Abstract
Purpose
The primary endpoint was to determine the plasma concentration of alpha-linolenic acid (ALA), and its metabolites, following milled flaxseed consumption at four doses. Secondary outcomes focused on plasma enterolignan concentrations and the effects on tolerability, platelet aggregation, plasma lipids and urinary thromboxane levels.
Methods
Healthy, younger adults (n = 34; 18–49 years old) were randomized into four groups consuming one muffin daily for 30 days fortified with 10, 20, 30 or 40 g of milled flaxseed. Blood and urine were collected at baseline and 4 weeks.
Results
Plasma ALA concentrations increased with all flaxseed doses (P < 0.01), except the 20 g/day dose (P = 0.10), yet there was no significant dose-dependent response (P = 0.81). Only with the 30 g/day diet were n-3 polyunsaturated fatty acids (P = 0.007), and eicosapentaenoic acid (EPA) (P = 0.047) increased from baseline values. Docosapentaenoic acid and docosahexaenoic acid were not detected at any dose. Plasma total enterolignan concentrations significantly increased over time in all treatment groups, yet despite a dose-dependent tendency, no between-group differences were detected (P = 0.22). Flaxseed was well tolerated, even at the highest dose, as there were no reported adverse events, changes in cholesterol, platelet aggregation or urinary 11-dehydro-thromboxane B2.
Conclusions
In healthy, younger adults, 10 g/day of milled flaxseed consumption is sufficient to significantly increase circulating ALA and total enterolignan concentrations; however, 30 g/day is required to convert ALA to EPA. Although all doses were well tolerated, 40 g/day is too low to attenuate cholesterol in this population.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Flaxseed (Linum usitatissimum), an oilseed predominantly produced in North America, has gained interest as a potential therapeutic agent in the treatment of risk factors for cardiovascular disease (CVD) [1, 2]. Animal studies involving dietary flaxseed have demonstrated improved vascular reactivity [3], inhibition of atherosclerosis [4], regression of preexisting atherosclerotic plaques [5], inhibition of arrhythmias during ischemia/reperfusion injury [6] and reduced plasma cholesterol [7]. Human trials have shown antihypertensive [8, 9] and cholesterol-lowering effects [10, 11] of dietary flaxseed.
Several bioactive components within flaxseed are thought to provide these beneficial cardioprotective properties. Alpha-linolenic acid (ALA; C18:3n-3) is an n-3 fatty acid that is enriched in flaxseed. Approximately 50 % of the overall total fatty acid content consists of ALA [1]. Several clinical studies have demonstrated the antihypertensive properties of dietary ALA [12–14]. Flaxseed also contains the highest amount of the plant-based lignan, secoisolariciresinol diglucoside (SDG) [15]. Next highest food sources of SDG include asparagus (180-fold less) [16], rye and wheat (360- and 870-fold less, respectively) [17], followed by various nuts [15] and legumes [18]. The SDG metabolites, enterodiol (END) and enterolactone (ENL) are enterolignans that undergo enterohepatic circulation in vivo. They are initially formed in the gut, are absorbed by the intestines, conjugated within the liver, excreted in bile and then reabsorbed and repackaged as sulfate or glucuronide conjugates in the liver [19, 20]. These are the bioactive forms of SDG that circulate systemically [21] and demonstrate antioxidative properties in vitro [22] and in vivo [23]. Clinical studies using dietary SDG or high-lignan flaxseed have demonstrated improved glycemic control [24], reduced plasma cholesterol and glucose concentrations [25] as well as lowered diastolic blood pressure [26]. Flaxseed is also 28 % dietary fiber by weight [27]. Dietary fiber can reduce circulating levels of cholesterol [28], which is also a predictive risk factor for CVD.
Since flaxseed contains these important bioactives in large quantities, gaining an understanding of the factors that will optimize their presence in plasma is critical to optimize their health-related actions. Flaxseed form is one factor that can influence plasma concentration. Plasma ALA and enterolignan concentrations are improved when the seed is milled compared to its whole form [29, 30]. However, the optimal dose of milled flaxseed to provide significant concentrations of ALA and enterolignan within a healthy human population has not yet been defined. Most human studies use upwards of 25 g/day of flaxseed [8, 27, 29, 31–35] with as much as 50 g/day [36, 37]. Studies using flaxseed at doses <25 g/day are limited [38–41]. Therefore, the primary endpoint of this study was to investigate the concentration of ALA in plasma as a function of the dosage of milled flaxseed with secondary endpoints focused on enterolignans and the associated effects of flaxseed ingestion on blood lipids, platelet aggregation, urinary thromboxane levels and tolerability. Although flaxseed has attained generally recognized as safe (GRAS) status [42], other reports using milled flaxseed in a dose-dependent manner do not include incidences of gastrointestinal-related adverse events [41, 43, 44] other than increased laxation [40]. We hypothesized that plasma ALA, its longer chain metabolites eicosapentaenoic acid (EPA) and docosapentaenoic acid (DPA) but not docosahexaenoic acid (DHA), and enterolignan concentrations would be significantly elevated in a dose-dependent manner. Additionally, adverse event reporting and attenuations in platelet aggregation, total cholesterol and LDL cholesterol and urinary thromboxane levels would be most pronounced at 40 g/day of administered flaxseed.
Materials and methods
Study participants
Healthy subjects, male and female, 18–49 years of age were recruited. Younger adults (<50 years) were selected as a representative healthy population as they are less likely to be using prescription medications for chronic disease management, as are older adults (≥50 years). In addition, maintaining participant age within the indicated range reduced confounding factors from age-related conditions. All subjects provided written informed consent prior to recruitment including any study-related screening procedures, and without restriction to gender, race or socioeconomic status. During screening, interested individuals provided information related to age, height and weight and were asked to provide a complete medical history inclusive of current medications, vitamins or herbal remedies. Participants could be included in the study if they were deemed healthy by a physician, were willing to refrain from taking any vitamin, herbal or oil supplements for 1 month prior to and during the course of the study, were not eating fish more than one time per week, were not eating flaxseed fortified foods and were willing to comply with protocol requirements. Subjects were instructed by the study coordinator to continue with their normal dietary patterns throughout the course of the study bearing in mind the inclusion criteria. Individuals were required to store the flaxseed-enriched food products in a −20 °C freezer for a 4-week period and be willing to consume one thawed food product daily for the 1-month duration of the study. A daily diary was also required to record muffin consumption dates and times as well as any comments or possible adverse events. Subjects were excluded from this study if any of the following criteria were met: cigarette smoking (tobacco products within the last 6 months), ingestion of supplementary vitamins or oils or more than one fish meal/week for 1 month prior to the start of the study, planned use of any herbal/antioxidant/fatty acid/nutritional supplements at any time in the duration of the study, were pregnant or planning to become pregnant, experienced diabetes, cardiovascular, renal or gastrointestinal problems, were using cholesterol-lowering drugs, hypertension drugs, antihistamines or hormone therapy, and experienced platelet abnormalities or abnormal blood clotting/bleeding times. Similarly, subjects were excluded if they did not adhere to study diet requirements. Two visits to the clinic would be required during the course of the study. The baseline visit (visit 1) would involve the individuals providing a fasted blood sample after which time they were randomized using a computer-generated randomization procedure into one of the four dietary groups and given a 1-month supply of the diet. The final visit (week 4) also required a fasting blood sample. Individuals were asked to submit their record books as well as any remaining supply of the diet. Both visits also discussed any changes in individual medical histories. Blood samples were analyzed for any changes in lipids (cholesterol, triglycerides, fatty acids), lignan metabolites and platelet aggregation. Urine was analyzed for 11-dehydro-thromboxane B2 concentrations.
Ethics and study design
The University of Manitoba Research Ethics Board and the St. Boniface Hospital Research Review Committee approved the study design, which was done in accordance with the ethical standards established in the 1964 Declaration of Helsinki and its amendments thereafter. A total of 40 healthy, male and female volunteers were recruited. Ten subjects were randomized into one of four study groups. Subjects consumed one muffin per day containing a distinct dose of either 10 g, 20 g, 30 g or 40 g of milled flaxseed, respective of study group. Study participants were asked to thaw and consume one muffin per day for a total of 4 weeks. Both the research team and the subject were blinded to the flaxseed dosage group from the time the subject was randomized until completion of the study, which included data analysis.
Experimental diet
The Canadian International Grains Institute, Winnipeg, Canada, prepared the muffins for this study. Muffins were stored in sealed plastic bags at −20 °C. Organic milled flaxseed was supplied by Bioriginal Food and Science Corporation (Saskatoon, SK, Canada). The same batch was used to prepare all study muffins. Muffins were offered in two different flavors: banana chocolate chip and cranberry–orange. Formulation details and a sensory evaluation of the cranberry–orange muffin (30 g) have been reported [45]. The calculated ALA and SDG contents for each 10 g addition of milled flaxseed are represented in Table 1 as are the formulation, energy and nutrient compositions of the diets.
Compliancy
Only one subject in the 10-g group withdrew from the study and that was done so voluntarily prior to the first blood draw. Subjects were asked to maintain a treatment diet record containing the dates that they consumed the muffin and the time of day at which it was consumed. These were submitted and collected at their final appointment. In addition, study foods that were not consumed were returned to the study coordinator who recorded this. Individuals were deemed compliant if at least one of ALA, END or ENL increased significantly in circulating plasma.
Sample collection and processing
Fasted venous blood samples (15 ml) were collected at both baseline and 4 weeks. Blood was collected in a total of three tubes. One tube contained 1 mg EDTA/ml which was centrifuged at 1800×g for 5 min at 4 °C after which the plasma was aliquoted into several cryovials (VWR International, Mississauga, ON) (P/N 479-0822). Samples were flash frozen in liquid nitrogen and stored at −80 °C until subsequent lipid and lignan metabolite analyses could be performed. Blood collected into the two sodium citrate tubes (10 ml) was gently inverted several times and maintained at room temperature for 30 min prior to immediate preparation for platelet aggregation measurements.
Urine was collected into certified urine sample containers (VWR International, Mississauga, ON) (P/N CA73240-106) at both 0 and 4 weeks at St. Boniface Hospital. The study coordinator then immediately transferred the samples on ice for storage at −80 °C.
Blood and urine measurements
Plasma fatty acids were extracted from thawed plasma aliquots and derivatized to their methyl esters using the method of Lepage and Roy [46]. Plasma fatty acid methyl esters (FAME’s) were quantified using an Agilent CP-3800 gas chromatograph (GC), equipped with a flame ionization detector (FID) and Agilent CP-Sil 88 capillary column (60 m × 0.25 mm × 0.20 μm). The methyl esters, in toluene (1 μl), were injected using a CP-8400 autosampler at a split ratio of 1:50. The flow rate of the helium carrier gas was 1.5 ml/min. The initial oven temperature was programmed at 80 °C and was held for 1 min. It was then raised 30 °C/min to 140 °C, further increased by 5 °C/min to 225 °C and held there for 10 min. The total run time for each sample was 30 min. C17:0 was used as internal standard, and the fatty acid contents of the sample were identified by comparison with an authentic standard, GLC-462 (Nu-Chek Prep, Inc., Elysian, MN, USA).
A detailed description of plasma enterolignan extraction and measurement has been described previously using a method developed and validated within our laboratory [47]. Briefly, 300 μL of human plasma, 300 μL of sodium acetate buffer (0.1 M, pH 5.0) and 60 μL of 2600 units β-glucuronidase–sulfatase from H-Pomatia (G1512) in 0.5 M, pH 5.0 sodium acetate solution were added to a silanized vial containing 1 μM hexadeuterated enterodiol (2H6-END) and hexadeuterated enterolactone (2H6-ENL) as internal standards. The solution was incubated at 37 °C for 4 h, and the resulting mixture separated using an Isolute SLE + 1 ml supported liquid extraction (SLE) column (Biotage, Charlotte, NC, USA), contained on a 24-port vacuum manifold, using 70:30 diethyl ether:ethyl acetate (4 × 1.25 ml). Following solvent evaporation under a steady stream of nitrogen gas with tube bases warmed to 37 °C, the analytes were silanized to their trimethylsilyl derivatives using 120 μl of pyridine and 120 μl of N,O-bis(trimethylsilyl)trifluoroacetamide (BSTFA). The reaction proceeded at 90 °C for 30 min, and the cooled samples were analyzed using gas chromatography/mass spectrometry in micro-selected ion storage mode (GC/MS-μSIS).
Total cholesterol (total-C) and triglycerides (TG) were measured enzymatically using commercial kits from Point Scientific Inc. (Canton, MI, USA). High-density lipoprotein cholesterol (HDL-C) measurements were taken enzymatically using a BioVision commercial kit (Milpitas, CA, USA). Low-density lipoprotein cholesterol (LDL-C) was calculated using the Friedewald equation [48].
Blood collected into sodium citrate tubes was gently inverted and then stored at room temperature for 30 min after which platelet aggregation studies were immediately performed. Platelet-rich plasma (PRP) was obtained by a low-speed centrifugation of the samples at 100×g for 15 min. Removal of the upper PRP layer and further centrifugation of the remaining blood at 2400×g for 15 min yielded the platelet-poor plasma (PPP) which would behave as the blank for the aggregation experiments. Equal volumes of PRP and PPP were aliquoted into individual cuvettes. Collagen (4 μg/ml) or 0.5 units of thrombin was then added to the PRP in separate controlled experiments. Both the PRP and PPP were stirred, and spectrophotometric changes were monitored upon the addition of the aggregate using a Chrono-log Aggregometer, model 490 (Chrono-log Corp.). The Aggro/Link (vs 4.75) software was used to determine both percent aggregation and rate of aggregation.
Urine creatinine was measured using a modified Jaffe method based on the creatinine–picrate reaction [49]. The thromboxane B2 metabolite, 11-d-TxB2 (11-dehydro-TxB2), was assayed using stable isotope dilution negative ion/chemical ionization gas chromatography mass spectrometry as previously described [50]. Final results are presented as 11-d-TxB2 in ng/g of creatinine.
Adverse effects monitoring
During the entire study, secondary adverse events associated with consuming dietary flaxseed (i.e., gastrointestinal discomfort, diarrhea, excessive gas, cramping) were monitored. These were grouped and classified using Palmer’s method of adverse event monitoring where symptom severity was graded from 0 to 4: 0 = none, 1 = mild, 2 = moderate, 3 = severe and 4 = death [51]. An adverse event was considered mild if it was transient and inconsequential, whereas it was considered moderate if it was persistent and/or systemic. A severe adverse event would be life threatening or require hospitalization and death if an individual died from the direct consumption of flaxseed.
Outcomes and sample size calculations
The primary endpoint was to determine the change in plasma ALA concentration within and between groups after 4 weeks of consuming various doses of milled flaxseed. Secondary endpoints included changes in plasma enterolignan, cholesterol, triglyceride, fatty acid and urinary thromboxane concentrations as well as percent and maximum rate of aggregation of platelets. Each individual at 0 week behaved as their own control for the study. Accounting for an estimated 20 % sample loss, a sample size of 10 per group was assigned that would provide a minimum of 80 % power to detect any significant differences in ALA between the groups. A two-tailed α of 0.5 was used with an estimated standard deviation of 35 μM based on published results [32].
Statistical analysis and efficacy
Results are represented as means ± the standard error of the mean (SEM). All data were analyzed using IBM SPSS Statistics version 22.0 (International Business Machines, Armond, NY, USA). Categorical variables were compared with a χ 2 test. A repeated measure ANOVA, using the Greenhouse–Geisser test, was used to detect group effects and time and group by time interactions. Time was the repeated measure with two levels set at 0 and 4 weeks, and group was the fixed factor. A Tukey multiple comparison post hoc test was used to detect significant differences between groups. All biomarkers were measured as a function of time and their interactions, or lack thereof, were determined using averaged values applied to bi-plots with visualization of interactions determined by intersecting lines. Paired t tests were used to compare changes between baseline and end of treatment, and a one-way ANOVA was used to compare mean values across the same time point. Differences were considered significant when P values were ≤0.05. Trends were noted if P ≤ 0.1. The efficacy of the data was included in the statistical analysis if subjects had consumed a minimum of 80 % of the supplied muffins, donated blood samples at both time points and maintained study instructions as originally detailed during their screening visit.
Results
Participant characteristics and anthropometric measurements at baseline
Ninety-eight percent of the enrolled subjects indicated that they had successfully completed the dietary study; however, only 85 % (34 subjects) were included in the data analysis. One participant in the 10-g group dropped out of the study prior to starting the diet. Another five individuals had to be excluded from the study due to various reasons. Three individuals, one from each of the 20-, 30- and 40-g groups, were removed as baseline plasma ALA concentrations were elevated (>200 μM). Baseline concentrations of ALA are typically <75 μM for healthy, age-matched adults in the Winnipeg vicinity [29, 32]. Consumption of flaxseed-based supplements prior to study onset was previously established as part of the exclusion criteria. Two individuals were also excluded from the 40-g group as they had no detectable ALA (limit of detection, LOD = 1.1 μM) or enterolignans (LOD for END = 9.9 nM and ENL = 10.1 nM) in their plasma after 4 weeks strongly suggesting non-adherence. Therefore, a total of 34 individuals were included in the study (Table 2). Power calculations were performed again on each of the groups to ensure that 80 % power was still being attained for ALA. Using the same criteria as described previously, a minimum of 80 % power was achieved for ALA in each of the four groups. Patient characteristics at baseline, namely age, gender distribution, BMI, weight and height, were similar between all four of the intervention groups (P > 0.05) (Table 2).
Plasma fatty acid content
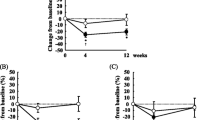
Plasma ALA concentrations were measured as the primary endpoint in the present study and significantly increased over time in the 10, 30 and 40 g/day dosage groups after 4 weeks of consuming milled flaxseed (P < 0.01) (Fig. 1a). A similar trend was observed in the 20 g/day group (P = 0.096). Each 10-g increment in flaxseed led to a mild dose-dependent response as observed by the 1.5-, 1.6-, 1.7- and 1.9-fold increases in plasma ALA concentrations after 4 weeks on the respective diets. The remaining fatty acids, as percent total fatty acids, were analyzed using repeated measures ANOVA (Table 3). No significant main effects for time or group were observed in total saturated fatty acids (SFA’s), total monounsaturated fatty acids (MUFA’s) or omega-6 polyunsaturated fatty acids (n-6 PUFA’s). However, n-3 PUFA’s increased after 4 weeks compared to baseline in all study groups, with significance achieved in the 30 g/day group (P = 0.007). This increase in n-3 PUFA’s led to an attenuation in the n-6/n-3 ratio in the 10 g/day (P = 0.045), 30 g/day (P < 0.01) and 40 g/day (P = 0.008) groups. Eicosapentaenoic acid (EPA, C20:5n-3) significantly increased after 4 weeks of consuming 30 g/day of milled flaxseed (P = 0.047), but not with 40 g/day (P = 0.12). No significant differences between groups or changes over time were observed for either docosapentaenoic acid (DPA, C22:5n-3) or docosahexaenoic acid (DHA, C22:6n-3).
Plasma ALA (a) and enterolignans (b–d) at baseline and following 4 weeks of consuming 10, 20, 30 or 40 g/day of milled flaxseed. There were no between-group differences, P ≥ 0.05. Within-group changes over time, † P < 0.05, ‡ P < 0.01. ALA alpha-linolenic acid, END enterodiol, ENL enterolactone, END + ENL total enterolignans, wk week
Plasma lignan metabolite content
One individual in the 20 g/day milled flaxseed group was taking antibiotics for the first 11 days of the study and was therefore excluded from all enterolignan measurements as antibiotics are known to interfere with the gut micro-flora required to produce these compounds [52]. As expected, this individual had no measurable plasma ENL and only trace amounts of END. Baseline enterolignan concentrations were consistent between all study groups (P > 0.05). Each of the plasma enterolignans increased over time as represented by a significant main effect for time determined using repeated measures ANOVA: END (F = 31.026, P < 0.001), ENL (F = 15.392, P < 0.001) and END + ENL (F = 47.738, P < 0.001). Despite these increase over time, no group differences in plasma enterolignan concentrations were observed as a result of increased flaxseed consumption: END (F = 0.58, P = 0.63), ENL (F = 1.32, P = 0.29) and END + ENL (F = 1.58, P = 0.22). When mean 4-week values were compared to baseline concentrations in each of the flaxseed dosage groups, END increased significantly in the 10 g/day (P = 0.041), 20 g/day (P = 0.014) and 30 g/day (P = 0.005) groups with a trend in the 40 g/day group (P = 0.073) (Fig. 1b). Final END concentrations in the 20 g/day (213.5 ± 63.2 nM), 30 g/day (209.1 ± 46.6 nM) and 40 g/day (206.4 ± 93.5 nM) groups were practically doubled compared to those in the 10 g/day (114.5 ± 45.7 nM) group (Fig. 1b). ENL was significantly increased in the 10 g/day (P = 0.018) and 20 g/day (P = 0.040) groups with trends in the 30 g/day (P = 0.055) and 40 g/day (P = 0.063) groups (Fig. 1c). Final ENL concentrations were highest in the 30 g/day group (303.8 ± 126.5 nM) and lowest in the 10 and 20 g/day groups (106 nM) (Fig. 1c). Subsequently, total enterolignans (END + ENL) increased significantly in all four flaxseed groups (P < 0.05) (Fig. 1d). Overall, a 5- to 31-fold increase in plasma enterolignan concentration was detected following 1 month of milled flaxseed consumption.
Plasma cholesterol and triglyceride content
Healthy, younger adults consuming various doses of milled flaxseed did not exhibit any significant changes after 1 month in total-C, LDL-C, HDL-C, triglycerides or in the total-C/HDL-C ratio (Table 4). However, a trend for a main effect over time was measured for total-C concentrations (P = 0.058). Significant group differences were detected for both total-C and LDL-C. This was attributed to significantly attenuated baseline values in the 40 g/day flaxseed group relative to the 20 g/day group for total-C (P = 0.030) and LDL-C (P = 0.047).
Platelet aggregation
The maximum platelet aggregation potential of plasma from individuals who had consumed 10–40 g/day of milled flaxseed was examined (Table 4). No differences in percent aggregation or rate of aggregation were observed between groups when either collagen (4 μg/ml) or thrombin (0.5 units) was used as agonists. However, a main effect for time was detected in percent aggregation with collagen, which was attributed to the 40 g/day group as aggregation was inhibited after 4 weeks as displayed by a trend in our data (P = 0.058). Rate of aggregation over time was unaffected by collagen. No significant changes over time in platelet aggregation were observed when thrombin was used.
Urine 11-d-TxB2
No changes over time or differences between groups were observed in 11-d-TxB2 (ng/g of creatinine) concentrations (P ≥ 0.05) (Table 4).
Adverse events
There were no significant differences between the four dosage groups in the number of reported adverse events as assessed by Chi-square tests (data not shown).
Discussion
This study demonstrates that consuming doses of milled flaxseed higher than 10–20 g/day may be unnecessary for improving plasma ALA and enterolignan concentrations in healthy adults with no preexisting clinical evidence of disease. It was also determined that milled flaxseed doses ranging from 10 to 40 g/day do not impact blood lipids, platelets, urinary thromboxane markers or adverse event reporting in individuals who are younger and already healthy.
ALA concentration in plasma is a measure of tissue availability. In the present study, significant increases in plasma ALA were observed after 4 weeks in all treatment groups but the 20 g/day group. This may be attributed to elevated baseline ALA concentrations in this group. A larger sample size would likely resolve this. Increased serum or plasma ALA concentrations, as a result of milled flaxseed consumption, have been reported in a number of human studies using doses as low as 13 g/day [41], 26–30 g/day [8, 29, 32, 41], 40 g/day [27, 33, 53] and as high as 50 g/day [36]. Although a dose-dependent fold increase in ALA was calculated with each 10-g increment in milled flaxseed, it was surprising that there were no significant differences between any of the treatment groups. In a crossover trial involving lupus nephritis patients, flaxseed was administered at doses of 15, 30 or 45 g/day over a similar 4-week period [40]. Unfortunately, these authors did not define the flaxseed form (i.e., milled or whole) used in the trial, so it is difficult to fully compare these two studies. However, no differences in circulating ALA were detected between any of the flaxseed treatment groups, similar to our study. In another trial involving overweight, prediabetic individuals, plasma ALA concentrations significantly increased in a dose-dependent manner when a diet containing 0, 13 and 26 g/day of milled flaxseed was ingested for 12 weeks [41]. This would suggest that the health status of the subject, and not the treatment duration [8, 29], may influence ALA concentration as a result of flaxseed dose. As doses ≥30 g/day were not examined [41], it is unknown whether higher doses would elevate plasma ALA concentrations beyond those achieved with the 26 g/day dose.
Another interest of the present study was to determine whether ALA metabolism, to the longer chain polyunsaturated fatty acids EPA, DPA and DHA, could be influenced in a dose-dependent manner as a result of milled flaxseed dose. It was surprising that only 30 g/day, and not 10, 20 or 40 g/day, significantly increased circulating EPA concentrations. Plasma EPA concentration, as a result of milled flaxseed dose, has only increased in one other human study to our knowledge at this same dose [8] yet not in others [29, 32]. Other studies using both higher doses (40 g/day) [27, 53] and lower doses (13 and 26 g/day) [41] of milled flaxseed found no significant changes compared to placebo. However, when EPA was measured in plasma or serum phospholipids, it increased significantly when the flaxseed dose was 30 g/day [40, 54] and 50 g/day [36], but not 15 g/day [40]. These results, along with ours, suggest that milled flaxseed doses ≥40 or <30 g/day are insufficient to elevate plasma EPA concentrations, yet do appear more favorable in improving plasma phospholipid concentrations. Interestingly, the same ALA doses administered as pure ALA or added to a variety of food products in the form of flax oil, all increased EPA concentrations in plasma [55–57], serum [58], plasma phospholipids [54] and erythrocyte phospholipids [59]. This suggests that ALA taken as milled flaxseed is not an efficient approach to increase circulating EPA concentrations. Unfortunately, DPA was not reported in most of these studies, yet in both trials that used 40 g/day of milled flaxseed, despite no measurable changes in EPA, significant increases in plasma DPA were detected [27, 53]. When plasma phospholipids were measured, only the 50 g/day dose increased DPA [36]. Future studies using dietary milled flaxseed should report plasma DPA concentrations as DPA can inhibit platelet aggregation [60] and angiogenesis [61] and can improve endothelial cell migration and proliferation [62]. The null effect of flaxseed dose on DHA is consistent with other clinical trials noting the poor conversion of ALA to DHA in humans [8, 27, 29, 32, 36, 41, 53, 54]. The benefits of reducing the n-6/n-3 ratio as in the present study, or conversely improving the n-3/n-6 ratio, have also been demonstrated as this ratio has been correlated with improved plaque composition, reduced progression and improved regression of plaques within the coronary arteries [63].
This is the first study to examine milled flaxseed dose on plasma concentrations of END and ENL. Similar to ALA, we were interested in the dose of milled flaxseed that would provide maximum concentrations of circulating enterolignans and whether increased doses would yield a dose-dependent response. Although dietary consumption of milled flaxseed increased plasma lignan metabolites in all four intervention groups, it was once again surprising that no between-group differences were observed. An apparent dose-dependent response in total enterolignan concentrations was observed when doses of 10–30 g/day were consumed (Fig. 1d), yet not at 40 g/day. As no group differences existed, this would suggest that 10 g/day of milled flaxseed is sufficient to significantly increase all reported enterolignans in the plasma of healthy adults. Similar to ALA, higher doses of 40 g/day may be unnecessary to achieve maximum circulating plasma enterolignan concentrations. Flaxseed dose may, however, exhibit a dose-dependent response in urinary enterolignan excretion [43, 44]. It would seem necessary that future studies include measurements for both urinary enterolignan output and circulating plasma concentrations as both values would be useful in assessing milled flaxseed dose.
High serum ENL concentrations have been associated with reduced mortality due to cardiovascular-related diseases [64]. It was hypothesized that the increased bulk fiber coming from higher flaxseed dose would alter transit time thereby converting more END to ENL. The ratio of END to ENL as a factor of flaxseed dose, however, remained relatively constant between all four groups despite the increased fiber content which is consistent with similar reports at a 25 g/day dose [65].
BMI and self-reported incidences of adverse events were also analyzed in relation to milled flaxseed dose. Averaged BMI’s did not change within or between groups after consuming any of the four flaxseed doses. As the total fat content of muffins increased with each 10 g addition of milled flaxseed, it was important to see this null effect on BMI. Furthermore, participants reported only mild to moderate adverse events when consuming milled flaxseed with no particular difference noted at any dose. It was hypothesized that most of the adverse events such as bloating, increased flatulence or cramping would be reported with the highest flaxseed dose due to its elevated fiber content. However, there were no statistical differences in adverse event reporting between groups within this study of healthy, young adults.
Health Canada has a cholesterol-lowering health claim for 40 g/day of flaxseed [66]. This was based on studies at this dose in postmenopausal [67, 68], menopausal [35] and hypercholesterolemic [27] adults. Other studies using 30 g/day of milled flaxseed report no effect in healthy adults [29, 32] and significant decreases in total cholesterol and LDL cholesterol in patients with peripheral artery disease [11]. As the participants in the present study were healthy and younger, it was not surprising to see no changes in these two lipid variables at doses ≤30 g/day. However, 40 g/day was still too low, despite having the highest fiber load. Only higher doses of 50 g/day have been shown to provide cholesterol-lowering effects in healthy, young adults [37].
Platelet aggregation, a critical process in wound healing, was explored in relation to flaxseed dose. It was hypothesized that platelet aggregation would be inhibited with higher milled flaxseed dose as flaxseed oil at higher doses has been shown to dramatically reduce platelet activity [69]. Percent aggregation was only mildly attenuated with 40 g/day of flaxseed when collagen (P = 0.058), not thrombin, was used as the agonist. This lack of change in rate and extent of platelet aggregation with increasing amounts of milled flaxseed is very important for individuals that desire the health benefits of flaxseed, yet have or anticipate problems with bleeding times. These results confirm that consuming increased doses of milled flaxseed (10–40 g/day) does not affect bleeding times in healthy individuals which is critical for patients requiring surgery or that may have a predisposition to cardiovascular or thrombotic diseases.
In conclusion, this is the first study to present the effects of milled flaxseed dose, ranging from 10 to 40 g/day, on circulating concentrations of n-3 fatty acids, enterolignans and blood lipids, in healthy, younger adults. Additionally, it provides insight into the safety of consuming such doses in relation to reported adverse events, platelet aggregation and urinary thromboxane concentrations. The results indicate that milled flaxseed doses as low as 10 g/day may be sufficient to significantly increase circulating ALA, END, ENL and total enterolignan concentrations and that higher doses may be unnecessary. However, only 30 g/day of milled flaxseed significantly improved plasma concentrations of EPA and total n-3 PUFA content. DPA and DHA were unaltered by any dose. Total cholesterol and LDL cholesterol were unchanged at even the highest flaxseed dose, suggesting that 50 g/day [36], not 40 g/day, is required to reduce cholesterol levels in this study population. However, in older adults presenting lipid abnormalities, lower flaxseed doses of 40 g/day [66] and 30 g/day [11] were successful in lowering cholesterol levels. Dosing studies in elderly, healthy adults and elderly adults with elevated cholesterol levels are warranted as they are most at risk of developing CV-related diseases. With few reported adverse events, and minimal changes in platelet aggregation and urinary thromboxane markers, milled flaxseed at both lower (10 g/day) and higher doses (40 g/day) appears safe for consumption in healthy, younger adults.
References
Prasad K (2009) Flaxseed and cardiovascular health. J Cardiovasc Pharmacol 54:369–377. doi:10.1097/FJC.0b013e3181af04e5
Bloedon LT, Szapary PO (2004) Flaxseed and cardiovascular risk. Nutr Rev 62:18–27
Dupasquier CM, Weber AM, Ander BP, Rampersad PP, Steigerwald S, Wigle JT, Mitchell RW, Kroeger EA, Gilchrist JS et al (2006) Effects of dietary flaxseed on vascular contractile function and atherosclerosis during prolonged hypercholesterolemia in rabbits. Am J Physiol Heart Circ Physiol 291:H2987–H2996. doi:10.1152/ajpheart.01179.2005
Dupasquier CM, Dibrov E, Kneesh AL, Cheung PK, Lee KG, Alexander HK, Yeganeh BK, Moghadasian MH, Pierce GN (2007) Dietary flaxseed inhibits atherosclerosis in the LDL receptor-deficient mouse in part through antiproliferative and anti-inflammatory actions. Am J Physiol Heart Circ Physiol 293:H2394–H2402. doi:10.1152/ajpheart.01104.2006
Francis AA, Deniset JF, Austria JA, LaVallee RK, Maddaford GG, Hedley TE, Dibrov E, Pierce GN (2013) The effects of dietary flaxseed on atherosclerotic plaque regression. Am J Physiol Heart Circ Physiol. doi:10.1152/ajpheart.00606.2012
Ander BP, Weber AR, Rampersad PP, Gilchrist JS, Pierce GN, Lukas A (2004) Dietary flaxseed protects against ventricular fibrillation induced by ischemia-reperfusion in normal and hypercholesterolemic Rabbits. J Nutr 134:3250–3256
Lucas EA, Lightfoot SA, Hammond LJ, Devareddy L, Khalil DA, Daggy BP, Smith BJ, Westcott N, Mocanu V et al (2004) Flaxseed reduces plasma cholesterol and atherosclerotic lesion formation in ovariectomized Golden Syrian hamsters. Atherosclerosis 173:223–229. doi:10.1016/j.atherosclerosis.2003.12.032
Rodriguez-Leyva D, Weighell W, Edel AL, LaVallee R, Dibrov E, Pinneker R, Maddaford TG, Ramjiawan B, Aliani M et al (2013) Potent antihypertensive action of dietary flaxseed in hypertensive patients. Hypertension 62:1081–1089. doi:10.1161/hypertensionaha.113.02094
Caligiuri SP, Edel AL, Aliani M, Pierce GN (2014) Flaxseed for hypertension: implications for blood pressure regulation. Curr Hypertens Rep 16:499. doi:10.1007/s11906-014-0499-8
Pan A, Yu D, Demark-Wahnefried W, Franco OH, Lin X (2009) Meta-analysis of the effects of flaxseed interventions on blood lipids. Am J Clin Nutr 90:288–297. doi:10.3945/ajcn.2009.27469
Edel AL, Rodriguez-Leyva D, Maddaford TG, Caligiuri SPB, Alejandro Austria J, Weighell W, Guzman R, Aliani M, Pierce GN (2015) Dietary flaxseed independently lowers circulating cholesterol and lowers it beyond the effects of cholesterol-lowering medications alone in patients with peripheral artery disease. J Nutr. doi:10.3945/jn.114.204594
Paschos GK, Magkos F, Panagiotakos DB, Votteas V, Zampelas A (2007) Dietary supplementation with flaxseed oil lowers blood pressure in dyslipidaemic patients. Eur J Clin Nutr 61:1201–1206. doi:10.1038/sj.ejcn.1602631
Takeuchi H, Sakurai C, Noda R, Sekine S, Murano Y, Wanaka K, Kasai M, Watanabe S, Aoyama T, Kondo K (2007) Antihypertensive effect and safety of dietary alpha-linolenic acid in subjects with high-normal blood pressure and mild hypertension. J Oleo Sci 56:347–360
Djoussé L, Arnett DK, Pankow JS, Hopkins PN, Province MA, Ellison RC (2005) Dietary linolenic acid is associated with a lower prevalence of hypertension in the NHLBI family heart study. Hypertension 45:368–373. doi:10.1161/01.HYP.0000154679.41568.e6
Thompson LU, Boucher BA, Liu Z, Cotterchio M, Kreiger N (2006) Phytoestrogen content of foods consumed in Canada, including isoflavones, lignans, and coumestan. Nutr Cancer 54:184–201. doi:10.1207/s15327914nc5402_5
Penalvo JL, Haajanen KM, Botting N, Adlercreutz H (2005) Quantification of lignans in food using isotope dilution gas chromatography/mass spectrometry. J Agric Food Chem 53:9342–9347. doi:10.1021/jf051488w
Smeds AI, Eklund PC, Sjöholm RE, Willför SM, Nishibe S, Deyama T, Holmbom BR (2007) Quantification of a broad spectrum of lignans in cereals, oilseeds, and nuts. J Agric Food Chem 55:1337–1346. doi:10.1021/jf0629134
Mazur W, Duke J, Wähälä K, Rasku S, Adlercreutz H (1998) Isoflavonoids and lignans in legumes: nutritional and health aspects in humans. J Nutr Biochem 9:193–200. doi:10.1016/S0955-2863(97)00184-8
Axelson M, Setchell KDR (1981) The excretion of lignans in rats—evidence for an intestinal bacterial source for this new group of compounds. FEBS Lett 123:337–342
Adlercreutz H, Hockerstedt K, Bannwart C, Bloigu S, Hamalainen E, Fotsis T, Ollus A (1987) Effect of dietary components, including lignans and phytoestrogens, on enterohepatic circulation and liver metabolism of estrogens and on sex hormone binding globulin (SHBG). J Steroid Biochem 27:1135–1144
Setchell KDR, Brown NM, Zimmer-Nechemias L, Wolfe B, Jha P, Heubi JE (2014) Metabolism of secoisolariciresinol-diglycoside the dietary precursor to the intestinally derived lignan enterolactone in humans. Food Funct 5:491–501. doi:10.1039/C3FO60402K
Kitts DD, Yuan YV, Wijewickreme AN, Thompson LU (1999) Antioxidant activity of the flaxseed lignan secoisolariciresinol diglycoside and its mammalian lignan metabolites enterodiol and enterolactone. Mol Cell Biochem 202:91–100
Vanharanta M, Voutilainen S, Nurmi T, Kaikkonen J, Roberts LJ, Morrow JD, Adlercreutz H, Salonen JT (2002) Association between low serum enterolactone and increased plasma F2-isoprostanes, a measure of lipid peroxidation. Atherosclerosis 160:465–469
Pan A, Sun J, Chen Y, Ye X, Li H, Yu Z, Wang Y, Gu W, Zhang X et al (2007) Effects of a flaxseed-derived lignan supplement in type 2 diabetic patients: a randomized, double-blind, cross-over trial. PLoS ONE 2:e1148. doi:10.1371/journal.pone.0001148
Zhang W, Wang X, Liu Y, Tian H, Flickinger B, Empie MW, Sun SZ (2008) Dietary flaxseed lignan extract lowers plasma cholesterol and glucose concentrations in hypercholesterolaemic subjects. Br J Nutr 99:1301–1309. doi:10.1017/S0007114507871649
Cornish SM, Chilibeck PD, Paus-Jennsen L, Biem HJ, Khozani T, Senanayake V, Vatanparast H, Little JP, Whiting SJ, Pahwa P (2009) A randomized controlled trial of the effects of flaxseed lignan complex on metabolic syndrome composite score and bone mineral in older adults. Appl Physiol Nutr Metab 34:89–98. doi:10.1139/h08-142
Bloedon LT, Balikai S, Chittams J, Cunnane SC, Berlin JA, Rader DJ, Szapary PO (2008) Flaxseed and cardiovascular risk factors: results from a double blind, randomized, controlled clinical trial. J Am Coll Nutr 27:65–74
Brown L, Rosner B, Willett WW, Sacks FM (1999) Cholesterol-lowering effects of dietary fiber: a meta-analysis. Am J Clin Nutr 69:30–42
Austria JA, Richard MN, Chahine MN, Edel AL, Malcolmson LJ, Dupasquier CM, Pierce GN (2008) Bioavailability of alpha-linolenic acid in subjects after ingestion of three different forms of flaxseed. J Am Coll Nutr 27:214–221
Kuijsten A, Arts IC, van’t Veer P, Hollman PC (2005) The relative bioavailability of enterolignans in humans is enhanced by milling and crushing of flaxseed. J Nutr 135:2812–2816
Coulman KD, Liu Z, Michaelides J, Quan Hum W, Thompson LU (2009) Fatty acids and lignans in unground whole flaxseed and sesame seed are bioavailable but have minimal antioxidant and lipid-lowering effects in postmenopausal women. Mol Nutr Food Res 53:1366–1375. doi:10.1002/mnfr.200900032
Patenaude A, Rodriguez-Leyva D, Edel AL, Dibrov E, Dupasquier CM, Austria JA, Richard MN, Chahine MN, Malcolmson LJ, Pierce GN (2009) Bioavailability of alpha-linolenic acid from flaxseed diets as a function of the age of the subject. Eur J Clin Nutr 63:1123–1129. doi:10.1038/ejcn.2009.41
Khalatbari Soltani S, Jamaluddin R, Tabibi H, Mohd Yusof BN, Atabak S, Loh S-P, Rahmani L (2013) Effects of flaxseed consumption on systemic inflammation and serum lipid profile in hemodialysis patients with lipid abnormalities. Hemodial Int 17:275–281. doi:10.1111/j.1542-4758.2012.00754.x
Patade A, Devareddy L, Lucas E, Korlagunta K, Daggy B, Arjmandi B (2008) Flaxseed reduces total and LDL cholesterol concentrations in Native American postmenopausal women. J Womens Health 17:355–366. doi:10.1089/jwh.2007.0359
Dodin S, Lemay A, Jacques H, Légaré F, Forest J-C, Mâsse B (2005) The effects of flaxseed dietary supplement on lipid profile, bone mineral density, and symptoms in menopausal women: a randomized, double-blind, wheat germ placebo-controlled clinical trial. J Clin Endocrinol Metab 90:1390–1397. doi:10.1210/jc.2004-1148
Cunnane SC, Hamadeh MJ, Liede AC, Thompson LU, Wolever TM, Jenkins DJ (1995) Nutritional attributes of traditional flaxseed in healthy young adults. Am J Clin Nutr 61:62–68
Cunnane SC, Ganguli S, Menard C, Liede AC, Hamadeh MJ, Chen ZY, Wolever TM, Jenkins DJ (1993) High alpha-linolenic acid flaxseed (Linum usitatissimum): some nutritional properties in humans. Br J Nutr 69:443–453
Mandasescu S, Mocanu V, Dascalita AM, Haliga R, Nestian I, Stitt PA, Luca V (2005) Flaxseed supplementation in hyperlipidemic patients. Rev Med Chir Soc Med Nat Iasi 109:502–506
Mani UV, Mani I, Biswas M, Kumar SN (2011) An open-label study on the effect of flax seed powder (Linum usitatissimum) supplementation in the management of diabetes mellitus. J Diet Suppl 8:257–265. doi:10.3109/19390211.2011.593615
Clark WF, Parbtani A, Huff MW, Spanner E, de Salis H, Chin-Yee I, Philbrick DJ, Holub BJ (1995) Flaxseed: a potential treatment for lupus nephritis. Kidney Int 48:475–480
Hutchins AM, Brown BD, Cunnane SC, Domitrovich SG, Adams ER, Bobowiec CE (2013) Daily flaxseed consumption improves glycemic control in obese men and women with pre-diabetes: a randomized study. Nutr Res 33:367–375. doi:10.1016/j.nutres.2013.02.012
Expert Panel (2009) United States Food and Drug Administration. United States Department of Health & Human Services. Determination of the GRAS status of the addition of whole and milled flaxseed to conventional foods and meat and poultry products [cited 2015 February 2015]. http://fda.gov/ucm/groups/fdagov-public/@fdagov-foods-gen/documents/document/ucm269248.pdf
Nesbitt PD, Lam Y, Thompson LU (1999) Human metabolism of mammalian lignan precursors in raw and processed flaxseed. Am J Clin Nutr 69:549–555
Hutchins AM, Martini MC, Olson BA, Thomas W, Slavin JL (2000) Flaxseed influences urinary lignan excretion in a dose-dependent manner in postmenopausal women. Cancer Epidemiol Biomark Prev 9:1113–1118
Aliani M, Ryland D, Pierce GN (2011) Effect of flax addition on the flavor profile of muffins and snack bars. Food Res Int 44:2489–2496. doi:10.1016/J.Foodres.2011.01.044
Lepage G, Roy CC (1986) Direct transesterification of all classes of lipids in a one-step reaction. J Lipid Res 27:114–120
Edel AL, Aliani M, Pierce GN (2013) Supported liquid extraction in the quantitation of plasma enterolignans using isotope dilution GC/MS with application to flaxseed consumption in healthy adults. J Chromatogr B Anal Biomed Life Sci 912:24–32. doi:10.1016/j.jchromb.2012.10.030
Friedewald WT, Levy RI, Fredrickson DS (1972) Estimation of the concentration of low-density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clin Chem 18:499–502
Heinegard D, Tiderstrom G (1973) Determination of serum creatinine by a direct colorimetric method. Clin Chim Acta 43:305–310
McAdam BF, Byrne D, Morrow JD, Oates JA (2005) Contribution of cyclooxygenase-2 to elevated biosynthesis of thromboxane A2 and prostacyclin in cigarette smokers. Circulation 112:1024–1029. doi:10.1161/circulationaha.105.542696
Palmer ME, Haller C, McKinney PE, Klein-Schwartz W, Tschirgi A, Smolinske SC, Woolf A, Sprague BM, Ko R, Everson G et al (2003) Adverse events associated with dietary supplements: an observational study. Lancet 361:101–106. doi:10.1016/S0140-6736(03)12227-1
Kilkkinen A, Pietinen P, Klaukka T, Virtamo J, Korhonen P, Adlercreutz H (2002) Use of oral antimicrobials decreases serum enterolactone concentration. Am J Epidemiol 155:472–477
Dodin S, Cunnane SC, Mâsse B, Lemay A, Jacques H, Asselin G, Tremblay-Mercier J, Marc I, Lamarche B et al (2008) Flaxseed on cardiovascular disease markers in healthy menopausal women: a randomized, double-blind, placebo-controlled trial. Nutrition 24:23–30. doi:10.1016/j.nut.2007.09.003
Taylor CG, Noto AD, Stringer DM, Froese S, Malcolmson L (2010) Dietary milled flaxseed and flaxseed oil improve n-3 fatty acid status and do not affect glycemic control in individuals with well-controlled type 2 diabetes. J Am Coll Nutr 29:72–80
Harper CR, Edwards MJ, DeFilipis AP, Jacobson TA (2006) Flaxseed oil increases the plasma concentrations of cardioprotective (n-3) fatty acids in humans. J Nutr 136:83–87
Goyens PL, Spilker ME, Zock PL, Katan MB, Mensink RP (2006) Conversion of α-linolenic acid in humans is influenced by the absolute amounts of α-linolenic acid and linoleic acid in the diet and not by their ratio. Am J Clin Nutr 84:44–53
Zhao G, Etherton TD, Martin KR, Gillies PJ, West SG, Kris-Etherton PM (2007) Dietary α-linolenic acid inhibits proinflammatory cytokine production by peripheral blood mononuclear cells in hypercholesterolemic subjects. Am J Clin Nutr 85:385–391
Egert S, Kannenberg F, Somoza V, Erbersdobler HF, Wahrburg U (2009) Dietary α-linolenic acid, EPA, and DHA have differential effects on LDL fatty acid composition but similar effects on serum lipid profiles in normolipidemic humans. J Nutr 139:861–868. doi:10.3945/jn.108.103861
Barceló-Coblijn G, Murphy EJ, Othman R, Moghadasian MH, Kashour T, Friel JK (2008) Flaxseed oil and fish-oil capsule consumption alters human red blood cell n-3 fatty acid composition: a multiple-dosing trial comparing 2 sources of n-3 fatty acid. Am J Clin Nutr 88:801–809
Akiba S, Murata T, Kitatani K, Sato T (2000) Involvement of lipoxygenase pathway in docosapentaenoic acid-induced inhibition of platelet aggregation. Biol Pharm Bull 23:1293–1297
Tsuji M, Murota SI, Morita I (2003) Docosapentaenoic acid (22:5, n-3) suppressed tube-forming activity in endothelial cells induced by vascular endothelial growth factor. Prostaglandins Leukot Essent Fatty Acids 68:337–342
Kaur G, Cameron-Smith D, Garg M, Sinclair AJ (2011) Docosapentaenoic acid (22:5n-3): a review of its biological effects. Prog Lipid Res 50:28–34. doi:10.1016/j.plipres.2010.07.004
Nozue T, Yamamoto S, Tohyama S, Fukui K, Umezawa S, Onishi Y, Kunishima T, Sato A, Nozato T et al (2013) Effects of serum n-3 to n-6 polyunsaturated fatty acids ratios on coronary atherosclerosis in statin-treated patients with coronary artery disease. Am J Cardiol 111:6–11. doi:10.1016/j.amjcard.2012.08.038
Vanharanta M, Voutilainen S, Rissanen TH, Adlercreutz H, Salonen JT (2003) Risk of cardiovascular disease-related and all-cause death according to serum concentrations of enterolactone: Kuopio Ischaemic Heart Disease Risk Factor Study. Arch Intern Med 163:1099–1104. doi:10.1001/archinte.163.9.1099
Morton MS, Wilcox G, Wahlqvist ML, Griffiths K (1994) Determination of lignans and isoflavonoids in human female plasma following dietary supplementation. J Endocrinol 142:251–259
Bureau of Nutritional Sciences Food Directorate, Health Products and Food Branch (2014) Summary of Health Canada’s assessment of a health claim about ground whole flaxseed and blood cholesterol lowering. Ontario (Canada) Bureau of Nutritional Sciences; [cited 2015 February 17]. http://www.hc-sc.gc.ca/fn-an/alt_formats/pdf/label-etiquet/claims-reclam/assess-evalu/flaxseed-graines-de-lin-eng.pdf
Arjmandi BH, Khan DA, Juma S, Drum ML, Venkatesh S, Sohn E, Wei L, Derman R (1998) Whole flaxseed consumption lowers serum LDL-cholesterol and lipoprotein(a) concentrations in postmenopausal women. Nutr Res 18:1203–1214. doi:10.1016/S0271-5317(98)00100-6
Lucas EA, Wild RD, Hammond LJ, Khalil DA, Juma S, Daggy BP, Stoecker BJ, Arjmandi BH (2002) Flaxseed improves lipid profile without altering biomarkers of bone metabolism in postmenopausal women. J Clin Endocrinol Metab 87:1527–1532. doi:10.1210/jcem.87.4.8374
Barre DE, Griscti O, Mizier-Barre KA, Hafez K (2005) Flaxseed oil and lipoprotein (a) significantly increase bleeding time in type 2 diabetes patients in Cape Breton, Nova Scotia, Canada. J Oleo Sci 54:347–354
United States Department of Agriculture (USDA) Agricultural Research Service (2007) National nutrient database for standard reference, Release 27 [cited 2015 February 17]. http://ndb.nal.usda.gov/ndb/foods/show/3745?fg=&man=&lfacet=&format=&count=&max=25&offset=&sort=&qlookup=flaxseed
Acknowledgments
We would like to acknowledge the assistance of Ms. Elaine Sopiwnyk, the encouragement of Ms. Kelley Fitzpatrick at Flax2015 and clinical support from the Office of Clinical Research at St Boniface Hospital. We would also like to thank Mr. Doug Staley for his statistical advice. This study was supported by grants from the Canadian Institutes for Health Research (GNP), Flax 2015 (GNP), the Flax Council of Canada (GNP), Agri-Food Research and Development Initiative (GNP and MA), Manitoba Medical Service Foundation (MA) and indirectly from St Boniface Hospital and Research Foundation. Andrea Edel was a recipient of the Banting and Best Canada Graduate Scholarship Doctoral Award from Canadian Institutes for Health Research and a Doctoral Research Scholarship from the Heart and Stroke Foundation of Canada.
Conflict of interest
The authors declare that they have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Edel, A.L., Patenaude, A.F., Richard, M.N. et al. The effect of flaxseed dose on circulating concentrations of alpha-linolenic acid and secoisolariciresinol diglucoside derived enterolignans in young, healthy adults. Eur J Nutr 55, 651–663 (2016). https://doi.org/10.1007/s00394-015-0885-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00394-015-0885-2