Abstract
Social media technologies have become increasingly useful tools for research-based interventions. However, participants and social media users have expressed ethical concerns with these studies, such as risks and benefits of participation, as well as privacy, confidentiality, and informed consent issues. This study was designed to follow up with and assess experiences and perceptions of ethics-related issues among a sample of 211 men who have sex with men who participated in the Harnessing Online Peer Education (HOPE) Peru study, a randomized controlled HIV prevention intervention conducted in Peru. We found that after adjusting for age, highest educational attainment, race, sexual orientation, and prior HIV research experience, participants in the intervention group were more likely than those in the control group to have safe sex (p = 0.0051) and get tested for HIV regularly (p = 0.0051). As a result of their participation, those in the intervention group benefited more positively than participants in the control group in improving HIV care (p = 0.0077) and learning where to receive sexual health services (p = 0.0021). Participants in the intervention group expressed higher levels of comfort than those in the control group in joining and seeing other people in the Facebook group (p = 0.039), seeing other people’s posts (p = 0.038) and having other group members talk to them online (p = 0.040). We discuss the implications of these results as they relate to social media-based HIV research.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Social media use has expanded rapidly in the past decade. According to one survey, an estimated 74% of American adults who are online use some form of social media (Pew Research Center 2013). Social media technologies can be used to reach a large number of individuals rapidly. Increasing numbers of research studies are now implemented over social media. However, recent social media-based studies have raised ethical questions (Bull et al. 2011; Young 2012). For example, a 2014 Facebook experiment assessed whether positive or negative content would affect users’ emotions and subsequent updates among 700,000 uninformed users (Albergotti 2014). This experiment sparked outrage among social media users (Albergotti 2014; The Muse 2014), with many commentators questioning the legality of the study and expressing concerns about informed consent, privacy, and confidentiality regarding online studies (Goel 2014; Meyer 2014) (Fig. 1).
Although corporate use of social media for research has caused controversy, public health researchers, seeking to use social media for public good, have successfully used social media to study and modify risk behaviors among high-risk populations, especially for stigmatized diseases (Bull et al. 2012; Young and Jaganath 2013; Young et al. 2013). For example, the Harnessing Online Peer Education (HOPE) study is a peer-led HIV intervention delivered over Facebook targeting African American and Latino men who have sex with men (MSM) at high risk of HIV (Young et al. 2013). The study found that participants in the intervention group were significantly more likely to request HIV home-testing kits compared with participants in the control group. These results have been replicated in international settings, such as Peru (Young et al. 2015). However, to date, no studies have focused on the ethics of social media-based interventions and participant experiences in these studies. Studies on research ethics have documented a process for determining whether and how research practices are conducted in an ethical manner. For example, ethics researchers have stated the need for researchers to conduct follow-up studies to assess whether studies adhere to the Belmont principles, citing the Common Rule such as benefiting participants more than harming them (Fisher 2011). Studies on the ethics of research are especially vital in the new and growing field of social media research because they will provide information on whether study participants find social media-based studies safe and acceptable, which will help inform future conduct of social media-based research. In addition, these issues are especially important to study in the global settings, such as Peru, where social media-based interventions are rapidly increasing in use.
A number of specific ethical issues with social media-based public health interventions like the HOPE study have been discussed by researchers and Institutional Review Boards and need to be addressed. First, as listed as a requirement in the Belmont principles, it is unknown whether the benefits of participating in social media-based HIV studies outweigh the risks. Second, it is unknown whether participants believe their privacy and confidentiality can be maintained as needed throughout study participation. Third, it is unknown whether participants believe social media-based public health research is justified in that it can benefit societal well-being. Finally, it is unknown whether participants in the control and intervention groups may have differing perspectives on these issues.
Therefore, this study was designed to evaluate the ethics of social media research according to the Belmont principles. We sought to re-contact Peruvian MSM participants from a Facebook-based HIV intervention (HOPE Peru) and assess their experiences and perceptions of ethics-related issues based on whether they were in a control or intervention group.
Methods
The Institutional Review Boards at UCLA and Epicentro approved the study protocol for the original HOPE study and the current study. Participants received information about the study and completed an informed consent online. The study adheres to the current recommendations on using social media in HIV research (Young 2012).
The HOPE Peru study was a 12-week HIV Facebook-based peer-led HIV intervention that sought to increase HIV testing and prevention behaviors among Peruvian MSM at high risk of HIV. It is a peer-led intervention, whereby participants in the HIV intervention were assigned to closed, secret, Facebook groups and assigned peer leaders (other Peruvian MSM) to encourage them to get an HIV test at a local clinic. Participants in the control group joined closed, secret Facebook groups but did not receive any additional information. The HOPE Peru study included 556 participants who were (1) male, (2) 18 years of age or older, (3) residents of greater Lima, (4) current Facebook users, and (5) had had sex with a man in the past 12 months. Consented participants were then randomized into one of two conditions: an HIV intervention Facebook group or a standard-of-care control Facebook group (general health). At 1-year follow-up (June 2014), participants were re-contacted using email, social media, and phone and invited to complete a survey to assess their ethical experiences in the HOPE Peru study.
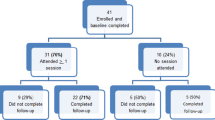
This ethics-related follow-up study provided enough funding to recruit the first 211 participants (out of 556 Peruvian MSM from the HOPE Peru study) who responded to the solicitation. Thus, the study includes 109 participants from the control group and 102 participants from the intervention group. Participants were compensated with an equivalent of $11 (USD) in Peruvian Sol upon completion of the survey.
Measures
The 40-item survey assessed the participants’ ethics-relevant perceptions and experiences in the social media-based study related to recruitment, informed consent, intervention, and follow-up/post-intervention. Participants from the control and the intervention groups completed the same survey. Because no existing items had been validated in studying the ethics of using social media in HIV prevention research, the authors created their own items based on feedback from other researchers, participants, and internal review boards on the ethical issues associated with social media-based research. Measures were generated in English. Translation was conducted by a bilingual Peruvian physician (co-investigator). Back translation was conducted by a bilingual investigator who viewed the Spanish content to assess whether it maintained the meaning of the original English text. Translation was not word for word, but rather tailored to the dialect and idioms of Peruvian Spanish.
Basic Demographics
Basic demographics included age, sexual identity, highest educational attainment, race/ethnicity, and prior HIV research experience.
Recruitment (11 Items)
Participants were asked questions to better understand the ethical issues associated with the recruitment process for an online social media-based intervention. For example, using a five-point Likert-type scale, participants were asked, “How comfortable were you about the idea of participating when you first read or heard about the study?” and “How comfortable were you clicking on the advertisement?” In addition, participants were asked to indicate their levels of comfort/discomfort (five-point Likert-type scale) regarding key study characteristics.
Informed Consent (Three Items)
Participants were asked to rate how much they understood the consent form and study process (did not understand at all to completely understood) and how similar/dissimilar their experiences participating in the study were from the consent form. In addition, participants were also asked about the completeness of the consent form (“Thinking back, is there any information that you were not told and think you needed to know about the study beforehand?”).
Intervention (15 Items)
Participants were asked to indicate their comfort/discomfort levels while participating in the study intervention. For example, participants were asked, using a five-point Likert-type scale, how comfortable they were (1) completing the baseline survey, (2) being sent a Facebook group invitation, (3) joining a social media-based research study with a group of strangers, (4) seeing other people’s posts on the group wall, (5) posting on the group wall, and (6) talking with other group members online.
Follow-Up/Post-Intervention (21 Items)
We asked participants how they felt after the study was completed in order to gain an understanding of the lasting ethical issues related to study participation. For example, using a five-point Likert-type scale (very negative to very positive), participants were asked how much they benefited from the study in (1) learning more about health, (2) learning about sexual health, (3) gaining new friends, 4) feeling closer to the MSM community, (5) feeling better about themselves, (6) learning about HIV status, (7) improving HIV care, (8) learning where to receive sexual health services, (9) gaining a job, (10) learning about research, (11) increasing trust in other people, and (12) increasing trust in research. Participants were also asked whether the study impacted their HIV testing decisions.
Analysis
All analyses were conducted using the R statistical package (version 3.1.0). A total of 211 participants were included in the study. Two-sample t test and chi-square (Fisher’s exact for n < 5) tests were used to determine the binary association between group (intervention vs. control) and basic demographics (age, prior HIV research experience, race, sexual orientation, and highest educational attainment). A simple logistic regression model for binary outcomes and cumulative logit regression model for ordinal outcomes were used to assess the binary association between group- and ethics-related outcomes. The final model was adjusted for age, sexual orientation, race, highest educational attainment, and prior HIV research experience. Only significant results are included in Tables 1, 2, and 3.
Results
Basic Demographics
Participants in the control group (M = 31.94, SD = 7.66) were slightly older than participants in the intervention group (M = 31.69, SD = 7.75) (Table 1). Most participants from both groups were gay, mixed race, and university educated. A greater number of participants in the intervention group (37.3%) compared with the control group (28.4%) had previously participated in a HIV study. No significant associations between basic demographic variables and group were found.
Benefits of Study Participation and Understanding of Consent
Compared to participants in the intervention group, participants in the control group expressed higher levels of understanding in the consent form and study process (p = 0.0067) (Table 2). Participants in the intervention group expressed higher levels of comfort in joining and seeing other people in the Facebook group (p = 0.028), seeing other people’s posts (p = 0.038) and having other group members talk to them online (p = 0.0032). After the study, a greater number of participants in the intervention group compared to participants in the control group expressed that they were likely or more likely to have safe sex (p = 0.034) and get tested for HIV regularly (p = 0.021). Participants in the intervention group were more likely than those in the control group to indicate that they benefited positively or very positively from the study in gaining new friends (p = 0.023), improving HIV care (p = 0.029) and they learned where to receive sexual health services (p = 0.0062).
Adjusted Odds Ratios for Group on Ethics-Related Outcomes
Table 3 presents the statistical results on the adjusted analysis of participants’ ethics-related concerns. After adjusting for age, highest educational attainment, race, sexual orientation, and prior HIV research experience, participants in the intervention group, compared to those in the control group, expressed lower levels of understanding in the consent form and study process. In addition, participants in the intervention group expressed higher levels of comfort than participants in the control group in joining and seeing other people in the Facebook group, seeing other people’s posts, and having other group members talk to them online. Participants in the intervention group were more likely than participants in the control group to have safe sex and to get tested for HIV regularly. They also reported that they benefited more positively than those in the control group in improving HIV care and learning where to receive sexual health services.
Discussion
To the best of our knowledge, this is the first study to examine participants’ experiences and ethics-related perceptions between intervention and control groups in a social media-based HIV intervention. Previous studies have found social media to be effective in delivering HIV prevention education (Young and Jaganath 2013; Young et al. 2013). In this study, participants in the intervention group also perceived the study to be beneficial, demonstrating additional ethical data. The intervention positively impacted participants’ HIV-related knowledge (e.g., locations for sexual health services and HIV care) and prevention behaviors (e.g., safe sex and regular HIV testing). Participants in the study also perceived the social media-based HIV intervention to be acceptable, providing important data on the ability for researchers to conduct these studies in the future. Participants in the intervention group felt more comfortable joining and interacting with others in an HIV-focused Facebook group than those in the control group. Given that stigma and discrimination remain important barriers to Peruvian MSM in accessing HIV prevention and education, social media, with over 12.4 million Peruvian users (55% of Peru’s population), provide a promising platform not only to reach individuals at risk for HIV but also to create a supportive community to increase prevention behavior (Pereyra and Santillana 2014).
Although only a small number of participants from both groups expressed concerns over privacy, confidentiality, and safety, the study found that online informed consent remains a challenge. Participants in the control group expressed higher levels of understanding in the consent form and study process than participants in the intervention group. In online settings, the lack of interaction between participants and researchers makes it difficult for researchers to ascertain whether participants have read and understood the consent form (Pequegnat et al. 2007; Simon Rosser et al. 2009). Previous online studies suggested that additional measures are needed to ensure participants’ understanding of the material. Online informed consent should embrace social media technologies and present materials to address the needs of each participant, such as the use of multimedia content, a preview of the study, bullet points, and a post-reading assessment (Pequegnat et al. 2007; Simon Rosser et al. 2009).
The most important implication of this study is that participants appear to believe that social media is a valuable tool for HIV prevention research. It appears that participants did not experience the ethical concerns reported from corporate research studies, such as the 2014 Facebook study. Participants’ reports of the benefits they received are also important for researchers planning work in this area, so that they can tailor interventions to benefit both participants and society.
This study has a few limitations. First, the study might suffer from recall bias as participants were contacted approximately 1.5 years after the study began to complete surveys regarding their experiences. Qualitative interviews with participants could help to address recall by helping to better understand when and how recall issues might affect participant responses. Qualitative methods could also be used to gain a more thorough understanding of the ethics-related issues identified in this analysis. Mixed methods approaches are therefore recommended for ethics-based research. Second, the results lack generalizability outside of Peru, Peruvian MSM, and HIV prevention studies. Future research can explore whether the current findings related to social media and HIV interventions extend to areas outside of Peru.
Social media use continues to grow worldwide, and the percentages of social media users among Internet users in many low- and middle-income countries have bypassed rates in the USA (Rainie and Poushter 2014). Given this explosion, social media has become a cost-effective tool in HIV research in the global health settings. While participants in this study found Facebook to be a safe, acceptable, and effective platform for delivery of an HIV intervention, more studies are needed to assess participants’ perceptions of ethics-related issues in social media-based studies. Future social media-based studies should consider assessing ethics-related questions regularly to ensure the safety and comfort of participants.
References
Albergotti, R. (2014, 7/30/14). Furor erupts over Facebook’s experiment on users. The Wall Street Journal. Retrieved 11 Apr 2015, from http://online.wsj.com/articles/furor-erupts-over-facebook-experiment-on-users-1404085840.
Bull, S. S., Breslin, L. T., Wright, E. E., Black, S. R., Levine, D., & Santelli, J. S. (2011). Case study: An ethics case study of HIV prevention research on Facebook: The Just/Us study. Journal of Pediatric Psychology, 36, 1082–1092.
Bull, S. S., Levine, D. K., Black, S. R., Schmiege, S. J., & Santelli, J. (2012). Social media–delivered sexual health intervention: A cluster randomized controlled trial. American Journal of Preventive Medicine, 43, 467–474.
Fisher, C. (2011). Addiction research ethics and the Belmont principles: Do drug users have a different moral voice? Substance Use and Misuse, 46, 728–741.
Goel, V. (2014, 06/29/14). Facebook tinkers with users’ emotions in news feed experiment, stirring outcry. New York Times. Retrieved 10 Oct 2015, from http://www.nytimes.com/2014/06/30/technology/facebook-tinkers-with-users-emotions-in-news-feed-experiment-stirring-outcry.html?_r=0.
Meyer, R. (2014, 09/24/14). Facebook’s mood manipulation experiment might have been illegal. The Atlantic. Retrieved 12 Sept 2015, from http://www.theatlantic.com/technology/archive/2014/09/facebooks-mood-manipulation-experiment-might-be-illegal/380717/.
Pequegnat, W., Rosser, B. R., Bowen, A. M., Bull, S. S., DiClemente, R. J., Bockting, W. O., et al. (2007). Conducting internet-based HIV/STD prevention survey research: Considerations in design and evaluation. AIDS and Behavior, 11, 505–521.
Pereyra, R. C., & Santillana, S. A. (2014, 3/21/14). Facebook apunta a premiar a las empresas que paguen por publicidad [in Spanish]. Gestion. Retrieved 10 Dec 2015, from http://gestion.pe/tendencias/facebook-apunta-premiar-empresas-que-paguen-publicidad-segun-futuro-labs-2086840.
Pew Research Center. (2013, 12/27/2013). Social networking fact sheet. Retrieved 11 Nov 2015, from http://www.pewinternet.org/fact-sheets/social-networking-fact-sheet/.
Rainie, L., & Poushter, J. (2014). Emerging nations catching up to U.S. on technology adoption, especially mobile and social media use. Retrieved 11 Nov 2015, from http://www.pewresearch.org/fact-tank/2014/02/13/emerging-nations-catching-up-to-u-s-on-technology-adoption-especially-mobile-and-social-media-use/.
Simon Rosser, B. R., Gurak, L., Horvath, K. J., Oakes, J. M., Konstan, J., & Danilenko, G. P. (2009). The challenges of ensuring participant consent in internet‐based sex studies: A case study of the Men’s INTernet Sex (MINTS‐I and II) studies. Journal of Computer-Mediated Communication, 14, 602–626.
The Muse. (2014). The Facebook experiment: What it means for you. Forbes. Retrieved 12 Apr 2016, from http://www.forbes.com/sites/dailymuse/2014/08/04/the-facebook-experiment-what-it-means-for-you/.
Young, S. D. (2012). Recommended guidelines on using social networking technologies for HIV prevention research. AIDS and Behavior, 16, 1743–1745.
Young, S. D., & Jaganath, D. (2013). Online social networking for HIV education and prevention: A mixed methods analysis. Sexually Transmitted Diseases, 40, 162–167.
Young, S. D., Cumberland, W., Lee, S., Jaganath, D., Szekeres, G., & Coates, T. (2013). Social networking technologies as an emerging tool for HIV prevention: A cluster randomized trial. Annals of Internal Medicine, 159, 318–324.
Young, S. D., Cumberland, W., Nianogo, R., Menacho, L., Galea, J., & Coates, T. (2015). HOPE social media intervention: Emerging tools for HIV intervention in Peru. The Lancet HIV, 2, e27–e32.
Acknowledgments
The authors would like to thank researchers at the Center for Digital Behavior (CDB) at the University of California, Los Angles (UCLA) for their input on this study. We wish to acknowledge Jason Chiu for his data analysis and help developing the manuscript while he was on staff.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The Institutional Review Boards at UCLA and Epicentro approved the study protocol for the original HOPE study and the current study. Participants received information about the study and completed an informed consent online. The study adheres to the current recommendations on using social media in HIV research.
Conflict of Interest
The authors declare that they have no conflict of interest.
Funding
This study was funded by the National Institute of Mental Health (Young: K01 MH090884) and by the Fordham University HIV and Drug Abuse Prevention Research Ethics Training (National Institute on Drug Abuse R25 DA031608-01).
Rights and permissions
About this article
Cite this article
Garett, R., Menacho, L. & Young, S.D. Ethical Issues in Using Social Media to Deliver an HIV Prevention Intervention: Results from the HOPE Peru Study. Prev Sci 18, 225–232 (2017). https://doi.org/10.1007/s11121-016-0739-z
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11121-016-0739-z