Abstract
To search for plant foodstuffs with potent anti-obese activity, we conducted a large scale screening based on the inhibitory activity on adipogenesis and the facilitating activity on adipolysis in vitro. That is, inhibition of intracellular lipid accumulation and facilitation of lipid degradation in 3T3-L1 adipocytes were extensively screened from ethanol and hexane extracts of approximately 100 kinds of plant foodstuffs marketed in Okinawa prefecture, which has been famous for the highest prevalence of exceptionally long-lived individuals in the world. Among them thirty one foodstuffs showed potent inhibitory activity on intracellular lipid accumulation in 3T3-L1 adipocytes, whereas only four foodstuffs showed clear facilitating effect on lipid degradation in 3T3-L1 adipocytes. Although further study to examine the in vivo effects on adipogenesis and adipolysis is required, this is the first study to investigate anti-obese characteristics of wide range of traditional Okinawa foodstuffs so that the results give useful information to take another look at Okinawa food culture.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
As the provocation of a World Longevity Region was advocated in 1995, Okinawa, an isolated island prefecture of Japan, has been famous for the highest prevalence of exceptionally long-lived individuals in the world. A cohort study on the elderly living in the village of Ogimi in Okinawa reported that a significantly greater number of this group maintained regular eating habit over a 10-year span, and consumed more seaweed and fish [1]. Furthermore, Okinawan food culture has been focused because its consumers have the longest life expectancies and low disability rates [2]. That is, with a core of Chinese food culture, the culture as modified through food trade with South-East Asia and the Pacific and strong Japanese influences. More recently, it has been postulated that low caloric intake and negative energy balance at younger ages, and little weight gain with age, life-long low body mass index (BMI), and relatively high plasma dehydroepiandrosterone (DHEA) levels at older ages, lead to low risk for mortality from age-related diseases, and survival patterns consistent with extended mean and maximum life span [3]. However, more recently cross-sectional results of an annual physical checkup demonstrated that metabolic syndrome, a risk factor for the development of cardiovascular disease, has been prevalent among men in Okinawa, and for the five risk factors, the highest rate of abdominal obesity was found possibly because the European and American lifestyle with high-fat diet has been widely adopted by the younger generations [4].
These findings and postulations based on Okinawa food culture and recent prevalence of metabolic syndrome tempted us to take another look at traditional Okinawa food culture. Beside the culture, the geographic feature of Okinawa is also unique in Japan. Okinawa, an elongated main island and a dozens of small islands, is located between the East China Sea on the west and the North Pacific Ocean on the east. The terrain of the northern two-thirds of the main island is mountainous and forested. The islands’ subtropical climate is hot and humid, so that biologists would expect to find a number of endemic species within the archipelago. The feature would also reflect the uniqueness on the Okinawa’s traditional food. Since our final goal is to develop dietary supplements using novel functional foodstuffs with potent anti-obese activity of desirable characteristics, we conducted a large scale screening in terms of the prevention of adipogenesis and the promotion of adipolysis in vitro at the first step.
Materials and Methods
Reagents
Reagents were purchased from the following sources: Dulbecco’s modified Eagle’s medium (DMEM) from Sigma-Aldrich (St. Louis, MO); calf serum (CS) from Thermo Trace Ltd. (Victoria, Australia); fetal calf serum (FCS) from Equitech-Bio, Inc. (Kerrville, TX, USA); Adipogenesis Assay Kit including adipocytic agents (0.5 mM 3-isobutyl-1-methylxanthine (IBMX), 1 μM dexamethasone (DEX), and 1 μM insulin) and oil red O, and adipolysis assay kit including the adipocytic agents from Chemicon International, Inc. (Temecula, CA, USA). All other reagents used were of analytical grade.
Preparation of Ethanol and Hexane Extracts from the Plant Foodstuffs
A hundred and two plant foodstuffs were collected from the market in Okinawa prefecture. Dried materials were rehydrated, and a suitable amount of water was added to some of raw materials. They were pulverized in a millser, and then were freeze-dried. The resultant freeze-dried materials were again pulverized in the millser. Almost 5–10 times volume of ethanol or hexane was added to each pulverized material, and was mixed for 2–3 h. The liquid layer of the mixture was filtrated using a Buchner funnel with sea sand. The residue was subjected to the same procedure. The collected filtrate was then concentrated by using an evaporator, and stored under −20 °C until used. When used in assays, each sample was dissolved and diluted with ethanol, followed by filtration through a membrane filter (pore size 0.2 μm).
All of the plant names and the parts used are not shown (too numerous to show).
Cell Culture and Adipogenesis Assay
Murine 3T3-L1 fibroblasts (Dainippon Pharma Co., Ltd., Osaka, Japan) were adjusted to be 30,000 cells/ml in DMEM supplemented with 10% CS, and 200 μl of the cell suspension were planted into 96-well culture plate and incubated at 37 °C in a humidified 5% CO2 incubator for 2 days. The medium was changed to DMEM supplemented with 10% FCS, 0.5 mM IBMX and 1 μM DEX, and further incubated for 2 days (initiation of differentiation). The medium was changed to DMEM supplemented with 10% FCS and 10 μg/ml of insulin, and was further incubated for 2 days. Thereafter, the medium was changed to normal culture medium (DMEM supplemented with 10% FCS), and was freshly replaced every 24 h. Each sample solution was added to be 1% (v/v) from the initiation of differentiation (day 0) to day 10. On day 10, 50 μl of oil red O working solution (provided by the manufacture of the assay kit) was added to each well, and incubated at room temperature for 15 min followed by washing 3 times with washing solution (provided by the manufacture of the assay kit). Then 50 μl of dye extraction solution (provided by the manufacture of the assay kit) was added to each well, followed by stirring on a platform rocker for 15 to 30 min. Extracted dye solution was then transferred to 96-well culture plate for reading the optical density at 520 nm.
Cell Culture and Adipolysis Assay
Cell culture was performed in the same way as in the adipogenesis assay. Each sample solution was added to be 1% (v/v) on day 9 (the 9th day after the initiation of differentiation), and after incubation for 24 h the culture medium was recovered to measure glycerol concentration. In detail, 25 μl of the medium was transferred to 96-well culture plate, and 200 μl of Free Glycerol Assay Reagent (provided by the manufacture of the assay kit) was added to the well. After incubation at room temperature for 15 min, optical density was read at 540 nm.
Results and Discussion
The biochemical pathways of adipogenesis have been well documented with the use of 3T3-L1 cell line [5, 6]. According to the characterization of regulatory regions of adipose-specific genes, the transcription factors peroxisome proliferator-activated receptor-γ (PPARγ) and CCAAT/enhancer binding protein (C/EBP), which play a key role in the complex transcriptional cascade during adipocyte differentiation, have been identified. DEX activates C/EBP, and IBMX inhibits soluble cyclic nucleotide phosphodiesterases that lead to increase in intracellular cAMP levels [7], resulting in activation of C/EBP family genes and PPARγ gene. C/EBPα and PPARγ activate expression of adipocyte-specific genes such as fatty acid synthetase, fatty acid binding protein, leptin and adiponectin. Insulin or insulin-like growth factor-1 activates PI3-kinase and Akt activity, resulting in promoting adipocyte differentiation. In contrast, cytokines such as tumor necrosis factor-α (TNF-α) and transforming growth factor β interfere with adipocyte differentiation. In this study, we used TNF-α as a positive control that inhibited intracellular lipid accumulation of 3T3-L1 cells treated with adipogenic agents (0.5 mM IBMX, 1 μM DEX, and 10 μg/ml of insulin). By using the 3T3-L1 model of adipogenesis, we screened for active foodstuffs harvested in Okinawa prefecture. Figure 1a demonstrates the representative data showing that optical density at 520 nm was decreased by the addition of sample solution. Figure 1b shows the converted data in which the solvent control is regarded as 0% and TNF-α used as the positive control is regarded as 100% inhibition. The figure indicates that the hexane extract of Artemisia indica willd. inhibited lipid accumulation of 3T3-L1 cells treated with the adipogenic agents in a concentration dependent manner. Table 1 shows a list of plant foodstuffs that showed 50% or more inhibition of adipogenesis at any concentrations tested (1, 10, 100, and 1,000 μg/ml). Among 102 plant foodstuffs tested, ethanol or hexane extracts of 31 foodstuffs showed potent inhibitory activity on intracellular lipid accumulation in 3T3-L1 adipocytes. In the study, we also examined the effect of α-lipoic acid as a known substance that exerts inhibitory action on adipogenesis [8]. The potency of α-lipoic acid is comparable to that of foodstuffs listed in Table 1.
a The representative data showing decreased optical density at 520 nm by the addition of sample solution (hexane extract of Artemisia indica willd.). b The converted data in which the solvent control is regarded as 0% and TNF-α used as the positive control is regarded as 100% inhibition. Each value represents the mean of duplicate measurements
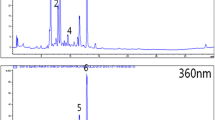
Adipolysis means the degradation of triglyceride stores in differentiated adipocytes. Isoproterenol is a nonselective agonist of the β-adrenergic class of G protein coupled receptors, which stimulate intracellular cAMP levels in adipocytes [9], has been reported to stimulate adipolysis in 3T3-L1 adipocytes [10]. Elevated level of cAMP activates protein kinase A, which results in phosphorylation of perilipin located at the surface of lipid storage droplet, and resultant phosphorylated perilipin induces the translocation of hormone-sensitive lipase (HSL) from the cytosol to the surface of the lipid storage droplet [11, 12]. HSL catalyzes triglycerides to fatty acids and glycerol as an index of adipolysis. In this study, we used isoproterenol as a positive control that increased glycerol concentration in the culture medium of 3T3-L1 cells treated with the adipogenic agents. By using the 3T3-L1 model of adiolysis, we screened for active foodstuffs harvested in Okinawa prefecture. Figure 2a demonstrates the representative data showing that glycerol concentration in the medium was increased by the addition of sample solution. Figure 2b shows the converted data in which the solvent control is regarded as 0% and isoproterenol used as the positive control is regarded as 100% stimulation. The figure indicates that the ethanol extract of Citrus depressa stimulated adiolysis of 3T3-L1 cells treated with the adipogenic agents in a concentration dependent manner. Table 2 shows a list of plant foodstuffs that showed 50% or more stimulation of adipolysis at any concentrations tested (1, 10, 100, and 1,000 μg/ml). Among one hundred and two plant foodstuffs tested, only four foodstuffs showed clear facilitating effect on lipid degradation in 3T3-L1 adipocytes. In the study, we also examined the effect of synephrin as a known substance that exerts stimulatory action on adipolysis [13]. The adipolytic activity of synephrin is very potent so that 10 μg/ml is enough to show 50% stimulation.
a The representative data showing increased glycerol concentration by the addition of sample solution (ethanol extract of Citrus depressa). b The converted data in which the solvent control is regarded as 0% and isoproterenol used as the positive control is regarded as 100% stimulation. Each value represents the mean of duplicate measurements
In the adipogenesis assay, 30% of foodstuffs tested exerted potent anti-adipogenesis activity in vitro. Although any tendency in the taxonomical relationship among active plant foodstuffs was not found, a relatively large percentage of foodstuffs were shown to be active. In contrast, in the adipolysis assay, only 3.9% of foodstuffs tested exerted clear adipolytic activity. Two of four active foodstuffs are fruit parts of Citrus species. It has been reported that citrus species are rich sources for various bioactive compounds such as flavonoids, adrenergic amines, limonoids, and coumarines [14, 15], and synephrine alkaloids, known to be adrenergic agonists [16, 17], are useful for the treatment of overweight and obesity by facilitating adipolysis [18]. Therefore, it seems to be natural that Citrus species exerted adipolytic effect in vitro.
Among the foodstuffs tested, the most famous and popular Okinawa traditional foodstuffs, Momordica charantia var. pavel Crantz, Caulerpa lentillifera, and Curcuma zedoaria showed neither inhibitory activity on adipogenesis nor stimulatory activity on adipolysis.
Although further study to examine the in vivo effects on adipogenesis and adipolysis by using a spontaneous obese animal model such as the obese diabetic KKAy mouse is frequently used as a model of metabolic syndrome [19–21], is required. This is the first study to investigate anti-obese characteristics of wide range of traditional Okinawa foodstuffs so that these results give useful information to take another look at Okinawa food culture.
Abbreviations
- DMEM:
-
Dulbecco’s modified Eagle’s medium
- CS:
-
calf serum
- FCS:
-
fetal calf serum
- IBMX:
-
3-isobutyl-1-methylxanthine
- DEX:
-
dexamethasone
References
Taira K, Tanaka H, Arakawa M, Nagashima N, Uza M, Shirakawa S (2002) Sleep health and lifestyle of elderly people in Ogimi, a village of longevity. Psychiatry Clin Neurosci 56:243–244. doi:10.1046/j.1440-1819.2002.01014.x
Sho H (2001) History and characteristics of Okinawan longevity food. Asia Pac J Clin Nutr 10:159–164. doi:10.1046/j.1440-6047.2001.00235.x
Willcox BJ, Willcox DG, Todoriki H, Fujiyoshi A, Yano K, He Q, Curb JD, Suzuki M (2007) Caloric restriction, the traditional Okinawa diet, and healthy aging. The diet of the world’s longest-lived people and its potential impact on morbidity and life span. N Y Acad Sci 1114:434–455. doi:10.1196/annals.1396.037
Tanaka H, Shimabukuro T, Shimabukuro M (2005) High prevalence of metabolic syndorome among men in Okinawa. J Atheroscler Thromb 12:284–288
Gregoire FM, Smas CM, Sul HS (1998) Understanding adipocyte differentiation. Physiol Rev 78:783–809
Rosen ED, Walkey CJ, Puigserver P, Spiegelman BM (2000) Transcriptional regulation of adipogenesis. Genes Dev 14:1293–1307
Elks ML, Manganiello VC (1985) A role for soluble cAMP phosphodiesterases in differentiation of 3T3-L1 adipocytes. J Cell Physiol 124:191–198. doi:10.1002/jcp.1041240204
Cho KJ, Moon HE, Moini H, Packer L, Yoon DY, Chung AS (2003) α-Lipoic acid inhibits adipocyte differentiation by regulating pro-adipogenic transcription factors via mitogen-activated protein kinase pathways. J Biol Chem 278:34823–34833. doi:10.1074/jbc.M210747200
Robidoux J, Martin TL, Collins S (2004) Beta-adrenergic receptors and regulation of energy expenditure: a family affair. Annu Rev Pharmacol Toxicol 44:297–323. doi:10.1146/annurev.pharmtox.44.101802.121659
Kawamura M, Jensen DF, Wancewicz EV, Joy LL, Khoo JC, Steinberg D (1981) Hormone-sensitive lipase in differentiated 3T3-L1 cells and its activation by cyclic AMP-dependent protein kinase. Proc Natl Acad Sci U S A 78:732–736. doi:10.1073/pnas.78.2.732
Sztalryd C, Xu G, Dorward H, Tansey JT, Contreras JA, Kimmel AR, Londos C (2003) Perilipin A is essential for the translocation of hormone-sensitive lipase during lipolytic activation. J Cell Biol 161:1093–1103. doi:10.1083/jcb.200210169
Zhang HH, Souza SC, Muliro KV, Kraemer FB (2003) Lipase-selective functional domains of perilipin A differentially regulate constitutive and protein kinase A-stimulated lipolysis. J Biol Chem 278:51535–51542. doi:10.1074/jbc.M309591200
Tsujita T, Takaku T (2007) Lipolysis induced by segment wall extract from Satsuma mandarin orange (Citrus unshu Mark). J Nutr Sci Vitaminol (Tokyo) 53:547–551. doi:10.3177/jnsv.53.547
Tang F, Tao L, Luo X, Ding L, Guo M, Nie L, Yao S (2006) Determinations of octopamine, synephrine and tyamine in Citrus herbs by ionic liquid improved ‘green’ chromatography. J Chromatogr A 1125:182–188. doi:10.1016/j.chroma.2006.05.049
Avula B, Upparapalli SK, Khan IA (2007) Simultaneous analysis of adrenergic amines and flavonoids in citrus peel jams abd fruit juices by liquid chromatography: part 2. J AOAC Int 90:633–640
Jordan R, Midgley JM, Thonoor CM, Williams CM (1987) Beta-adrenergic activities of octopamine and synephrine stereoisomaers on guinea-pig atria and trachea. J Pharm Pharmacol 39:752–754
Carpéné C, Bousquet-Mélou A, Galitzky J, Berlan M, Lafontan M (1998) Lipolytic effects β 1-, β 2-, β 3-adrenergic agonists in white adipose tissue of mammals. Ann N Y Acad Sci 15:186–189. doi:10.1111/j.1749-6632.1998.tb10756.x
Haaz S, Fontaine KR, Cutter G, Limdi N, Perumean-Chaney S, Allison DB (2006) Citrus aurantium and synephrine alkaloids in the treatment of overweight and obesity: an update. Obes Rev 7:79–88. doi:10.1111/j.1467-789X.2006.00195.x
Sundbom M, Kaiser C, Björkstrand E, Castro VM, Larsson C, Selén G, Nyhem CS, James SR (2008) Inhibition of 11betaHSD1 with the S-phenylethylaminothiazolone BVT116429 increases adiponectin concentrations and improves glucose homeostasis in diabetic KKAy mice. BMC Pharmacol 8:3. doi:10.1186/1471-2210-8-3
Maeda H, Hosokawa M, Sashima T, Funayama K, Miyashita K (2005) Fucoxanthin from edible seaweed, Undaria pinnatifida, shows antiobesity effect through UCP1 expression in white adipose tissues. Biochem Biophys Res Commun 332:392–397
Masuzaki H, Ogawa Y, Aizawa-Abe M, Hosoda K, Suga J, Ebihara K, Satoh N, Iwai H, Inoue G, Nishimura H, Yoshimasa Y, Nakao K (1999) Glucose metabolism and insulin sensitivity in transgenic mice overexpressing leptin with lethal yellow agouti mutation: usefulness of leptin for the treatment of obesity-associated diabetes. Diabetes 48:1615–1622. doi:10.2337/diabetes.48.8.1615
Acknowledgments
This work was supported by a grant of the Industry-Academia-Government Collaboration, Okinawa 2006.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Niwano, Y., Beppu, F., Shimada, T. et al. Extensive Screening for Plant Foodstuffs in Okinawa, Japan with Anti-Obese Activity on Adipocytes In Vitro . Plant Foods Hum Nutr 64, 6–10 (2009). https://doi.org/10.1007/s11130-008-0102-z
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11130-008-0102-z