus, the results from the present study are important to the characterization of the anti-adipogenesis mechanism of the bioactive compounds in maté, because these transcription factors are critical to the final stages of adipocyte differentiation and progression. In addition, Hsu and Yen [30] found that rutin exhibits anti-adipogenic activity mediated by the inhibition of the expression of PPARγ2 and C/EBPα.
Moreover, it is well known that obesity leads to the increased production of several inflammatory cytokines, which play a critical role in obesity-related inflammation and metabolic pathologies. TNF-α is a potent cytokine that induces the production of IL-6, which is the major determinant of the acute phase response [31]. It has been reported that in obese individuals and animal models, the levels of TNF-α and IL-6 are persistently elevated and that a reduction of adipose mass leads to a decrease in the expression levels of these genes. Additionally, TNF-α can modulate leptin secretion by increasing its expression and circulating levels [32, 33]. Our data demonstrated a down-regulation of TNF-α and leptin mRNA expression after the treatment when compared to the control group. In a related finding, Cho et al. [34] showed that supplementation with chlorogenic acid in a diet-induced obesity model in rats enhanced lipid metabolism and the hormones related to obesity, such as leptin.
Conclusions
To the best of our knowledge, this is the first time that the effects of fractionated I. paraguariensis extracts were tested on preadipocytes to identify the compounds responsible for the anti-adipogenic activity that was previously demonstrated in crude maté extracts in vivo. Our results suggest that the polyphenol extract inhibits the lipid storage in adipocytes, in part by suppressing the expression of several genes related to adipogenesis. Therefore, an extract enriched in active compounds was obtained having potential to be used in the food and pharmaceutical industries.
Abbreviations
- DMEM:
-
Dulbecco’s modified Eagle’s medium
- IBMX:
-
3-isobutyl-1-methylxanthine
- TNFα:
-
Tumor necrosis factor-alpha
- FE:
-
Fresh leaf ethanol extract
- DE:
-
Dried leaf ethanol extract
References
Bracesco N, Sanchez AG, Contreras V, Menini T, Gugliucci A (2011) Recent advances on Ilex paraguariensis research: Minireview. J Ethnopharmacol 136:378–384
Heck AI, Mejia EG (2007) Yerba mate tea (Ilex paraguariensis): A comprehensive review on chemistry, health implications, and technological considerations. J Food Sci 72:138–151
Matsumoto RLT, Bastos DHM, Mendonça S, Nunes VS, Bartchewsky W, Ribeiro ML (2009) Effects of mate tea (Ilex paraguariensis) ingestion on mRNA expression of antioxidant enzymes, lipid peroxidation, and total antioxidant status in healthy young women. J Agric Food Chem 57:1775–1780
Miranda DD, Arçari DM, Pedrazzoli J Jr, Carvalho PO, Cerutti SM, Bastos DHM, Ribeiro ML (2008) Protective effects of mate tea (Ilex paraguariensis) on H2O2-induced DNA damage and repair in mice. Mutagenesis 23:261–265
Arçari DP, Bartchewsky W, Dos Santos TW, Oliveira K, Funck A, Pedrazzoli J, De Souza MFF, Saad MJ, Bastos DHM, Gambero A, Carvalho PO, Ribeiro ML (2009) Antiobesity effects of yerba maté extract (Ilex paraguariensis) in high-fat diet–induced obese mice. Obesity 17:2127–2133
Arçari DP, Bartchewsky W, Dos Santos TW, Oliveira K, Oliveira C, Gotardo E, Pedrazzoli J, Gambero A, Ferraz LFC, Carvalho PO, Ribeiro ML (2011) Anti-inflammatory effects of yerba maté extract (Ilex paraguariensis) ameliorate insulin resistance in mice with high fat diet-induced obesity. Mol Cell Endocrinol 335(2):110–115
Rössner S (2002) Obesity: The disease of the twenty–first century. Int J Obes 26(4):S2–S4
Haslam DW, James WP (2005) Obesity. Lancet 366:1197–1209
Furukawa S, Fujita T, Shimabukuro M, Iwaki M, Yamada Y, Nakajima Y, Nakayama O, Makishima Y, Shimomura I (2004) Increased oxidative stress in obesity and its impact on metabolic syndrome. J Clin Invest 114:1752–1761
Ahima RS, Flier JS (2000) Adipose tissue as an endocrine organ. Trends Endocrinol Metab 11(8):327–332
Ailhaud G, Hauner H (2004) Development of white adipose tissue. In: Bray AGB (ed) Handbook of obesity: Etiology and pathophysiology. Marcel Dekker, New York
Gosmann G, Guillaume D, Taketa ATC, Schenkel EP (1995) Triterpenoid saponins from Ilex paraguariensis. J Nat Prod 58:438–441
Stahl E (1969) Thin-layer chromatography, a laboratory handbook, 2nd edn. Springer, New York
Silva FA, Pavei C, Ortega GG, Lima EM, Diniz DGA, Moreira JCF, Bassani VL (2007) Validation of an LC method for polyphenol assay in extractive solutions from Ilex paraguariensis (Mate). J Liq Chromatogr Relat Technol 30:3119–3131
Beara IN, Lesjak MM, Jovin ED, Balog KJ, Anackov GT, Orcić DZ, Mimica-Dukić NM (2009) Plantain (Plantago L.) species as novel sources of flavonoid antioxidants. J Agric Food Chem 57(19):9268–9273
Moon HS, Chung CS, Lee HG, Kim TG, Choi YJ, Cho CS (2007) Inhibitory effect of (−)-epigallocatechin-3-gallate on lipid accumulation of 3T3-L1 cells. Obesity 15(11):2571–2582
Mosmann T (1983) Rapid colorimetric assay for cellular growth and survival: Application to proliferation and cytotoxicity assays. J Immunol Methods 65:55–63
Xin H, Bernal A, Amato FA, Pinhasov A, Kauffman J, Brenneman DE, Derian CK, Andrade-Gordon P, Plata-Salaman CR, Ilyin SE (2004) High-throughput Si-RNa-based functional target validation. J Biomol Screen 9:286–293
Livak KJ, Schmittgen TD (2001) Analysis of relative gene expression data using real-time quantitative PCR and the 2-(−Delta Delta C(T)) method. Methods 25:402–408
Chandra S, De Mejia EG (2004) Polyphenolic compounds, antioxidant capacity, and quinine reductase activity of an aqueous extract of Ardisia compressa in comparison to mate (Ilex paraguariensis) and green (Camellia sinensis) teas. J Agric Food Chem 52:3583–3589
Trayhurn P (2007) Adipocyte biology. Obes Rev 8(1):41–44
Park T, Kim Y (2011) Phytochemicals as potential agents for prevention and treatment of obesity and metabolic diseases. In: Atta-ur-Rahman, Choudhary MI (eds) Anti-obesity drug discovery and development. Bentham, Dubai, pp 150–185
González-Espinosa de los Monteros LA, Ramón-Gallegos E, Torres-Torres N, Mora-Escobedo R (2011) Effect of germinated soybean protein hydrolysates on adipogenesis and adipolysis in 3T3-L1 cells. Plant Foods Hum Nutr 66:355–362
Konieczny SF, Emerson CP (1984) 5-azacytidine induction of stable mesodermal stem cell lineages from 10 T1/2 cells: Evidence for regulatory genes controlling determination. Cell 38:791–800
Niwano Y, Beppu F, Shimada T, Kyan R, Yasura K, Tamaki M, Nishino M, Midorikawa Y, Hamada H (2009) Extensive screening for plant foodstuffs in Okinawa, Japan with anti-obese activity on adipocytes in vitro. Plant Foods Hum Nutr 64:6–10
Tamori Y, Masugi J, Nishino N, Kasuga M (2002) Role of peroxisome proliferator-activated receptor-gamma in maintenance of the characteristics of mature 3T3-L1 adipocytes. Diabetes 51:2045–2055
Fajas L, Fruchart JC, Auwerx J (1998) Transcriptional control of adipogenesis. Curr Opin Cell Biol 10:165–173
Evans D, Aberle J, Wendt D, Wolf A, Beisiegel U, Mann WA (2001) A polymorphism, L162V, in the peroxisome proliferator-activated receptor α (PPARα) gene is associated with lower body mass index in patients with non-insulin dependent diabetes mellitus. J Mol Med 79:198–204
Fox KE, Fankell DM, Erickson PF, Majka SM, Crossno JT Jr, Klemm DJ (2006) Depletion of cAMP-response element-binding protein/ATF1 inhibits adipogenic conversion of 3T3-L1 cells ectopically expressing CCAAT/enhancer-binding protein (C/EBP) alpha, C/EBP beta, or PPAR gamma 2. J Biol Chem 281:40341–40353
Hsu CL, Yen GC (2007) Effects of flavonoids and phenolic acids on the inhibition of adipogenesis in 3T3-L1 adipocytes. J Agric Food Chem 55:8404–8410
Bulló M, García-Lorda P, Peinado-Onsurbe J, Hernández M, Del Castillo D, Argilés JM, Salas-Salvadó J (2002) TNF-alpha expression of subcutaneous adipose tissue in obese and morbid obese females: Relationship to adipocyte LPL activity and leptin synthesis. Int J Obes Relat Metab Disord 26:652–658
Kern PA, Ranganathan S, Li C, Wood L, Ranganathan G (2001) Adipose tissue tumor necrosis factor and interleukin-6 expression in human obesity and insulin resistance. Am J Physiol Endocrinol Metab 280:745–751
Okada Y, Okada M, Sagesaka Y (2010) Screening of dried plant seed extracts for adiponectin production activity and tumor necrosis factor-alpha inhibitory activity on 3T3-L1 adipocytes. Plant Foods Hum Nutr 65(8):225–232
Cho AS, Jeon SM, Kim MJ, Yeo J, Kl S, Choi MS, Lee MK (2010) Chlorogenic acid exhibits anti-obesity property and improves lipid metabolism in high-fat diet-induced-obese mice. Food Chem Toxicol 48:937–943
Acknowledgements
We are grateful to the Brazilian agencies: Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq) and Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES) for fellowships and financial support.
Author information
Authors and Affiliations
Corresponding authors
Electronic supplementary material
Below is the link to the electronic supplementary material.
ESM 1
(PDF 47 kb)
Rights and permissions
About this article
Cite this article
Gosmann, G., Barlette, A.G., Dhamer, T. et al. Phenolic Compounds from Maté (Ilex paraguariensis) Inhibit Adipogenesis in 3T3-L1 Preadipocytes. Plant Foods Hum Nutr 67, 156–161 (2012). https://doi.org/10.1007/s11130-012-0289-x
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11130-012-0289-x