Abstract
Ten postmenopausal women (age 55.6 ± 0.8 years, BMI 24.6 ± 1.1 kg/m2) ingested 25 g/day milled chia seed during a 7-week period, with six plasma samples collected for measurement of α-linolenic acid (ALA), eicosapentaenoic acid (EPA), docosapentaenoic acid (DPA), and docosahexaenoic acid (DHA). Subjects operated as their own controls with overnight fasted blood samples taken at baseline (average of two samples), and then after 1, 2, 3, 5, and 7 weeks supplementation. Plasma ALA increased significantly after one week supplementation and was 138 % above baseline levels by the end of the study (overall time effect, P < 0.001). EPA increased 30 % above baseline (overall time effect, P = 0.019) and was correlated across time with ALA (r = 0.84, P = 0.02). No significant change in plasma DPA levels was measured (overall time effect, P = 0.067). Plasma DHA decreased slightly by the end of the study (overall time effect, P = 0.030) and was not correlated with change in ALA. In conclusion, ingestion of 25 g/day milled chia seeds for seven weeks by postmenopausal women resulted in significant increases in plasma ALA and EPA but not DPA and DHA.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The n-3 fatty acids are a family of polyunsaturated fatty acids that have in common a final carbon-carbon double bond in the n-3 position [1]. The most common n-3 polyunsaturated fatty acids (n-3-PUFAs) are: α-linolenic acid (ALA; 18:3n-3), eicosapentaenoic acid (EPA; 20:5n-3), docosapentaenoic acid (DPA; 22:5n-3), and docosahexaenoic acid (DHA; 22:6n-3). The reluctance of U.S. adults to increase fish intake, and concerns over heavy metal accumulation in fish have accelerated interest in plant-derived sources of n-3 PUFAs (ALA) including flaxseed, walnuts, and algae [2, 3]. Plant n-3 PUFAs are abundant and readily available, and are often contained in foods that are high in dietary fiber and other components with potential health value.
The human body cannot synthesize n-3 fatty acids de novo, but can form EPA, DPA and DHA from ALA (especially when intakes of other plant oils rich in n-6 fatty acids are limited [4–6]. Reported ALA to EPA conversion rates vary between 0.2 to 21 %, depending on gender, age, and study methodology [4–6]. The conversion of ALA to DHA ranges from <1 to 9 % [7]. Women have a higher conversion rate of ALA to EPA and DHA when compared to men, with some of the gender difference related to a greater use of fatty acids for β-oxidation in men [5, 6].
Chia seed (Salvia hispanica L.) is an oilseed native to southern Mexico and northern Guatemala [8–11]. Chia seed has up to 4.11 g ALA, 6.73 g carbohydrates, 6.05 g protein, 141 kcal calories and 7.55 g of dietary fiber per 25 g serving. In our previous study, overnighted fasted plasma ALA increased 24.4 % after 12-weeks supplementation of whole chia seeds [12]. No statistically significant increase in plasma EPA or DHA was observed in the chia seed group. In that study, 76 subjects (28 men and 48 women, ages 20 to 70 years) were allowed to consume their normal diets without restrictions placed on fish and plant oil intake. These limitations in study design could have contributed to the variations in plasma responses.
We designed a study with a homogeneous population with tight control of the diet and serial collections of blood samples to investigate plasma responses in EPA, DPA, and DHA from ingesting ALA from milled chia seeds. We used milled chia seeds because other research with flaxseed suggests greater ALA delivery with milled compared to whole seeds [13]. The objective of the present study was to measure sequential plasma fatty acid responses to 7-weeks supplementation with milled chia seeds in ten postmenopausal women.
Materials and Methods
Subjects
Subjects included 10 postmenopausal women, ages 53–60 years who were recruited through local advertising. Subject inclusion criteria included the following: healthy without known disease; a body mass index (BMI) no greater than 35 kg/m2 and not currently on a weight reduction diet; a history of not using fish oil supplements or not consuming two or more fish dishes per week; willingness to avoid all forms of fish oil and flaxseed oil supplements, limit fish meat and sea food to no more than once per week, and restrict intake of plant oils rich in n-6 fatty acids such as sunflower, safflower, soybean, corn oil, and similar plant oils. Subjects were instructed to use moderate amounts of olive oil and canola during the study. Written informed consent was obtained from each subject, and the experimental procedures were approved by the institutional review board of Appalachian State University. We certify that all applicable institutional and governmental regulations concerning the ethical use of human volunteers were followed during this research.
Research Design
Subjects operated as their own controls with overnight fasted blood samples taken at baseline (two samples, two days apart), and then after 1, 2, 3, 5, and 7 weeks supplementation of 25 g milled chia seeds per day. Body weight, 3-day diet records, and questionnaire responses to assess potential adverse effects and adherence to the supplementation regimen were monitored prior to the study, and then again at 3 and 7 weeks after chia seed supplementation. Subjects agreed to follow their normal dietary and physical activity patterns during the 7-week study, and make no formal attempts to lose weight.
Chia Seed Supplements
Milled chia seeds were supplied by Chia Farms, Inc. (Orlando, FL). Subjects were instructed to consume 25 g of milled chia seeds per day for seven weeks by sprinkling the contents of the seed packets into fruit juices, yogurt, breakfast cereal, salads, and other dishes at one meal or spread throughout the day. Compliance to the supplementation regimen was monitored by counting the empty supplement packets that were requested from the subjects during blood sampling at three and seven weeks.
Blood Collection and Blood Pressure
Blood samples were drawn from an antecubital vein with subjects in the seated position for at least 15 min. Blood samples were drawn at 7:00–9:00 am, with all subjects having avoided food and beverage intake other than water for at least 9 h. Blood samples were centrifuged in sodium heparin tubes, plasma aliquots were snap-frozen in liquid nitrogen, and then stored at −80 °C prior to analysis.
Chia Seed Nutrient Analysis
Fatty acids from milled chia seeds were first extracted with hexane, than esterified and methyl esters were analyzed by gas chromatography time of flight mass spectrometry (GC-TOF-MS). Polyphenolic compounds from defatted milled chia seeds were extracted with methanol and analyzed by high performance liquid chromatography time of flight mass spectrometry (HPLC-TOF-MS). Milled chia seeds were digested with hydrochloric acid and sulfuric acid and the residue was dissolved for elemental analysis by inductively coupled plasma mass spectrometry (ICP-MS). Proximates including moisture, energy, protein, total lipid and total dietary fiber were assayed following multiple AOAC methods by Microbac Laboratories, Inc. (Wilson, NC).
Plasma ALA, EPA, DPA and DHA
Plasma samples were analyzed for ALA, EPA, DPA, and DHA as previously described [12] using an HP 6890 N gas chromatograph (Agilent Technologies, Palo Alto, CA) equipped with a 5975B Inert XL MSD mass spectrometer detector. A DB-23 GC column (60 m × 250 μm X 0.15 μm) from Agilent Technologies (Palo Alto, CA) was used to separate the methyl esters of the extracted fatty acids.
Food Records and Analysis
During orientation, a nutritionist instructed the subjects regarding completion of the 3-day food records. Subjects recorded 3-day food intake pre-study, and then prior to the 3- and 7-week blood sampling time points. The food records were analyzed using a computerized dietary assessment program (Food Processor, ESHA Research, Salem, OR).
Symptom Log
Subjects completed symptom logs pre-study, and after 3- and 7-weeks supplementations. The symptom logs included questions on digestive health (constipation, heartburn, bloating, diarrhea, and nausea), hunger levels (morning, afternoon, and evening), energy levels (morning, afternoon, and evening), sickness (fever, cough, sore throat, stuffy nose, runny nose, and headache), pain (joint, muscle, and back), allergies, stress level, focus/concentration, and overall well-being. Subjects indicated responses using a 12-point Likert scale, with 1 relating to “none at all”, 6 “moderate”, and 12 “very high”.
Statistical Procedures
Data are presented as mean ± SE. The two pre-study measures were averaged to represent baseline, and data from weeks 5 and 7 were averaged to represent end-of-study measures. Repeated measures ANOVA was used to calculate overall time effects for each of the fatty acids measured in this study (with five within subject time points). Changed from baseline was compared across each time point using paired t-tests with Bonferroni adjustment (P < 0.0125). Plasma fatty acids data were normalized through Z-score transformation so that all fatty acids are shown in the same scale for a clearer view of overall correlation (Fig. 2). To calculate Z score, each concentration was subtracted by the mean concentration of that fatty acid, and then divided by the standard deviation of that fatty acid. Pearson correlation coefficients were calculated between 1) ALA and EPA, 2) ALA and DPA, 3) ALA and DHA, with p < 0.03 indicating significance.
Results
Subjects (n = 10) ranged in age from 52 to 60 years (55.6 ± 0.8 years) with a BMI of 17 to 29 kg/m2 (24.6 ± 1.1 kg/m2). Subjects consumed all of the chia seed supplied to them for the study as assessed by each of the five laboratory visits following baseline testing. Nutrient data are presented in Table 1 for the milled chia seed supplement used in this study. These data indicate that the chia seed supplement was high in total fat (64.4 % of energy) and ALA, protein (17.2 % of energy), dietary fiber, and a variety of minerals. The predominate polyphenol in the chia seed supplement was protocatechuic acid.
Analysis of 3-day food records not including the chia seed supplement (pre-study, and after 3- and 7-weeks supplementation) showed that the percent of energy consumed as total fat did not change significantly (29.9 ± 1.9 %, 30.5 ± 2.5 %, and 29.1 ± 2.7 %, respectively). Dietary intake of ALA, EPA, DPA, and DHA fatty acids did not change during the 7-week study (data not shown), and EPA, DPA, and DHA were either absent or at low levels before and during the study. Symptoms for digestive health, hunger, energy level, illness, pain, allergies, stress, focus/concentration, and overall well-being as assessed by symptoms logs did not differ significantly between pre- and post chia seed supplementation (data not shown). Body mass did not change significantly during the 7-week study (pre-study 69.4 ± 13.8 kg, 3-weeks 69.3 ± 13.7 kg, and 7-weeks 69.1 ± 13.4 kg).
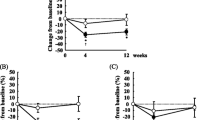
Mean changes from baseline in plasma ALA, EPA, DPA, and DHA are depicted in Fig. 1. Plasma ALA increased 138 % when comparing baseline with the average of weeks 5 and 7 (end of study) (overall time effect, P < 0.001), and was also significantly above baseline after 1-, 2-, and 3-weeks chia seed supplementation (all contrasts, P < 0.0125). Plasma EPA increased 30 % above baseline levels in the present study (overall time effect, P = 0.019), with significant elevations after 1 week and 5–7 weeks chia seed supplementation (both P < 0.0125). Individual plots for ALA and EPA indicated large variations both pre-study and in response to chia seed supplementation (data not shown).
Overnight-fasted plasma fatty acid concentration for subjects tested pre-study and during the 7-weeks supplementation with chia seeds. Values are mean ± SE (n = 10, μg/mL). * P < 0.0125 relative to the average of two pre-study measures. Abbreviations: α-linolenic acid (ALA), eicosapentaenoic acid (EPA), docosapentaenoic acid (DPA), and docosahexaenoic acid (DHA)
Figure 2 shows a plot of the normalized data (Z-score transformation) comparing the patterns of change of ALA and EPA on the same scale. Correlation data indicate a significant positive relationship between plasma EPA and ALA during the first 3-weeks of chia seed supplementation (r = 0.97, P = 0.01) and across all time points (r = 0.84, P = 0.02). The overall time effect for change in plasma DPA level was not significant (P = 0.067) (Fig. 1).
Plasma DHA was not different from baseline until the end of the study with a 12 % decrease measured (overall time effect, P = 0.030, with P = 0.049 for the end-of-study contrast). No statistically significant correlation was found between plasma ALA and DPA although a weak correlation was seen up to week 3 (r = 0.86, P = 0.06) (Fig. 2). For plasma DHA, a negative but insignificant correlation with ALA was found across all time points (r = −0.66, P = 0.11).
Discussion
Postmenopausal women consuming 25 g/day of milled chia seeds for seven weeks experienced significant increases in plasma ALA and EPA, but not DPA and DHA. The increase in plasma ALA with milled chia seed consumption occurred within the first week, and increased 138 % above baseline levels by the end of the study. The increase in plasma EPA was smaller proportionately (30 %), but was correlated across time with ALA.
Other studies using flaxseed supplements report plasma ALA increases of 40 to 160 % [2, 5, 13–15]. In our previous study with whole chia seeds (soaked 10 min in water), overnight fasted plasma ALA increased 24.4 % [12]. The greater increase measured in the present study may be related to several factors including the use of a milled chia seed supplement, a more homogeneous subject group, and tighter diet control [12]. In a previous study using flaxseed supplements, subjects receiving milled compared to whole flaxseed had significantly higher levels of plasma ALA [13]. These results suggest that ALA is more easily incorporated into human plasma from milled oil seeds (chia or flaxseed) than from whole seeds.
Plasma EPA increased 30 % above baseline levels in the present study, but individuals varied widely both pre-study and in response to milled chia seed supplementation. These data support previous findings that the metabolic distribution of ALA (i.e., tissue incorporation, EPA conversion, and energy substrate) varies substantially between individuals [14, 16]. After ALA supplementation, plasma EPA has been reported to increase 20–100 % depending on subject characteristics, dietary restraint, and the ALA dosing regimen [5, 6, 14, 15, 17]. The 30 % increase in plasma EPA in our subjects (postmenopausal women) following seven weeks of chia seed ALA supplementation (4.1 g/day) is comparable to other similar studies [2, 13].
Chia seed supplementation was not associated with significant increases in plasma DPA or DHA in our postmenopausal female subjects. Conversion of ALA to DPA and DHA has been studied in both animals and humans [5, 6, 14, 16–19]. Most previous studies report limited conversion of ALA to DPA in both men and women, and no conversion of ALA to DHA in men. Young women and postmenopausal women who are receiving hormone replacement treatment may convert ALA to DHA better than other subgroups [6]. In the present study, plasma EPA, DPA and DHA were not significantly higher in two of the 10 subjects who took sex hormone replacement medications (data not shown). We speculate that the restriction of fish and seafood products during our study may have contributed to the decrease in plasma DHA.
Conclusions
In summary, plasma ALA and EPA, but not DPA or DHA, increased significantly in 10 postmenopausal women after taking 25 g milled chia seeds/day for seven weeks. The pattern of increase in plasma ALA and EPA during milled chia seed supplementation was significantly correlated. The increase in plasma ALA in response to ingesting chia seed supplement reached its highest plateau after five weeks. The health implications of increasing plasma ALA through milled chia seeds or other plant sources are still being debated, but two randomized studies (10–12 weeks) by our research team have failed to show changes in disease risk factors including blood lipids, blood pressure, inflammation, oxidative stress, and arterial stiffness [12, 20]. A cardioprotective effect of high, long-term ALA intake has been suggested by a number of epidemiological studies, and an additive effect of ALA with n-3 long-chain PUFA from fish and fish oil has been observed [21].
Abbreviations
- ALA:
-
α-linolenic acid
- ANOVA:
-
Analysis of variance
- AOAC:
-
Association of Official Agricultural Chemists
- BMI:
-
Body mass index
- DHA:
-
Docosahexaenoic acid
- DPA:
-
Docosapentaenoic acid
- EPA:
-
Eicosapentaenoic acid
- GC-TOF-MS:
-
Gas chromatography time of flight mass spectrometry
- HPLC-TOF-MS:
-
High performance liquid chromatography time of flight mass spectrometry
- ICP-MS:
-
Inductively coupled plasma mass spectrometry
- n-3-PUFAs:
-
n-3 polyunsaturated fatty acids
References
Anderson BM, Ma DWL (2009) Are all n-3 polyunsaturated fatty acids created equal? Lipids Health Dis 8:33–52
Harper CR, Edwards MJ, Defilipis AP, Jacobson TA (2006) Flaxseed oil increases the plasma concentrations of cardioprotective (n-3) fatty acids in humans. J Nutr 136:83–87
Whelan J, Rust C (2006) Innovative dietary sources of n-3 fatty acids. Annu Rev Nutr 26:75–103
Burdge GC, Finnegan YE, Minihane AM, Williams CM, Wootton SA (2003) Effect of altered dietary n-3 fatty acid intake upon plasma lipid fatty acid composition, conversion of C-13 alpha-linolenic acid to longer-chain fatty acids and partitioning towards beta-oxidation in older men. Br J Nutr 90:311–321
Burdge GC, Jones AE, Wootton SA (2002) Eicosapentaenoic and docosapentaenoic acids are the principal products of alpha-linolenic acid metabolism in young men. Br J Nutr 88:355–363
Burdge GC, Wootton SA (2003) Conversion of alpha-linolenic acid to eicosapentaenoic, docosapentaenoic and docosahexaenoic acids in young women. Br J Nutr 88:411–420
Brenna JT, Salem N, Sinclair AJ, Cunnane SC (2009) Alpha-linolenic acid supplementation and conversion to n-3 long-chain polyunsaturated fatty acids in humans. Prostag Leukotr Ess 80:85–91
Ayerza R, Coates W (2007) Effect of dietary alpha-linolenic fatty acid derived from chia when fed as ground seed, whole seed and oil on lipid content and fatty acid composition of rat plasma. Ann Nutr Metab 51:27–34
Ayerza R, Coates W, Lauria M (2002) Chia seed (Salvia hispanica L.) as an omega-3 fatty acid source for broilers: influence on fatty acid composition, cholesterol and fat content of white and dark meats, growth performance, and sensory characteristics. Poult Sci 81:826–837
Chicco AG, D’Alessandro ME, Hein GJ, Oliva ME, Lombardo YB (2009) Dietary chia seed (Salvia hispanica L.) rich in alpha-linolenic acid improves adiposity and normalises hypertriacylglycerolaemia and insulin resistance in dyslipaemic rats. Br J Nutr 101:41–50
Vuksan V, Whitham D, Sievenpiper JL, Jenkins AL, Rogovik AL, Bazinet RP, Vidgen E, Hanna A (2007) Supplementation of conventional therapy with the novel grain Salba (Salvia hispanica L.) improves major and emerging cardiovascular risk factors in type 2 diabetes: results of a randomized controlled trial. Diabetes Care 30:2804–2810
Nieman DC, Cayea EJ, Austin MD, Henson DA, McAnulty SR, Jin F (2009) Chia seed does not promote weight loss or alter disease risk factors in overweight adults. Nutr Res 29:414–418
Austria JA, Richard MN, Chahine MN, Edel AL, Malcomson LJ, Dupasquier CMC, Pierce GN (2008) Bioavailability of alpha-linolenic acid in subjects after ingestion of three different forms of flaxseed. J Am Coll Nutr 27:214–221
Goyens PL, Spilker ME, Zock PL, Katan MB, Mensink RP (2006) Conversion of alpha-linolenic acid in humans is influenced by the absolute amounts of alpha-linolenic acid and linoleic acid in the diet and not by their ratio. Am J Clin Nutr 84:44–53
Taylor CG, Noto AD, Stringer DM, Froese S, Malcolmson L (2010) Dietary milled flaxseed and flaxseed oil improve n-3 fatty acid status and do not affect glycemic control in individuals with well-controlled type 2 diabetes. J Am Coll Nutr 29:72–80
Plourde M, Cunnane SC (2007) Extremely limited synthesis of long chain polyunsaturates in adults: implications for their dietary essentiality and use as supplements. Appl Physiol Nutr Metab 32:619–634
Pawlosky R, Hibbeln J, Lin Y, Salem N (2003) N-3 fatty acid metabolism in women. Br J Nutr 90:993–994
Vermunt SH, Mensink RP, Simonis MM, Hornstra G (2000) Effects of dietary alpha-linolenic acid on the conversion and oxidation of 13 C-alpha-linolenic acid. Lipids 35:137–142
Welch AA, Shakya-Shrestha S, Lentjes MA, Wareham NJ, Khaw KT (2010) Dietary intake and status of n-3 polyunsaturated fatty acids in a population of fish-eating and non-fish-eating meat-eaters, vegetarians, and vegans and the precursor-product ratio of alpha-linolenic acid to long-chain n-3 polyunsaturated fatty acids: results from the EPIC-Norfolk cohort. Am J Clin Nutr 92:1040–1051
Nieman DC, Gillitt N, Jin F, Henson DA, Kennerly K, Shanely A, Ore B, Su MM, Schwartz S (2012) Chia seed supplementation and disease risk factors in overweight women: a metabolomics investigation. J Alt Comp Med (in press).
Vedtofte MS, Jakobsen MU, Lauritzen L, Heitmann BL (2011) Dietary α-linolenic acid, linoleic acid, and n-3 long-chain PUFA and risk of ischemic heart disease. Am J Clin Nutr 94:1097–1103
Acknowledgments
We acknowledge the assistance of Dustin Dew of Appalachian State University and Tondra Blevins of the University of North Carolina at Chapel Hill for their assistance in this project. We also acknowledge Raymond P. Glahn, Michael A. Rutzke, YongPei Chang, and Mary Bodis from the USDA/ARS at Cornell University for their assistance in the ICP-MS mineral analysis of the milled chia seed supplement. Financial support was provided in part by Chia Farms, Inc. (Orlando, FL, USA).
Conflict of Interest
All authors declare no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Jin, F., Nieman, D.C., Sha, W. et al. Supplementation of Milled Chia Seeds Increases Plasma ALA and EPA in Postmenopausal Women. Plant Foods Hum Nutr 67, 105–110 (2012). https://doi.org/10.1007/s11130-012-0286-0
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11130-012-0286-0