Abstract
Fe(II) cations bind with high efficiency and specificity at the high-affinity (HA), Mn-binding site (termed the “blocking effect” since Fe blocks further electron donation to the site) of the oxygen-evolving complex (OEC) in Mn-depleted, photosystem II (PSII) membrane fragments (Semin et al. in Biochemistry 41:5854, 2002). Furthermore, Fe(II) cations can substitute for 1 or 2Mn cations (pH dependent) in Ca-depleted PSII membranes (Semin et al. in Journal of Bioenergetics and Biomembranes 48:227, 2016; Semin et al. in Journal of Photochemistry and Photobiology B 178:192, 2018). In the current study, we examined the effect of Ca2+ cations on the interaction of Fe(II) ions with Mn-depleted [PSII(-Mn)] and Ca-depleted [PSII(-Ca)] photosystem II membranes. We found that Ca2+ cations (about 50 mM) inhibit the light-dependent oxidation of Fe(II) (5 µM) by about 25% in PSII(-Mn) membranes, whereas inhibition of the blocking process is greater at about 40%. Blocking of the HA site by Fe cations also decreases the rate of charge recombination between QA− and YZ•+ from t1/2 = 30 ms to 46 ms. However, Ca2+ does not affect the rate during the blocking process. An Fe(II) cation (20 µM) replaces 1Mn cation in the Mn4CaO5 catalytic cluster of PSII(-Ca) membranes at pH 5.7 but 2 Mn cations at pH 6.5. In the presence of Ca2+ (10 mM) during the substitution process, Fe(II) is not able to extract Mn at pH 5.7 and extracts only 1Mn at pH 6.5 (instead of two without Ca2+). Measurements of fluorescence induction kinetics support these observations. Inhibition of Mn substitution with Fe(II) cations in the OEC only occurs with Ca2+ and Sr2+ cations, which are also able to restore oxygen evolution in PSII(-Ca) samples. Nonactive cations like La3+, Ni2+, Cd2+, and Mg2+ have no influence on the replacement of Mn with Fe. These results show that the location and/or ligand composition of one Mn cation in the Mn4CaO5 cluster is strongly affected by calcium depletion or rebinding and that bound calcium affects the redox potential of the extractable Mn4 cation in the OEC, making it resistant to reduction.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Oxygen in the atmosphere is generated during the light-dependent oxidation of water, which is catalyzed by the photosynthetic O2-evolving complex (OEC) of photosystem II (PSII) in plants, algae, and cyanobacteria. The catalytic center of the OEC is the inorganic cluster, Mn4CaO5 (Umena et al. 2011). It synthetizes the molecular bond linking the oxygen atoms of two water molecules and operates continuously by cycling through five redox states (the ‘Kok cycle’). During this cycle, the OEC extracts water protons and electrons that are used to drive ATP and NADPH formation. Molecular oxygen is released into biosphere as the byproduct of the cycle. Understanding of the Mn4CaO5 catalyst structure has rapidly advanced over the past few years due to novel X-ray crystallography technology. Umena et al. (Umena et al. 2011) performed X-ray diffraction analysis at a resolution of 1.9 Å on PSII core preparations from Thermosynechococcus vulcanus (a thermophilic cyanobacterium) in the dark-stable S1-state. Such high resolution allowed them to determine the structure of the cluster, to reveal four molecules of water (W1–W4) within the cluster (they provide ligands to Mn4 and Ca) and to identify a number of polypeptide ligands to Mn4 and Ca. The Mn4CaO5 cluster is a distorted cube formed by 3Mn cations and 1Ca cation connected by oxygen bridges, and the 4th Mn cation is bound to the cluster by two oxygen bridges labeled O4 and O5. Recently, a “radiation-damage-free” structure of PSII, which eliminates the X-ray-induced reduction of the Mn cations during the measurements, was determined using X-ray pulses of femtosecond duration (Suga et al. 2015). Furthermore, the OEC structure of in higher S states than the dark-stable S1-state has also been investigated using an X-ray laser source at room temperature (Suga et al. 2017; Kern et al. 2018). Nevertheless, the exact mechanism of the PSII water-splitting reaction remains unclear despite the availability of an atomic structure of the OEC.
Due to similarities between the redox and atomic properties of manganese and iron cations, Fe(II) cations under oxidizing conditions can bind to unoccupied Mn-binding sites [as Fe(III)] with very high specificity and affinity in Mn-depleted, PSII membrane fragments [PSII(-Mn), hereafter] when they are illuminated (Semin et al. 2002; Semin and Seibert 2004, 2006). Interaction of Fe(II) cations with the donor side of the PSII(-Mn) membrane is reminiscent in fact of the Mn-photoligation steps of the photoactivation process (Tamura and Cheniae 1987; Ananyev and Dismukes 1996). An Fe(II) cation binds to the high-affinity (HA), Mn-binding site at micromolar concentrations, and the bound cation is oxidized by YZ•+ under weak illumination since light is a necessary factor for cation ligation (Semin et al. 2002). The resultant Fe(III) cation blocks the HA site (i.e., prevents the interaction of additional added Mn(II) cations with the site), but 4–5 Fe(II) cations must be oxidized in order to observe the blocking effect (Semin and Seibert 2004). The bound Fe(III) cation is coordinated at the HA site by a carboxylic group (Semin and Seibert 2006). All these results demonstrate the high specificity of Fe cation interactions with OEC Mn-binding sites.
In addition to the above method of Mn substitution with Fe, the binding of Fe cations at Mn-binding sites in the OEC can be accomplished using another simple method. Ca-depleted PSII (PSII[-Ca]) membranes contain the Mn cluster but lack two of the three extrinsic proteins (PsbP and PsbQ, Ghanotakis et al. 1984a) that normally cover and protect the Mn cluster from destruction. As a result, the Mn cluster is open to reduction by exogenous reductants (Ghanotakis et al. 1984b). If a Mn(II) cation is reduced, it is rapidly released from its binding site. If exogenous Fe(II) cations are the reductant, Fe(III) forms and occupies the empty Mn site, because Fe has a higher specific affinity for the HA Mn-binding site than Mn itself. Using such an approach, we found that Fe(II) cations can substitute for 2Mn cations in PSII(-Ca) membranes at pH 6.5 (Semin and Seibert 2016) and 1Mn ion at pH 5.7 (Semin et al. 2018). Another metal cation essential in the OEC for the water-splitting reaction is the redox-inactive metal, Ca2+ (see Najafpour et al. 2016, for a review). Although its role has not been fully elucidated, there have been several recent structural and functional hypotheses. Brudvig and his co-workers proposed that Ca2+ acts as a Lewis acid, binding a water substrate molecule and hence tuning its reactivity (Vrettos et al. 2001a). Kim and Debus (2017), using FTIR difference spectroscopy, identified water molecule, W3, bound to a Ca cation, as the substrate becoming part of the O–O bond of the O2 released from the OEC during a water-splitting cycle. Recently Tsui and Agapie (2013) found a linear dependence between the heterometallic metal–oxido cluster potential and the Lewis acidity of redox-inactive metal cations. They suggested that this correlation provides evidence for the role of the Ca2+ ion in modulating redox potential of the manganese cluster. Taking this into account, we investigated the interaction of Ca- and Mn-binding sites and the effect of Ca2+ cations on Fe cation substitution for Mn, based on the reduction of Mn by Fe cations.
Materials and methods
Spinach PSII-enriched membranes were prepared according to Ghanotakis and Babcock (1983). The rates of O2 evolution (450–550 μmol O2 mg chlorophyll (Chl)−1 h−1) were measured polarographically with 0.2 mM 2,6-dichloro-1,4-benzoquinone as the exogenous electron acceptor. Samples were stored at − 80 °C in 50 mM 2-(N-morpholino)-ethanesulfonic acid (MES) at pH 6.5, 15 mM NaCl, and 400 mM sucrose (buffer A). Chlorophyll concentrations were measured in 80% acetone (Porra et al. 1989).
The photoreduction rate of the exogenous electron acceptor 2,6-dichlorophenolindophenol (DCPIP) was determined spectrophotometrically from the change in absorbance at 600 nm, using the molar extinction coefficient for the deprotonated form of DCPIP (ε = 21.8 mM−1 cm−1 Armstrong 1964).
Tris-HCl extraction of manganese [PSII(-Mn) samples] from the OEC. PSII membranes (0.5 mg Chl ml−1) were incubated in a 1 M Tris-HCl buffer (pH 9.4), containing 0.4 M sucrose, for 30 min at 4 °C under room (fluorescent) light (Preston and Seibert 1991). The pelleted membranes were washed twice with buffer A and resuspended in the same buffer. The functional manganese cluster and the three extrinsic polypeptides (17, 23, and 33 kDa) required for photosynthetic oxygen evolution were removed by this treatment (Ghirardi et al. 1996).
Ca-depleted PSII [PSII(-Ca)] membranes were prepared by incubating PSII membranes in buffer, containing 2 M NaCl, 0.4 M sucrose, and 25 mM MES (pH 6.5) (Ono and Inoue 1990). The preparations were incubated in the buffer for 15 min at room temperature under low illumination (4–5 μE m−2 s−1, room fluorescent light). The resulting material was then washed twice with buffer A. Besides, Ca2+ PSII(-Ca) membranes lack the PsbQ and PsbP extrinsic proteins, which prevent exogenous reducing agents from attacking the Mn/Ca cluster.
PSII samples with the HA Mn-binding site blocked by Fe cations [PSII(-Mn,+Fe)] were obtained according to the method described in Semin et al. (2002). PSII(-Mn) membranes (25 μg Chl ml−1) were incubated in buffer A (pH 6.5) containing 5 μM Fe(II) for 3 min under low light (5 μE m−2 s−1) and constant stirring. After incubation, the preparations were precipitated by centrifugation, washed, and resuspended in buffer A.
PSII preparations with a heterogeneous 2Mn–2Fe cluster in the OEC [PSII(2Mn,2Fe)] were obtained by the method developed by Semin and Seibert (2016). PSII(-Ca) membranes (100 μg Chl ml−1) were incubated with ferrous sulfate (20 μM) in buffer A (pH 6.5) for 120 min at 4 °C in the dark. After incubation, the membranes were precipitated by centrifugation, washed, and resuspended in buffer A. The resulting membrane fragments contained precisely two Mn cations and two Fe(III) cations per reaction center in every center (Semin and Seibert 2016).
Flash-probe fluorescence (for measurement of the flash-induced Chl fluorescence decay kinetics) was monitored at 22 °C using a lab-built apparatus (Ghirardi et al. 1996). Membrane suspensions (25 μg Chl ml−1 in buffer A) were excited with a saturating actinic, single-turnover xenon flash (3 μs) passed through a Corion LS-650 low-bandpass filter. Weak monitoring flashes were provided by an array of Hewlett-Packard light-emitting diodes. Dark-adapted samples contained 40 μM DCMU. Fluorescence emission (F), normalized to the fluorescence emitted by the samples due to weak monitoring flashes prior to excitation with a saturating flash (the fluorescence termed F0), was represented in the figures as the ratio (F − F0)/F0 (Ghirardi et al. 1996).
Mn concentrations in different samples were measured as described in a previous publication (Semin and Seibert 2009). Samples (100 μg Chl) were suspended in 1 ml of buffer A and then incubated with 50 mM CaCl2 for 2 min in the dark at 5 °C. After incubation, the samples were microfuged to remove any non-specifically bound Mn. The membrane pellet was resuspended in 90 μl of 0.6 N HCl (pellet volume, about 10 μl) to solubilize the functional Mn remaining in the pellet. Next, 0.9 ml of deionized/glass-distilled water was added to the membrane suspension, and finally, the microfuge tube was centrifuged for 3 min at 15,000×g (22 °C). The supernatant (0.9 ml) was filtered through a 13-mm Acrodisc syringe filter containing a 0.2 μm nylon membrane (Pall Life Sciences, Ann Arbor, USA). The filtrate (in a 1-ml glass/quartz cuvette) was mixed consecutively according to Serrat (1998) with 40 μl of 2 M NaOH, 40 μl of a stock solution of 3,3′,5,5′-tetramethylbenzidine (TMB) (100 mg TMB in 100 ml of 0.1 M hydrochloric acid), and 40 μl of 5.3 M phosphoric acid. The absorbance at 450 nm was used to calculate Mn(II) concentrations (extinction coefficient of 34 mM−1 cm−1) in the samples (Serrat 1998). PSII reaction center (RC) concentrations were calculated in μM using 250 molecules of Chl/RC in the PSII samples (Ghanotakis et al. 1984c; Xu and Bricker 1992).
Fluorescence induction kinetics (FIK) were measured under constant saturating (1200 μE m−2 s−1) light using a Hansatech Instruments, Ltd. (England), Plant Efficiency Analyzer. LEDs (with λmax at 650 nm and a spectral range of 580–710 nm) were used as the source of excitation light. The fluorescence signal, measured 50 μs after switching on the steady light source, was used as the F0 value. The sample was illuminated for 2 s. A common logarithmic scale was used to construct the FIK graphs.
Results and Discussion
Ca2+ effects on the interaction of Fe(II) cations with the HA Mn-binding site in the OEC
The HA Mn-binding site contains a carboxylic group ligand (amino acid residue, D1–D170 Nixon and Diner 1992). According to X-ray diffraction analysis (Umena et al. 2011), the HA site in intact membranes is occupied by a Mn cation denoted as Mn4. D1–E333 also participates in the coordination of Mn4. The same carboxylic group(s) participate in the ligation of either manganese or iron. Interestingly, aspartic acid (D1–D170) also provides a ligand to a Ca2+ cation and is also the bridge between these two cations (Umena et al. 2011). Therefore, we suggest the existence of a Ca2+-cation effect on the interaction of Fe(II) with the HA site.
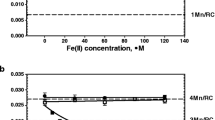
The blocking process of the HA site by iron cation(s) in PSII(-Mn) membranes commences with the weak binding of an Fe(II) to the HA Mn-binding site; the weakly bound Fe(II) cation is then oxidized by a light-induced YZ•+ radical (Semin et al. 2002). This is the first step of blocking process. The efficiency of Fe(II) oxidation at the HA site can be measured separately using the flash-probe fluorescence method. While only one Fe(III) cation blocks the HA site, the oxidation of 4–5 Fe(II) cations is required to complete the blocking process (Semin and Seibert 2004). This bound Fe(III) as mentioned above prevents the further interaction of exogenous Mn(II) cations with this site and hence the donation of electrons to YZ•+ via this site. This is the second step of the blocking process. Completion of both steps is termed the entire blocking process. The efficiency of blocking can also be estimated by measuring electron donation efficiency of via the HA site by Mn(II) cations after blocking. Taking this mechanism of blocking into account, we studied the effect of Ca2+ cations on the donation of electrons by Fe(II) cations to YZ•+ during the light-dependent oxidation process and then on the entire blocking process of the HA sites by Fe(III) cations. Donation of electrons was monitored using flash-probe fluorescence (Ghirardi et al. 1996). The Fmax and Ffin values were used to determine the efficiency of donation, using the equation, [(Fmax − Ffin)/Fmax]−Fe(II) − [(Fmax − Ffin)/Fmax]+Fe(II) (Semin et al. 2002). Here, Ffin is the final fluorescence yield detected after decay of the flash-induced Fmax and Fmax is the maximum fluorescence yield following actinic flash excitation. Ca2+ inhibition of the flash-induced donation of electrons by Fe(II) cations (5 µM) to YZ•+ is shown in Fig. 1 (curve 1). The results show the inhibition of Fe(II) oxidation, which reaches a maximum level of 25% at rather high Ca2+ concentration (about 25 mM, where the Ca/Fe ratio is equal to 5000).
Effect of Ca2+ concentration on the donation to (first step in the text) and blocking of (second step in the text) PSII(-Mn) membranes by iron as determined by the flash-probe fluorescence method. 1 Ca2+ inhibition of flash-induced donation of electrons by exogenous Fe(II) cations (5 µM) in PSII(-Mn) membranes to YZ•+. 2 Ca2+ inhibition of the entire blocking process (see text) associated with the HA site: PSII(-Mn) membranes (25 µg Chl ml−1) were incubated in buffer A with FeSO4 (5 µM) + Ca2+ under room light (4 μE m−2 s−1) for 3 min. The samples were then pelleted and resuspended in buffer A (at 25 µg Chl ml−1), and the flash-fluorescence decay profile was measured in the presence of 40 µM DCMU. The level of blocking in % was determined by estimating Fe(II) (10 µM) donation in blocked membranes, using the equation noted in the text, relative to the 100% control (donation in non-blocked PSII(-Mn) membranes). Data are average of three independent experiments
The Ca2+ concentration dependence on the entire blocking process [electron donation plus tight binding of the Fe(III)] is also shown in Fig. 1 (curve 2). Blocking of the HA site in the presence of different concentrations of Ca2+ was carried out by incubating PSII(-Mn) membranes with Fe(II) for 3 min under weak light. The efficiency of blocking was estimated as the efficiency of donation after blocking compared to that before, measured as described above. The results demonstrate that Ca2+ added together with Fe(II) to PSII(-Mn) samples before incubation decreased the efficiency of blocking. The maximal level of inhibition was observed in the same Ca2+ concentration region as was the case for donation (curve 1), but the efficiency of inhibition was greater (about 40% compared to 25%). By comparing the results (inhibition by Ca2+ of both Fe(II) oxidation and Fe(III) blocking), we note the following: (1) Since the efficiency of these two processes is different, this means that oxidation of Fe(II) cations at the HA site (first step of the blocking process, which can be measured in the absence of the second) is only the initial part of the blocking process. (2) The second step of the blocking process following Fe(II) oxidation is the strong binding of a newly generated Fe(III) cation with the Mn-binding site. The efficiency of the inhibition of the second step is about 15% (40%–25%). (3) The concentration dependence of Fe(II) oxidation (curve 1) and the entire blocking process by Ca2+(curve 2) are similar, saturating at 25–50 mM Ca2+. The same concentration dependence occurs in the case of the activation of oxygen evolution by Ca2+ in PSII(-Ca) membranes (Tamura et al. 1989; Miyao-Tokutomi and Inoue 1992). These results suggest that the effect of Ca on the entire blocking process is the result of conformational changes induced by the interaction of the Ca2+ ion with its specific binding site, which in turn affects the HA site.
In the above-described experiments, Ca2+ was present in the sample during the blocking process. We also investigated the Ca2+ effect when incubation occurred before and after blocking the PSII(-Mn) membranes (Table 1, rows 4, 5). Ca2+ does not change the fluorescence decay curve very much, if the Ca ions were added after the PSII(-Mn) membranes were blocked (Fig. 2). Ca2+ addition to blocked PSII(-Mn) membranes (Table 1, row 5) also had little effect on electron donation. Furthermore, the efficiency of Fe(II) oxidation did not change, if Ca2+ was added before addition of Fe(II) (Table 1, row 4). In this case, membranes were incubated with Ca2+ for 15 min to bind a Ca2+ ion to the Ca-binding site, then unbound Ca2+ was removed by centrifugation, and the membranes were washed with buffer A prior to incubating with Fe(II) to block the HA site. However, the absence of a tightly bound Ca2+ ion to Ca-binding site in this experiment cannot be excluded.
Ca2+ effects on the charge recombination rate between QA− and YZ•+ in blocked PSII(-Mn) membranes as determined by flash-probe fluorescence. The concentration of the PSII(-Mn) membranes was 25 µg Chl ml−1 in buffer A. Fluorescence decays were measured in the presence of 40 µM DCMU without (black curve) or in the presence of 50 mM CaCl2 (red curve). Fluorescence was measured 2–3 min after Ca2+ addition. No Fe(II) was present in this experiment. Each fluorescence kinetic trace is an average of three decays
Nevertheless, we do note that Ca2+ ions significantly affect the rate of fluorescence decay, representing the charge recombination between QA− and YZ•+. The half-time (t1/2) of fluorescence decay is a time constant that reflects the rate of the recombination process. It is equal to 31 ± 1 ms in control PSII(-Mn) membranes, but Ca2+ addition decreases the recombination rate to 51 ± 1 ms (Table 1, rows 1 and 2). This is understandable since the Ca-binding site is close to Yz, and water molecule, W4 (a ligand to Ca), forms a hydrogen bond with YZ. Also, another water molecule, W3 bound to a Ca ion, is connected to YZ via water molecule, W7 (Kawakami et al. 2011). The saturation of this effect is observed beginning at a Ca2+ concentration of about 10 mM (Fig. 3). It should be noted that the t1/2 for a Ca2+ concentration of 50 mM is about 57 ms (the Ca2+ saturation level in Fig. 3). There is some difference (about 10–12%) between this value and the value for a similar sample presented in Table 1 (51 ± 1 ms). Possible reasons for this difference are noted in a footnote of Table 1. Interestingly, blocking of the HA site with iron also increases the rate fluorescence decay (to 46 ± 2 ms) as in the case with Ca2+ (Table 1, compare row 1 with rows 2 and 3). The HA site is also close to YZ and Mn4, the latter of which occupies the site in native PSII membranes. Mn4 also interacts with YZ via the hydrogen bond net, including water molecules W2 (ligand to Mn4), W3 (ligand to Ca2+), and W5–W7 (Kawakami et al. 2011). However, in the case of simultaneous application of both metal cations [Fe(II) and Ca2+], the decrease in the recombination rate is not the sum of the two effects but corresponds only to effect of one cation. So, for example, addition of Ca2+ to PSII(-Mn,+ Fe) membranes does not significantly influence the recombination rate (t1/2 = 42 ± 3 ms vs. 46 ± 2 ms). We have found that the pH dependence of t1/2 values in PSII(-Mn) membranes (Semin and Seibert 2004) changes rapidly in the pH region from 6.0 to 8.0.
Ca2+ effects on the substitution of Mn cations in the OEC with Fe cations
Incubation of PSII(-Ca) membranes with Fe(II) cations in the dark is accompanied by the substitution of one (at pH 5.7) or two (at pH 6.5) Mn ions with Fe cation(s) (Semin and Seibert 2016; Semin et al. 2018). Fe(II) cations chemically reduce available Mn cations in the Mn cluster; reduced Mn(II) cations are released from their binding site(s), and Fe(III) cation(s) formed by the oxidation of the Fe(II) cations occupy the vacant Mn site(s). Using this model of substitution (Semin and Seibert 2016; Semin et al. 2018), we investigated the effect of Ca2+ cations on the Fe for Mn exchange process. Table 2 clearly shows that Ca2+ ions prevent the substitution of 1Mn ion at pH 5.7 [the residual quantity of Mn after treatment with Fe(II) is 4Mn/reaction center (RC)] and also 1Mn ion at pH 6.5 [the residual quantity of Mn after treatment with Fe(II) is 3Mn/RC]. This effect is specific since it is observed not only for Ca2+ cations but also for Sr2+ cations (see Table 3). It is known that Sr2+ is the only other metal cation besides Ca2+ that can restore O2-evolving activity in the Ca-depleted PSII membranes (to about 40% of the control O2-evolution activity at saturating light intensity Ghanotakis et al. 1984a). According to Vrettos et al. (2001b), the ability of Sr2+ to substitute for Ca2+ in the OEC is the result of similarities in the pKa of the aqua ion bound to the cations and ionic radius of cations (see Table 3).
The effect of inhibition by Ca2+ on the substitution of 1Mn cation in the OEC with Fe(II) is also supported by measurements of fluorescence induction kinetics (Fig. 4). The FIK of the native PSII preparations (Fig. 4, curve 1) increases from the minimum level of O to the P level (maximum) due to the reduction of QA and QB by electrons coming from the OEC (Lazar 1999). Extraction of a Ca2+ cation from the OEC results in almost complete inhibition of oxygen release; however, the reduction of the exogenous electron acceptor, DCPIP, is inhibited much less efficiently (the remaining activity is about 70% Semin et al. 2008). This effect was termed decoupling (Semin et al. 2008). The study of the mechanism behind this (“decoupling” effect, not related to the uncoupling of ATP) has shown that electron transport in the absence of oxygen release is due to incomplete oxidation of water, which only proceeds to H2O2 instead of O2 (i.e., the oxidation of water to H2O2 ensures the generation of electrons in PSII(-Ca) preparations for donations to QA and QB) (Semin et al. 2013). The presence of electron flow in the PSII(-Ca) samples is shown by the FIK of this sample (Fig. 4, curve 2), which is similar to the fluorescence kinetics of intact PSII membranes (Semin et al. 2008). Substitution of 2 or 1Mn ions with Fe ions inhibits electron transport (DCPIP reduction) but not completely. Note in Fig. 4 that the sample with 3Mn/RC is more active than the sample with 2Mn/RC (Semin and Seibert 2016; Semin et al. 2018). Electron-transport activity after total Mn extraction by Tris treatment is absent (Semin and Seibert 2016; Semin et al. 2018). We measured the FIK curve of PSII(2Mn,2Fe) samples in Fig. 4, curve 4. The maximum P (Fmax) of this curve is significantly lower than that of curve 2 for PSII(-Ca) samples. Furthermore, if the sample contains 3Mn/1Fe (Table 2, row 3, pH 6.5), the fluorescence curve (Fig. 4, curve 3) has a larger Fmax than that seen in curve 4, but similar to FIK curve 2 in PSII(-Ca) membranes. These results show that the electron-transport activity of this sample is higher than the activity of the PSII(2Mn,2Fe) sample [i.e., during incubation with Fe(II) together with Ca2+ at pH 6.5, the OEC in PSII(-Ca) membranes lose only 1Mn, not 2, supporting results presented in Table 2].
Fluorescence induction curves of chlorophyll a in different membrane preparations of PSII (25 μg Chl ml−1) measured in buffer A at pH 6.5. 1 PSII [the rate of DCPIP reduction was 132 ± 5 μmol DCPIP mg Chl–1 h–1 (100%)]; 2 PSII(-Ca) membrane preparations (DCPIP reduction, 69 ± 3%); 3 PSII(3Mn,1Fe) sample prepared by incubation of PSII(-Ca) membranes (100 µg Chl ml−1) with 20 µM Fe(II) and 10 mM Ca2+ at pH 6.5 (DCPIP reduction, 22 ± 3%); 4 PSII (2Mn,2Fe) prepared at pH 6.5 (DCPIP reduction, 17 ± 2%); 5 PSII (-Mn) (DCPIP reduction, 5 ± 1%)
Other cations (La3+, Cd2+, Ni2+, and Mg2+) do not prevent the extraction of Mn cations in the OEC by Fe(II) (Table 3). It has been suggested that the value of the pK of the aqua metal cation is important for the ability of the cation to restore O2-evolution function in PSII(-Ca) membranes (Vrettos et al. 2001b). However, according to Table 3, the atomic radius of the cation may also be a factor. Our results show that one Mn cation in the cluster is under rather effective Ca2+ control. A bound Ca cation increases the resistance of this Mn cation to chemical reduction by exogenous reductants. In our previous work, we have shown that in the case of the substitution of 2Mn ions with Fe (at pH 6.5) (Semin and Seibert 2016), one of the extracted Mn is bound to the HA site (Mn4). The sensitivity of one of the Mn cations to Ca2+ action at both pH 5.7 and 6.5 (Table 2) allows us to conclude that this Mn binds to the HA site since there is structural proximity of the Ca-binding site. Both sites (for Mn4 and Ca2+) interact with each other via direct hydrogen bonds between water molecules, W2 (ligand to Mn4) and W3 (ligand to Ca), as well as with the hydrogen bond net, including the W5–W7 water molecules (Kawakami et al. 2011). Moreover, these two metal cations are mutually connected via the carboxylic group of D1–D170 and the oxygen bridge, O5, in native PSII preparations (Kawakami et al. 2011). However, it should be noted that such a tight interaction between Mn and Ca2+ cations via hydrogen bonds and ligands can occur only in the intact PSII. In our work, we used mainly PSII(-Mn) and Ca-depleted PSII membranes. It can be assumed that in Mn-depleted and Ca-depleted membranes, these hydrogen bonds are broken. In this regard, we note that the Zouni group (Zhang et al. 2017) recently found that extraction of the Mn/Ca cluster by hydroxylamine from PSII crystals (prepared from native PSII cores) leaves the positions of all the coordinating residues and most of the nearby water molecules largely unaffected. However, recently Gisriel et al. (2020) using cryo-EM methods have shown that the configuration of ligands coordinating the Mn4CaO5 cluster in apo-PSII from Synechocystis sp. PCC 6803 differs from the ligand sphere configuration in the native PSII. These results were supported by data obtained by Zabret et al. (2020) and Tokano et al. (2020). Apparently, the absence of changes in the ligand positions (after extraction of the OEC cluster by hydroxylamine from crystals of PSII) may be due to stabilization of the structure by crystal packing forces (Gisriel et al. 2020; Zabret et al. 2020). Taking these data into account, we suggest that in PSII(-Mn) membranes, the Ca2+ effect on Fe(II) oxidation/blocking is most likely due to competition of Fe and Ca cations for negatively charged amino acids, D1–D170 and D1–E333 associated with the Mn-binding HA site. In the case of PSII(-Ca) membranes, the situation is most likely different. Although a Ca2+ cation is extracted from the OEC, the Ca-binding site is still functional since it can bind a Ca2+ cation, and this binding is accompanied by the restoration of O2-evolution activity (Ghanotakis et al. 1984a). Therefore, Ca2+ effects on the substitution of Mn by Fe can be explained by the interaction of a bound Ca2+ cation with Mn ions through the OEC ligands and/or hydrogen bond net. Our results demonstrate that exogenous Ca2+ prevents the extraction of 1Mn cation (by Fe[II]) from the OEC at both pH 5.7 and pH 6.5 (Table 2). Since extraction of Mn from the OEC results from the reduction of Mn cations in the structure and their subsequent release, we also conclude that the bound calcium ion probably changes the redox potential of the extractable Mn cation (Mn4), making it resistant to reduction.
Abbreviations
- Chl:
-
Chlorophyll
- DCMU:
-
3-(3,4-Dichlorophenyl)-1,1-dimethylurea
- DCPIP:
-
2,6-Dichlorophenolindophenol
- HA:
-
High-affinity Mn-binding site [also known as the site where the “dangler” cation (Mn #4 of the Suga et al. 2015 structure) binds]
- F 0 :
-
Fluorescence emitted by a sample at low light levels prior to flash excitation
- (F − F 0)/F 0 :
-
Fluorescence yield
- F max :
-
Maximum fluorescence yield following actinic flash excitation
- F fin :
-
Final fluorescence yield detected after decay of the flash-induced fluorescence
- MES:
-
2-(N-Morpholino)-ethanesulfonic acid
- OEC:
-
Oxygen-evolving complex
- PSII:
-
Photosystem II
- PSII(-Ca):
-
Ca2+-depleted PSII membranes with 4 Mn cations in the OEC
- PSII(-Mn):
-
Mn-depleted PSII membranes
- PSII(-Mn,+Fe):
-
PSII(-Mn) membranes blocked by an iron cation at the high-affinity Mn-binding site
- RC:
-
Reaction center
- TMB:
-
3,3′,5,5′-Tetramethylbenzidine
- Tris:
-
Tris(hydroxymethyl)aminomethane
References
Ananyev GM, Dismukes GC (1996) High-resolution kinetic studies of the reassembly of the tetra-manganese cluster of photosynthetic water oxidation: proton equilibrium, cations, and electrostatics. Biochemistry 35:14608–14617. https://doi.org/10.1021/bi960894t
Armstrong JM (1964) The molar extinction coefficient of 2,6-dichlorophenolindophenol. Biochim Biophys Acta 86:194–197. https://doi.org/10.1016/0304-4165(64)90180-1
Dean JA (ed) (1985) Lange’s handbook of chemistry. McGraw-Hill Book Co, New York
Ghanotakis DF, Babcock GT (1983) Hydroxylamine as an inhibitor between Z and P680 in photosystem II. FEBS Lett 153:231–234. https://doi.org/10.1016/0014-5793(83)80154-9
Ghanotakis DF, Babcock GT, Yocum CF (1984a) Calcium reconstitutes high rates of oxygen evolution in polypeptide depleted photosystem II preparations. FEBS Lett 167:127–130. https://doi.org/10.1016/0014-5793(84)80846-7
Ghanotakis DF, Topper JN, Yocum CF (1984b) Exogenous reductants reduce and destroy the Mn-complex in photosystem II membranes depleted of the 17 and 23 kDa polypeptides. Biochim Biophys Acta 767:524–531. https://doi.org/10.1016/0005-2728(84)90051-3
Ghanotakis DF, Babcock GT, Yocum CF (1984c) Structural and catalytic properties of the oxygen-evolving complex. Correlation of polypeptide and manganese release with the behavior of Z+ in chloroplasts and a highly resolved preparation of the PSII complex. Biochim Biophys Acta 765:388–398. https://doi.org/10.1016/0005-2728(84)90180-4
Ghirardi ML, Lutton TM, Seibert M (1996) Interactions between diphenylcarbazide, zinc, cobalt, and manganese on the oxidizing side of photosystem II. Biochemistry 35:1820–1828. https://doi.org/10.1021/bi951657d
Gisriel CJ, Zhou K, Huang H-L, Debus RJ, Xiong Y, Brudvig GW (2020) Cryo-EM structure of monomeric photosystem II from Synechocystis sp. PCC 6803 lacking the water-oxidation complex. Joule 4(10):2131–2148. https://doi.org/10.1016/j.joule.2020.07.016
Kawakami K, Umena Y, Kamiya N, Shen J-R (2011) Structure of the catalytic, inorganic core of oxygen-evolving photosystem II at 1.9 Å resolution. J Photochem Photobiol B 104:9–18. https://doi.org/10.1016/j.jphotobiol.2011.03.017
Kern J, Chatterjee R, Young ID, Fuller FD, Lassalle L, Ibrahim M, Gul S, Fransson T, Brewster AS, Alonso-Mori R, Hussein R, Zhang M, Douthit L, de Lichtenberg C, Cheah MH, Shevela D, Wersig J, Seuffert I, Sokaras D, Pastor E, Weninger C, Kroll T, Sierra RG, Aller P, Butryn A, Orville AM, Liang M, Batyuk A, Koglin JE, Carbajo S, Boutet S, Moriarty NW, Holton JM, Dobbek H, Adams PD, Bergmann U, Sauter NK, Zouni A, Messinger J, Yano J, Yachandra VK (2018) Structures of the intermediates of Kok’s photosynthetic water oxidation clock. Nature 563:421–425. https://doi.org/10.1038/s41586-018-0681-2
Kim CJ, Debus RJ (2017) Evidence from FTIR difference spectroscopy that a substrate H2O molecule for O2 formation in photosystem II is provided by the Ca ion of the catalytic Mn4CaO5 cluster. Biochemistry 56:2558–2570. https://doi.org/10.1021/acs.biochem.6b01278
Lazar D (1999) Chlorophyll a fluorescence induction. Biochim Biophys Acta 1412:1–28. https://doi.org/10.1016/S0005-2728(99)00047-X
Miyao-Tokutomi M, Inoue Y (1992) Improvement by benzoquinones of the quantum yield of photoactivation of photosynthetic oxygen evolution: direct evidence for the two-quantum mechanism. Biochemistry 31:526–532. https://doi.org/10.1021/bi00117a032
Najafpour MM, Renger G, Hołyńska M, Moghaddam AN, Aro EM, Carpentier R, Nishihara H, Eaton-Rye JJ, Shen J-R, Allakhverdiev SI (2016) Manganese compounds as water-oxidizing catalysts: from the natural water-oxidizing complex to nanosized manganese oxide structures. Chem Rev 116:2886–2936. https://doi.org/10.1021/acs.chemrev.5b00340
Nixon PJ, Diner BA (1992) Aspartate 170 of the photosystem II reaction center polypeptide D1 is involved in the assembly of the oxygen-evolving manganese cluster. Biochemistry 31:942–948. https://doi.org/10.1021/bi00118a041
Ono T, Inoue Y (1990) Abnormal redox reactions in photosynthetic O2-evolving centers in NaCl/EDTA-washed PS II A dark-stable EPR multiline signal and an unknown positive charge accumulator. Biochim Biophys Acta 1020:269–277. https://doi.org/10.1016/0005-2728(90)90157-Y
Porra RJ, Thompson WA, Kriedemann PE (1989) Determination of accurate extinction coefficients and simultaneous-equations for assaying chlorophyll-A and chlorophyll-B extracted with 4 different solvents: verification of the concentration of chlorophyll standards by atomic-absorption spectroscopy. Biochim Biophys Acta 975:384–394. https://doi.org/10.1016/S0005-2728(89)80347-0
Preston C, Seibert M (1991) The carboxyl modifier 1-ethyl-3-[3-(dimethylamino)propyl] carbodiimide (EDC) inhibits half of the high-affinity manganese-binding site in photosystem II membrane fragments. Biochemistry 30:9615–9624. https://doi.org/10.1021/bi00104a008
Semin BK, Ghirardi ML, Seibert M (2002) Blocking of electron donation by Mn(II) to YZ• following incubation of Mn-depleted photosystem II membranes with Fe(II) in the light. Biochemistry 41:5854–5864. https://doi.org/10.1021/bi0200054
Semin BK, Seibert M (2004) Iron bound to the high-affinity Mn-binding site of the oxygen-evolving complex shifts the pK of a component controlling electron transport via Y(Z). Biochemistry 43:6772–6782. https://doi.org/10.1021/bi036047p
Semin BK, Seibert M (2006) A carboxylic residue at the high-affinity, Mn-binding site participates in the binding of iron cations that block the site. Biochim Biophys Acta 1757:189–197. https://doi.org/10.1016/j.bbabio.2006.02.001
Semin BK, Davletshina LN, Ivanov II, Rubin AB, Seibert M (2008) Uncoupling of processes of molecular synthesis and electron transport in the Ca2+-depleted PSII membranes. Photosynth Res 98:235–249. https://doi.org/10.1007/s11120-008-9347-5
Semin BK, Seibert M (2009) A simple colorimetric determination of the manganese content in photosynthetic membranes. Photosynth Res 100:45–48. https://doi.org/10.1007/s11120-009-9421-7
Semin BK, Davletshina LN, Timofeev KN, Ivanov II, Rubin AB, Seibert M (2013) Production of reactive oxygen species in decoupled, Ca2+-depleted PSII and their use in assigning a function to chloride on both sides of PSII. Photosynth Res 117:385–399. https://doi.org/10.1007/s11120-013-9870-x
Semin BK, Seibert M (2016) Substituting Fe for two of the four Mn ions in photosystem II: effects on water-oxidation. J Bioenerg Biomembr 48:227–240. https://doi.org/10.1007/s10863-016-9651-2
Semin BK, Davletshina LN, Seibert M, Rubin AB (2018) Creation of a 3Mn/1Fe cluster in the oxygen-evolving complex of photosystem II and investigation of its functional activity. J Photochem Photobiol B 178:192–200. https://doi.org/10.1016/j.jphotobiol.2017.11.016
Serrat FB (1998) 3,3′,5,5′-Tetramethylbenzidine for the colorimetric determination of manganese in water. Mikrochim Acta 129:77–80. https://doi.org/10.1007/BF01246852
Suga M, Akita F, Hirata K, Ueno G, Murakami H, Nakajima Y, Shimizu T, Yamashita K, Yamamoto M, Ago H, Shen J-R (2015) Native structure of photosystem II at 1.95 Å resolution viewed by femtosecond X-ray pulses. Nature 517:99–103. https://doi.org/10.1038/nature13991
Suga M, Akita F, Sugahara M, Kubo M, Nakajima Y, Nakane T, Yamashita K, Umena Y, Nakabayashi M, Yamane T, Nakano T, Suzuki M, Masuda T, Inoue S, Kimura T, Nomura T, Yonekura S, Yu L-J, Sakamoto T, Motomura T, Chen J-H, Kato Y, Noguchi T, Tono K, Joti Y, Kameshima T, Hatsui T, Nango E, Tanaka R, Naitow H, Matsuura Y, Yamashita A, Yamamoto M, Nureki O, Yabashi M, Ishikawa T, Iwata S, Shen J-R (2017) Light-induced structural changes and the site of O=O bond formation in PSII caught by XFEL. Nature 543:131–135. https://doi.org/10.1038/nature21400
Tamura N, Cheniae GM (1987) Photoactivation of the water-oxidizing complex in photosystem II membranes depleted of Mn and extrinsic proteins. I. Biochemical and kinetic characterization. Biochim Biophys Acta 890:179–194. https://doi.org/10.1016/0005-2728(87)90019-3
Tamura N, Inoue Y, Cheniae GM (1989) Photoactivation of the water-oxidizing complex in photosystem II membranes depleted of Mn, Ca and extrinsic proteins: II. Studies on the functions of Ca2+. Biochim Biophys Acta 976:173–181. https://doi.org/10.1016/S0005-2728(89)80227-0
Tokano T, Kato Y, Sugiyama S, Uchihashi T, Noguchi T (2020) Structural dynamics of a protein domain relevant to the water-oxidizing complex in photosystem II as visualized by high-speed atomic force microscopy. J Phys Chem B 124(28):5847–5857. https://doi.org/10.1021/acs.jpcb.0c03892
Tsui EY, Agapie T (2013) Reduction potentials of heterometallic manganese–oxido cubane complexes modulated by redox-inactive metals. Proc Natl Acad Sci USA 110:10084–10088. https://doi.org/10.1073/pnas.1302677110
Umena Y, Kawakami K, Shen J-R, Kamiya N (2011) Crystal structure of oxygen-evolving photosystem II at a resolution of 1.9 Å. Nature 473:55–65. https://doi.org/10.1038/nature09913
Vrettos JS, Limburg J, Brudvig GW (2001a) Mechanism of photosynthetic water oxidation: combining biophysical studies of photosystem II with inorganic model chemistry. Biochim Biophys Acta 1503:229–245. https://doi.org/10.1016/S0005-2728(00)00214-0
Vrettos JS, Stone DA, Brudvig GW (2001b) Quantifying the ion selectivity of the Ca2+ site in photosystem II. Evidence for direct involvement of Ca2+ in O2 formation. Biochemistry 40:7937–7945. https://doi.org/10.1021/bi010679z
Xu Q, Bricker TM (1992) Structural organization of proteins on the oxidizing side of photosystem I. Two molecules of the 33-kDa manganese-stabilizing proteins per reaction center. J Biol Chem 267:25816–25821
Zabret J, Bohn S, Schuller SK, Arnolds O, Möller M, Meier-Credo J, Liauw P, Chan A, Tajkhorshid E, Langer JD, Stoll R, Krieger-Liszkay A, Engel BD, Rudack T, Schuller JM, Nowaczyk MM (2020) How to build a water-splitting machine: structural insights into photosystem II assembly. bioRxiv. https://doi.org/10.1101/2020.09.14.294884
Zhang M, Bommer M, Chatterjee R, Hussein R, Yano J, Dau H, Kern J, Dobbek H, Zouni A (2017) Structural insights into the light-driven auto-assembly process of the water-oxidizing Mn4CaO5-cluster in photosystem II. eLife 6:e26933. https://doi.org/10.7554/eLife.26933
Acknowledgements
The work (MS) at the National Renewable Energy Laboratory (NREL) was carried out under US Department of Energy contract number DE-AC36-08-GO28308. MS also acknowledges the support of the NREL Emeritus Program.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Semin, B.К., Davletshina, L.N., Goryachev, S.N. et al. Ca2+ effects on Fe(II) interactions with Mn-binding sites in Mn-depleted oxygen-evolving complexes of photosystem II and on Fe replacement of Mn in Mn-containing, Ca-depleted complexes. Photosynth Res 147, 229–237 (2021). https://doi.org/10.1007/s11120-020-00813-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11120-020-00813-z