Abstract
Although terrestrial CO2 concentrations [CO2] are not expected to reach 1000 μmol mol−1 (or ppm) for many decades, CO2 levels in closed systems such as growth chambers and greenhouses can easily exceed this concentration. CO2 levels in life support systems (LSS) in space can exceed 10,000 ppm (1 %). In order to understand how photosynthesis in C4 plants may respond to elevated CO2, it is necessary to determine if leaves of closed artificial ecosystem grown plants have a fully developed C4 photosynthetic apparatus, and whether or not photosynthesis in these leaves is more responsive to elevated [CO2] than leaves of C3 plants. To address this issue, we evaluated the response of gas exchange, water use efficiency, and photosynthetic efficiency of PSII by soybean (Glycine max (L.) Merr., ‘Heihe35’) of a typical C3 plant and maize (Zea mays L., ‘Susheng’) of C4 plant under four CO2 concentrations (500, 1000, 3000, and 5000 ppm), which were grown under controlled environmental conditions of Lunar Palace 1. The results showed that photosynthetic pigment by the C3 plants of soybean was more sensitive to elevated [CO2] below 3000 ppm than the C4 plants of maize. Elevated [CO2] to 1000 ppm induced a higher initial photosynthetic rate, while super-elevated [CO2] appeared to negate such initial growth promotion for C3 plants. The C4 plant had the highest ETR, φPSII, and qP under 500–3000 ppm [CO2], but then decreased substantially at 5000 ppm [CO2] for both species. Therefore, photosynthetic down-regulation and a decrease in photosynthetic electron transport occurred by both species in response to super-elevated [CO2] at 3000 and 5000 ppm. Accordingly, plants can be selected for and adapt to the efficient use of elevated CO2 concentration in LSS.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
According to the intergovernmental panel on climate change (IPCC), the preindustrial levels of carbon in the atmosphere rose from 285 μmol l−1 to the current level of 384 μmol l−1 and the predicted rise in the atmospheric CO2 would approach 700 μmol l−1 by the year 2050 (IPCC 2007). Such an abnormal rise in the levels of atmospheric CO2 would result in direct and indirect global climate changes. Although terrestrial CO2 concentrations [CO2] are not expected to reach 1000 μmol mol−1 (or ppm) for many decades, CO2 levels in closed systems such as growth chambers and greenhouses can easily exceed this concentration. CO2 levels in life support systems (LSS) in space can exceed 10,000 ppm (1 %). For several years, plant growth has been studying in closed, controlled environments typical of what might been countered in space. In particular, we have been interested in the effects of super-elevated CO2 concentrations in these environments. In the space shuttle cabin atmosphere (~101 kPa total pressure), CO2 concentrations typically range between 4000 and 6000 μmol mol−1, but concentrations can exceed 10,000 μmol mol−1 with large crews (Wheeler et al. 1999). Thus, the CO2 levels for advanced life support systems may be at super-elevated levels, and CO2 may be used as a pressurizing gas for growing plants on Moon or Mars (Wheeler et al. 2001). Similar situations exist on the Russian Mir space station and will probably occur on the planned International Space Station (Mitchell et al. 1994). If plant research is to be carried out in these settings, and if plants are ultimately used to provide food, O2, clean water, and CO2 removal for long duration missions and bioregenerative life support system (BLSS) (Dong et al. 2014), the effects of super-elevated CO2 on plants will need to be understood.
In general, these studies have shown that increases in CO2 (e.g., a doubling from 350 to 700 μmol mol−1) increase photosynthetic rates for C3 species and decrease water use per unit area of vegetation for C3 and C4 species (Högy and Fangmeier 2008; Allen et al. 2011). The present atmospheric [CO2] limits growth of C3 crop plants, which show responses to elevated [CO2] via reduced photorespiration and enhanced photosynthetic rates, thereby increasing their growth and yield. The extensive literature on the impacts of elevated CO2 demonstrates both positive and negative photosynthetic responses in different groups of higher plants, could be attributed to differences in experimental technologies, plant species used for the experiments, age of the plant as well as duration of the treatment (Sage 2002; Davey et al. 2006; Reddy et al. 2010). During photosynthesis, plants transpire large quantities of water to the atmosphere in exchange for CO2 acquisition (Kocacinar 2014). Therefore, there should be a balance in plants for this exchange under each specific environmental niche (Tyree and Zimmermann 2002; Sperry 2003).
Though the C3 pathway of photosynthesis dominates most terrestrial ecosystems, another pathway, C4, is important in certain agricultural and natural systems. The C4 pathway is common among species native to tropical and subtropical grasslands, and important crop species such as corn, sorghum, sugar cane, and pasture grasses possess C4 photosynthesis. In general, these studies have shown that increases in CO2 (e.g., a doubling from 350 to 700 μmol mol−1) increase photosynthetic rates for C3 species and decrease water use per unit area of vegetation for C3 and C4 species (Drake et al. 1997). C4 differs from C3 photosynthesis in several important biochemical and physiological properties. In both types, rubisco fixes CO2 into the photosynthetic carbon reduction pathway common to all aerobic photosynthetic organisms, but the rubisco reaction is compartmented differently (Collatz et al. 1992). In C3 photosynthesis, CO2 fixed by rubisco is obtained directly from the intercellular spaces of the leaf by diffusion, whereas in C4 plants, CO2 is delivered to rubisco, which is located in the bundle sheath chloroplasts, by a metabolic pump that concentrates CO2 (Terashima et al. 2001). The elevated CO2 concentrations maintained in the bundle sheath cells at the cost of additional ATP have the benefit of inhibiting photorespiration. Consequently, C4 plants lack several features of C3 plants that are associated with photorespiration (Collatz et al. 1992). In addition, the kinetics of the photosynthetic CO2 response also differ, because for C4 metabolism, the initial CO2 fixation is via a more efficient catalyst, PEP carboxylase (Drake et al. 1997). These differences in function and compartmentation result in differing sensitivities of net photosynthesis to environmental conditions such as temperature, CO2 and O2 concentrations, light intensity, and nitrogen availability. Generally, these differences tend to favor C4 plants over C3 plants at high temperatures where photorespiration is stimulated.
The Permanent Astrobase life-support artificial closed ecosystem ground-based integrative experimental Facility of Lunar Palace 1 is an ideal site for experiments with agricultural crops under different elevated CO2, offering excellent environmental controls, and at the same time providing a growing environment that, because of the size of both its planting area and soil depth, is closer to mimicking field conditions than any other existing growth chamber. The system can produce water and fertilizer, process waste, and revitalize air. To develop decision support tools for growing plants on the Moon, Mars, or artificial closed ecosystem (Blüm et al. 1994), more studies on the effects of super-elevated [CO2] on different species plants are required under the environmental conditions of human involved expected in space agriculture. However, to date, not much attention has been paid to the study about the mechanisms by which gas exchange and photosynthetic efficiency of PSII respond to long-term super-elevated [CO2] between C3 and C4 plant in controlled environments for developing a safe, nutritious diet for BLSS. In particular, the effects of elevated CO2 on the photosynthesis and transpiration rates between C3 and C4 plant in artificial closed ecosystem are unclear.
In this study, our objective was to investigate the photosynthetic pigment, gas changes, photosynthetic efficiency of PSII, and water use efficiency of a typical C3 plant by soybean (Glycine max (L.) Merr., ‘Heihe35’) and maize (Zea mays L., ‘Susheng’) of C4 plants under a range of CO2 concentrations (500, 1000, 3000 and 5000 μmol mol−1) in controlled environmental conditions of Lunar Palace 1 and reveal plausible underlying mechanisms for the differential physiological and developmental responses to elevated [CO2] between C3 and C4 plants.
Materials and methods
Plant materials
Seeds of soybean (Glycine max (L.) Merr., ‘Heihe35’) and maize (Zea mays L., ‘Susheng’) provided by Heilongjiang Academy of Agricultural Sciences and China Agricultural University, respectively, were planted in stainless steel sinks containing a growth media of vermiculite. All seeds were screened for size, weight, uniformity, and lack of defects and subjected to the germination method by Fernandez-Orozco et al. (2008). The maize is a fast flowering mini variety. The height is only 55 ± 5.2 cm. Seeds were surface-sterilized by soaking in 80 mM hydrogen peroxide (H2O2) for 6 h, then rinsed five times in sterile distilled water (ddH2O), and blot dried (He and Gao 2009). The sterilized seeds were preserved in the dark (Temp. 20 °C, R.H. 85 %). The planting density of was 120 and 80 plants per m2 for soybean and maize, respectively.
Environmental conditions
Light systems were provided with mixtures of red plus white LEDs (R:W = 1:1) at a photosynthetic photon flux (PPF) of 500 ± 20 umol m−2 s−1 at the top of canopy, according to a light/dark regime of 12/12 h (light time from 8:00 a.m. to 8:00 p.m.) for both soybean and maize. Temperature (T) regime was established at 26/22 °C, (light/dark) and relative humidity (RH) in the light time was kept within the optimal range of 65–75 % for all treatments. A modified half-strength Hoagland solution (Table 1) was used as nutrient solution (Hoagland and Arnon 1950). The EC and pH were kept at 2.0 dS m−1 and 5.5 respectively (Wheeler et al. 2008). The air change and dehumidification were guaranteed by axial flow fans and dehumidifiers.
Experimental design and cultural procedures
Lunar Palace 1 could provide a largely closed environment for crop growth tests for BLSS and supply astronauts with basic living requirements. Oxygen, water, and food regenerate through biotechnology, making it possible for astronauts to live in space for long periods. Plants were cultivated in batches every 10 days in the stainless steel pots, which were equipped with the porous-tube nutrient delivery system (PTNDS) implementing water supply on demand (Hoehn et al. 2003) under controlled environmental conditions of Lunar Palace 1. At the flowering stages of plants, 500 ± 50, 1000 ± 50, 3000 ± 50, and 5000 ± 50 μmol mol−1 [CO2] were used in the plant cabin at least 7 days. On the seventh day, all characteristics were analyzed. Both soybean and maize under different treatments of elevated [CO2] (500–5000 ppm) were used in the cabin under 500 ppm [CO2] before flowering stages of plants.
Photosynthetic pigment
The content of Chlorophyll a (Chl a), Chlorophyll b (Chl b), Carotenoid (Car), and the total Chlorophyll (Chl tot) were detected by ultraviolet spectrophotometer (SP-75, Shanghai spectrum instruments co., LTD, China) (Mackinney 1941). Chlorophyll ratio (Chl a/b) is the ratio of Chl a–Chl b. Samples were frozen in liquid nitrogen and stored at −80 °C until use.
Gas exchange
Carbon dioxide gas exchange rates were measured on attached fully expanded third leaves after 7 days of elevated CO2 treatment, using the LI-COR portable infrared CO2 gas analyzer (LI-6400 XRT portable photosynthesis system, LI-COR Biosciences, Lincoln, NE, USA). Block temperature was kept at 25 °C during all measurements (registered leaf temperatures ranging 24–28 °C) and vapor pressure deficit (VPD) at around 1.5 kPa. Measurements were made at a photosynthetic photon flux density (PPFD) of 500 μmol m−2 s−1, as provided by a Q-Beam (blue and red diode) light source. Given the small area (2 cm2) of the leaf cuvette, the flow rate was adjusted to 150 mmol air min−1 to ensure that CO2 differentials between the reference and the sample. IRGAs were >4 μmol mol−1 air. Leaf gas exchange parameters were performed to determine their net photosynthesis (A), stomatal conductance (Gs), intercellular CO2 concentration (Ci), and transient transpiration rate (Tr) under different elevated CO2 concentrations. Water use efficiency (WUE) is the ratio of photosynthetic capacity to transpiration. The intrinsic water use efficiency (IWUE) was calculated by dividing A by Gs, and the instantaneous carboxylation efficiency (ICE) was calculated by dividing A by Ci (Pérez-López et al. 2013; Silva et al. 2010).
Chlorophyll fluorescence
After 7 days of elevated CO2 treatment, Chlorophyll fluorescence measurements were collected using LI-6400 portable photosynthesis systems with 6400-40 Leaf Chamber Fluorometers (LI-COR Inc., Lincoln, NE, USA) on fully expanded leaves. All daytime measurements were carried out between 10:00 and 19:00 h. On the same night after the daytime measurements, fluorescence characteristics F 0 and F m were measured between 02:00 and 04:00 h to obtain minimum and maximum fluorescence yields of fully relaxed samples. After the dark adaptation, minimal fluorescence (F 0) was measured with weak modulated irradiation (0.1 μmol m−2 s−1), and a pulse (800 ms) of saturating light (7000 μmol photons m−2 s−1) was applied to measure the maximum fluorescence yield (F m) and to calculate F v/F m. The fluorescence parameters F s and F m′ were determined as previously described (Baker and Rosenqvist 2004). F s is the steady fluorescence under light-adapted state (red-blue actinic beams, 500 μmol m−2 s−1). Revised parts are marked in yellow in the manuscript. The optimal photochemical efficiency of PSII (F v /F m) was calculated by dividing F v by F m, and the potential activity of PSII (F v /F o) was calculated by dividing F v by F o. The effective quantum yields of PSII (ΦPSII), photochemistry quenching (qP), nonphotochemistry quenching (qN), and photosynthetic electron transport (ETR) were calculated by the methods (Maxwell and Johnson 2000).
Statistical analysis
The measurements were replicated three times and randomly arranged in each block. Statistical analyses were conducted using Statistical Product and Service Solutions for Windows, version SPSS 20 (SPSS Inc., Chicago, Illinois). The data were analyzed using analysis of variance (ANOVA), and the differences between the means were tested using Duncan’s multiple range test (P < 0.05).
Results and discussion
Photosynthetic pigment
The analysis of the chlorophyll content in different elevated [CO2] showed that the chlorophyll patterns varied considerably between C3 and C4 plants. Elevated [CO2] from 500 to 1000 ppm did have a significant effect on the photosynthetic pigment of the leaves by soybean, a typical C3 plant, but no significance for maize, which is a typical C4 plant (Fig. 1). The contents of Chl a, Chl b, Car, and Chl tot by the C4 plants compared to that of the C3 plants are very high at 500 ppm [CO2], but no significance under 1000 and 3000 ppm [CO2] except Chl b under 3000 ppm (Fig. 1a). A higher chlorophyll content in C4 plant may be an energy utilization mechanism that would protect the photosynthetic apparatus against excess CO2. Our results also suggest that high CO2 levels may not always induce significant differences between C3 and C4 plant even when exposed to super-elevated CO2 as shown in this study. The content of photosynthetic pigment including Chl a, Chl b, and Car and Chl tot in both soybean and maize leaves were reduced drastically under 5000 ppm [CO2] (Fig. 1a–d), but the Chl a/b was not changed for C3 species(Fig. 1e). The value reducing of Chl b of C4 species was greater than that of chlorophyll a under 3000 and 5000 ppm [CO2], thus the Chl a/b increased (Fig. 1a, b, e). The leaves of maize under 3000 ppm [CO2] showed a progressively lower chlorophyll contents indicating a faster senescence of leaves that increased during the late growth period (Bindi et al. 2002). Photosynthetic pigment by the C3 plants was more sensitive to elevated [CO2] below 3000 μmol mol−1 than the C4 plants (Fig. 1), indicating that higher CO2 levels did not induced increased susceptibility of photosynthetic pigment composition for C4 plants. Thus, senescence typically also involves cessation of photosynthesis and degeneration of cellular structures, with strong losses of chlorophyll under super-elevated [CO2]. Our results show that super-elevated CO2 concentration of 5000 ppm may prompt loss of chlorophyll from the leaves for both soybean and maize which may accelerate the aging process. The negative CO2 effect on chlorophyll content might be explained with the hypothesis that super-elevated [CO2] induced a higher starch accumulation that disrupts chloroplasts and increases chlorophyll degradation or decreases chlorophyll synthesis (Delucia et al. 1985). Other hypotheses, however, cannot be excluded, such as that where CO2 induced limitation of transpiration might cause decreased in N uptake (Conroy and Hocking 1993) which could contribute to chlorophyll loss. Shangguan et al. (2000) also reported reduction in total chlorophyll content due to nitrogen deficiency. During the process of chlorophyll degradation, Chl b is converted in Chl a (Fang et al. 1998) and this may explain the increase of the ratio Chl a/b in 3000–5000 ppm stressed maize leaves together with the depression of chlorophyll content.
Response of chlorophyll a (Chl a, a), chlorophyll b (Chl b, b), chlorophyll carotenoid (Car, c), total chlorophyll content (Chl tot, d), and chlorophyll ratio (Chl a/b, e) of C3 and C4 plants to different elevated [CO2] conditions. Vertical bars are mean ± SD. Different letters in columns indicate statistically significant differences (P < 0.05)
Gas exchange
The gas exchange parameters were measured in the various C3 and C4 species under the growth conditions at 7 days after the CO2 enrichment for comparing their carbon assimilation efficiencies. As shown in Fig. 2, all gas exchange parameters except Ci/Ca showed significant differences between soybean and maize. The photosynthesis rate (A) of soybean which is typical of a C3 plant showed a steep initial slope in response to varying intercellular CO2 concentration (Ci) and saturated at high 1000 ppm CO2 concentrations (Fig. 2a, b). A of C3 plants increased by 96.8 % at 1000 ppm [CO2], but decreased by 14.1 % at 3000 ppm and 46.7 % at 5000 ppm compared to the values detected under 500 ppm. While photosynthesis in C3 species is stimulated by CO2 concentrations above current ambient levels, it may exhibit photosynthetic acclimation when grown under long-term CO2 enrichment, that is, reduced photosynthetic capacity under saturating light (Morgan et al. 1994). However, [CO2] ranged from 500 to 1000 ppm, there was no significant change of A for C4 plants of maize (Fig. 2a). The C3 species had the highest A at 1000 ppm, but C4 plants exhibited essentially no effect of A and Tr at 1000 ppm, presumably due to the CO2 concentration mechanism of the C4 pathway of photosynthesis. The effects of increased CO2 have been attributed to the assumption that the inherent CO2-concentrating mechanism in C4 plants renders these plants insensitive to elevated CO2 atmosphere (Reddy et al. 2010). Results from previous research with C4 plants demonstrate that C4 photosynthesis is a carbon-concentrating mechanism that evolved from C3 progenitors to many times (Sage et al. 2012), due to a coordinated series of anatomical and biochemical adjustments, established the compartmentation and enzyme activities required to efficiently concentrate CO2 around Rubisco (Monson and Rawsthorne 2000). Furthermore, the C3 and C4 showed approximately 1.6 and 2.7 times lower photosynthesis rate, respectively, at 5000 than 500 ppm (Fig. 2a). A decreased photosynthetic capacity or down-regulation of photosynthesis has been observed in super-elevated CO2 enrichment studies and is termed acclimation (Levine et al. 2008). Based upon findings from elevated [CO2], accelerated senescence has been proposed to explain this phenomenon (Miller et al. 1997; Ludewig and Sonnewald 2000).The mechanism underlying this acclimation is still very elusive. In addition, higher chlorophyll content is suggested to be an adaptation of the plants under elevated CO2 to increase the photosynthetic activity (Bhatt et al. 2010). Besides, under super-elevated [CO2] 5000 ppm by soybean, the CO2 assimilation rate decreased about 40 % of that in 500 ppm [CO2]. The results indicated that lower CO2 assimilation rates could potentially be due either to biochemical photosynthetic capacity or diffusional limitations, the latter involving both stomatal and mesophyll conductance to CO2 (Hanba et al. 2001; Grassi and Magnani 2005).
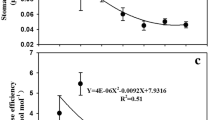
Response of photosynthetic rate (A, a), transpiration rate (Tr, B), intercellular carbon dioxide concentration (Ci, c), Ci/Ca ratio (Ci/Ca, d), stomatal conductance (Gs, e), instantaneous carboxylation efficiency (ICE, f), water use efficiency (WUE, g), and intrinsic water use efficiency (IWUE, h) of C3 and C4 plants to different elevated [CO2] conditions. Vertical bars are mean ± SD. Different letters in columns indicate statistically significant differences (P < 0.05)
Elevated [CO2] ranged from 1000 to 5000 ppm caused a larger increase of Ci and a greater reduction of transpiration rate (Tr) in both C3 and C4 species (Fig. 2b, c). In contrast, CO2 enrichment of 3000 and 5000 ppm decreased Ci/Ca ratio in soybean and maize compared to the values under 500 and 1000 ppm (Fig. 2d). The decreased A and the increased Ci under elevated [CO2] may indicate the non-stomatal limitation of photosynthesis. However,Meinzer et al. (1994) and Saliendra et al. (1996) found a Ci/Ca ratio relatively constant under adverse environmental conditions for sugarcane.
C3 and C4 plants exhibit significant differences in stomatal conductance (Gs) under 500 and 3000 ppm. There is no apparent inhibition of Gs by maize at 1000 ppm [CO2], but Gs decreased greatly above 3000 ppm for both C3 and C4 species. C4 plants in general have lower Gs than C3 plants (Fig. 2e). The C4 maize had significantly higher water use efficiencies (WUE) than the C3 soybean under all elevated [CO2]. At [CO2] of 500 ppm, WUE was about 2.1 times greater in the C4 than the C3 species, but the tendency gradually decreased as [CO2] increased up to 5000 ppm (Fig. 2g). While CO2-induced stomatal closure and improved water relations, with no doubt, contribute significantly to the enhanced growth of C4 species under CO2 enrichment, the possible direct benefits on CO2 fixation should not be discounted. This increased transpiration was found to be associated with increased Gs during both the light and dark cycles (Wheeler et al. 1999). WUE is known to be affected by atmospheric CO2 concentration. Under elevated CO2, Gs in most species would decrease, resulting in less transpiration per unit leaf area. Lower Gs coupled with higher photosynthetic capacity in leaves of C4 plants under ambient [CO2] results in higher water use efficiencies in comparison to leaves of C3 plants (Osborne and Sack 2012). Whereas photosynthesis and stomatal movement are physiological processes that occur within leaves but whose influence on CO2, water vapor, and sensible heat fluxes extends to canopy (Collatz et al. 1992). Moreover, plants grown under 500 ppm had higher Tr than those grown under elevated CO2 1000–5000 ppm, clearly the same of what would be expected (Jarvis and Davies 1998).
Instantaneous carboxylation efficiency (ICE) and intrinsic water use efficiency (IWUE) by soybean which is a typical C3 plant showed a steep initial slope in response to 1000 ppm [CO2] (Fig. 2f, h). As a result of reduced Gs or enhanced A, an increase in IWUE was observed when plants were exposed to atmospheric CO2 enrichment. Elevated CO2 typically increases the rate of photosynthesis in many C3 species by increasing the intercellular CO2 concentration, enhancing the carboxylation efficiency, and reducing photorespiration (Robredo et al. 2007). In contrast, ICE and IWUE at 1000 ppm by C4 maize responded slower to [CO2] compared to soybean (Fig. 2f, h). Additionally, ICEs in C3 soybean and C4 maize were declined fiercely by 200.53 and 89.4 %, respectively, at 3000 ppm compared to the values detected under 1000 ppm (Fig. 2f). When [CO2] was raised to 5000 ppm, IWUE increased in both C3 and C4 species, leading to approximately 131.4 and 62.1 % greater values at 500 ppm, respectively. However, [CO2] ranged from 500 to 3000 ppm, and IWUE had no significant change in maize (Fig. 2h). IWUE was higher in the C3 and C4 under 5000 ppm (Fig. 2h) that may be explained by the lowest Gs (Fig. 2e). Lower Gs is sometimes related to a drop in leaf water content (Robredo et al. 2007). In line with our findings, many articles have reported increases in IWUE ranging from 25 to 230 % as a consequence of both reduced stomatal conductance and enhanced photosynthesis (e.g., Drake et al. 1997; Saxe et al. 1998).
Chlorophyll fluorescence
Indeed, optimal photochemical efficiency of PSII (F v /F m) determined on the youngest fully expanded leaves was not changed. Elevated [CO2] did not significantly affected optimal photochemical efficiency of PSII (F v /F m) between soybean and maize (P > 0.05), whereas F v /F m was significantly higher in maize at lower 500 ppm [CO2] than in soybean. However, F v /F m decreased significantly with super-elevated [CO2] in both crops (Fig. 3a). The potential activity of PSII (F v /F o) for C3 and C4 plants leaves were significantly affected by elevated [CO2] (Fig. 3b). F v /F o was significantly lower at 500 than 1000 ppm [CO2] in both soybean and maize leaves, but declined fiercely at 3000 and 5000 ppm (Fig. 3b). The efficiency and stability of PS II, a major component of the photosynthetic apparatus, have been widely monitored through the measurement of fluorescence of PS II (F v /F m) in dark-adapted leaves (Singh and Agrawal 2014). In the present study, increase in F v /F m ratio under elevated [CO2] 1000 ppm by soybean indicates increased efficiency of PS II and also reduced risk of damage caused to PSII by oxidative stress. Zhao et al. (2010) also reported an increase in F v /F m ratio in Betula platyphylla exposed to 700 ppm CO2.
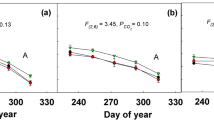
Response of optimal photochemical efficiency of PSII (F v/F m, a), potential activity of PSII (F v/F o, b), photosynthetic electron transport (ETR, c), effective quantum yield of PSII (φPSII, d), photochemistry quenching (qP, e), and nonphotochemistry quenching (qN, f) of C3 and C4 plants to different elevated [CO2] conditions. Vertical bars are mean ± SD. Different letters in columns indicate statistically significant differences (P < 0.05)
The C4 species Z. mays L. had the highest photosynthetic electron transport (ETR) and effective quantum yield of PSII (φPSII) under different [CO2] levels from 500 to 3000 ppm, and exhibited essentially no inhibition of ETR by 3000 ppm [CO2] (Fig. 3c). However, Glycine max (L.) had significantly higher ETR than C4 Z. mays L. under 5000 ppm [CO2] (Fig. 3c). ETR and φPSII showed a steep initial slope in response to 1000 ppm [CO2] and a drastically decrease at 5000 ppm by both species [CO2] (Fig. 3c, d). These results suggest that photosynthesis was improved by elevated CO2 concentration (1000 ppm) in both C3 and C4 plants, and such an improvement was associated with an improvement in stomatal conductance and the actual PSII efficiency. However, the results show that super-elevated [CO2] induced a decreasing φPSII for both C3 and C4 species, indicating that 5000 ppm [CO2] resulted in alterations in PSII photochemistry in the light-adapted leaves.
Under different elevated [CO2] levels, significant differences in photochemistry quenching (qP) and non-photochemical quenching (qN) were obvious for both C3 and C4 as compared with controls (500 ppm). The qP was similar under 3000 and 5000 ppm [CO2] between C3 and C4 species (Fig. 3e). Furthermore, the qP by both cereal crops increased significantly with increasing CO2 up to 1000 ppm and then decreased substantially at 5000 ppm [CO2] (Fig. 3e). This suggests that QA, the primary electron acceptor of PS II, be more oxidized at super-elevated [CO2] despite the much lower rate of photosynthesis in this treatment. A ubiquitous type of regulation is the de-excitation of singlet-excited light-harvesting pigments, measured as qN of chlorophyll fluorescence, which results in the harmless thermal dissipation of absorbed light energy. The maize had high qP, whereas lower qN compared to soybean under 500– 3000 ppm [CO2]. Glycine max (L.) had significantly higher qN than C4 Z. mays L. under 500 and 5000 ppm [CO2]. (Figure 3e, f). CO2 assimilation plays the role of a major sink for the reducing ATP and NADPH which generated by the primary photochemical reactions (Lu and Zhang 2000). When the rate of ATP and NADPH synthesis exceeds the demand for CO2 fixation, the regulation serves by a dissipation mechanism for excess excitation energy (Krause and Cornic 1987). Typically, qN is characterized by modulated pulse fluorometry and it is often assumed implicitly to be a good proxy for the actual physiological photoprotection capacity of the organism (Lambrev et al. 2012). We found that both C3 and C4 plants under 5000 ppm [CO2] showed a lower capacity of photosynthesis and a lower rate of PSII photosynthetic electron transport compared with the plants under 500–3000 ppm [CO2] as well as a corresponding increase in thermal energy of non-photochemical fluorescence quenching. The higher qN under higher [CO2] indicates that a higher proportion of absorbed photons is lost as thermal energy instead of being used to drive photosynthesis (Shangguan et al. 2000) for regulating and protecting photosynthesis in the closed environments in which light energy absorption exceeds the capacity for light utilization.
In this study, measured F v /F m, ϕPSII, qP, and Pn values were somewhat lower in relation to values previously published for the same or closely related species probably due to differences in the growing conditions. We consider that the main reasons may be the following aspects: Firstly, the experiments were carried out in an artificial closed environment ‘Lunar Palace 1.’ Although it can offer environmental controls (including temperature, humility, light identity, etc.), the ventilation of plants cabin by air circulation fans was still not very good compared to the field. It influenced possibilities for crop production and product quality, and reduces the use of chemicals for plant protection (Mistriotis et al. 1997). Second, the traits of photosynthetic characters, nutrient uptake, and biomass yield in most plants in controlled environments were significantly different from the field plantings by the same cultivar (Dong et al. 2015; Collier and Huntington 1983; Patterson et al. 1977). The reasons for this difference in incidence between field and controlled environments are not completely understood, but it is believed to be related to plant growth rate and PSII efficiency (Collier and Huntington 1983). Besides, relative sensitivity of stomata to elevated [CO2] has been described (Dong et al. 2015), and hence, the higher CO2 endured during the seedling stage may have caused some influence on the Gs, leading to somewhat higher qN than expected.
Conclusion
Our results demonstrate that both soybean and maize were cultivated successfully in Lunar Palace 1, and they are the main food crop species which could provide vegetable protein, minerals, and fiber for the diet. In contrast to C3 plants, chlorophyll content by the C4 plants was not sensitive to [CO2] under 1000 ppm, but highly sensitive to super-elevated [CO2] (3000–5000 ppm) for both crops. Elevated [CO2], ranging from 500 to 3000 ppm, in C4 plants had the highest ETR, φPSII, and qP, but photosynthesis efficiency declined fiercely above 5000 ppm [CO2] for both C3 and C4 plants. Therefore, photosynthetic down-regulation and a decrease in photosynthetic electron transport occurred by both species in response to super-elevated [CO2] at 3000 and 5000 ppm. A major challenge would be to develop a whole plant for optimal acclimation responses for increasing atmospheric CO2 concentrations and its interactions with various growth environments. Next we will conduct in-depth research on the high CO2 on soybean and other seed crops by a complex combination of high CO2 concentration and other environmental factors to optimize and use the space station source of high CO2 and improve crop productivity and quality in BLSS. This study also provides technical personnel, a way to improve photosynthetic efficiency by increasing [CO2], and thus to promote crop yields and quality for agricultural facilities (e.g., greenhouse and plant factory). Furthermore, it will play an essential reference to crop production and gas regulation of space technology in BLSS.
Abbreviations
- A:
-
Net photosynthetic rate
- LSS:
-
Life support systems
- Car:
-
Carotenoid content
- Chl a :
-
Chlorophyll a
- Chl b :
-
Chlorophyll b
- Chl a/b :
-
Chlorophyll ratio
- Chl tot:
-
Total chlorophyll content
- Ci:
-
Intercellular carbon dioxide concentration
- ETR:
-
Photosynthetic electron transport
- F v /F m :
-
Optimal photochemical efficiency of PSII
- F v /F o :
-
Potential activity of PSII
- Gs:
-
Stomatal conductance
- ICE:
-
Instantaneous carboxylation efficiency
- IWUE:
-
Intrinsic water use efficiency
- PTNDS:
-
Porous-tube nutrient delivery system
- qP:
-
Photochemistry quenching
- qN:
-
Nonphotochemistry quenching
- Tr:
-
Transpiration rate
- WUE:
-
Water use efficiency
- φPSII:
-
Effective quantum yield of PSII
- [CO2]:
-
Carbon dioxide concentration
References
Allen LH Jr, Kakani VG, Vu JC, Boote KJ (2011) Elevated CO2 increases water use efficiency by sustaining photosynthesis of water-limited maize and sorghum. J Plant Physiol 168(16):1909–1918
Baker NR, Rosenqvist E (2004) Applications of chlorophyll fluorescence can improve crop production strategies: an examination of future possibilities. J Exp Bot 55:1607–1621
Bhatt RK, Baig MJ, Tiwari HS (2010) Elevated CO2 influences photosynthetic characteristics of Avenasativa L. cultivars. J Environ Biol 31:813–818
Bindi M, Hacour A, Vandermeiren K, Craigon J, Ojanperä K, Selldén G, Fibbi L (2002) Chlorophyll concentration of potatoes grown under elevated carbon dioxide and/or ozone concentrations. Eur J Agron 17(4):319–335
IPCC, Climate Change, Fourth Assessment Report, Cambridge University Press, London, 2007
Collatz GJ, Ribas-Carbo M, Berry JA (1992) Coupled photosynthesis-stomatal conductance model for leaves of C4 plants. Funct Plant Biol 19(5):519–538
Collier GF, Huntington VC (1983) The relationship between leaf growth, calcium accumulation and distribution, and tipburn development in field-grown butterhead lettuce. Sci Hortic 21(2):123–128
Conroy J, Hocking P (1993) Nitrogen nutrition of C3 plants at elevated atmospheric CO2 concentrations. Plant Physiol 89:570–576
Davey PA, Olcer H, Zakhleniuk O, Bernacchi CJ, Calfapietra C, Long SP, Raines CA (2006) Can fast growing trees escape biochemical down-regulation of photosynthesis when grown throughout their complete production cycle in the open air under elevated carbon dioxide? Plant Cell Environ 29:1235–1244
Delucia EH, Sasek TW, Strain BR (1985) Photosynthetic inhibition after long-term exposure to elevated levels of atmospheric carbon dioxide. Photosynth Res 7:175–184
Dong C, Fu Y, Liu G, Liu H (2014) Low light intensity effects on the growth, photosynthetic characteristics, antioxidant capacity, yield and quality of wheat (Triticum aestivum L.) at different growth stages in BLSS. Adv Space Res 53:1557–1566
Dong C, Shao LZ, Fu YM, Wang MJ, Liu H (2015) Evaluation of wheat growth, morphological characteristics, biomass yield and quality in Lunar Palace-1, plant factory, green house and field systems. Acta Astronaut 111:102–109
Drake BG, Gonzàlez-Meler MA, Long SP (1997) More efficient plants: a consequence of rising atmospheric CO2? Annu Rev Plant Biol 48(1):609–639
Fang Z, Bouwkamp JC, Solomos T (1998) Chlorophyllase activities and chlorophyll degradation during leaf senescence in non-yellowing mutant and wild type of Phaseolus vulgaris L. J Exp B 49(320):503–510
Fernandez-Orozco R, Frias J, Zielinski H, Piskula MK, Kozlowska H, Vidal-Valverde C (2008) Kinetic study of the antioxidant compounds and antioxidant capacity during germination of Vigna radiata cv. Emmerald, Glycine max cv. Jutro and Glycine max cv. Merit. Food Chem 111:622–630
Grassi G, Magnani F (2005) Stomatal, mesophyll conductance and biochemical limitations to photosynthesis as affected by drought and leaf ontogeny in ash and oak trees. Plant Cell Environ 28(7):834–849
Hanba YT, Miyazawa SI, Kogami H, Terashima I (2001) Effects of leaf age on internal CO2 transfer conductance and photosynthesis in tree species having different types of shoot phenology. Aust J Plant Physiol 28:1075–1084
He L, Gao Z (2009) Pretreatment of seed with H2O2 enhances drought tolerance of wheat (Triticum aestivum L.) seedlings. Afr J Biotechnol 8(22):6151
Hoagland, D.R., Arnon, D.I. (1950). The water-culture method for growing plants without soil. Circular California Agricultural Experiment Station. 347
Hoehn, A., Scovazzo, P., Clawson, J., Geissinger, T., Kalinowski, W., & Pineau, J. (2003). Design, testing and operation of porous media for dehumidification and nutrient delivery in microgravity plant growth systems. SAE Technical Paper, 01-2614
Högy P, Fangmeier A (2008) Effects of elevated atmospheric CO2 on grain quality of wheat. J Cereal Sci 48(3):580–591
Jarvis AJ, Davies WJ (1998) The coupled response of stomatal conductance to photosynthesis and transpiration. J Exp Bot 49:399–406
Kocacinar, F. (2014). Photosynthetic, hydraulic and biomass properties in closely related C3 and C4 species. Physiol Plant
Krause GH, Cornic G (1987) CO2 and O2 interactions in photoinhibition. Elsevier, Amsterdam, pp 169–226
Lambrev PH, Miloslavina Y, Jahns P, Holzwarth AR (2012) On the relationship between non-photochemical quenching and photoprotection of photosystem II. Biochim et Biophysica Acta (BBA) 1817(5):760–769
Levine LH, Kasahara H, Kopka J, Erban A, Fehrl I, Kaplan F, Levine HG (2008) Physiologic and metabolic responses of wheat seedlings to elevated and super-elevated carbon dioxide. Adv Space Res 42(12):1917–1928
Lu C, Zhang J (2000) Photosynthetic CO2 assimilation, chlorophyll fluorescence and photoinhibition as affected by nitrogen deficiency in maize plants. Plant Sci 151(2):135–143
Ludewig F, Sonnewald U (2000) High CO2-mediated down-regulation of photosynthetic gene transcripts is caused by accelerated leaf senescence rather than sugar accumulation. FEBS Lett 479:19–24
Mackinney G (1941) Absorption of light by chlorophyll solutions. J Biol Chem 140:315–322
Maxwell K, Johnson GN (2000) Chlorophyll fluorescence—a practical guide. J Exp Bot 51:659–668
Meinzer FC, Plaut Z, Saliendra NZ (1994) Carbon isotope discrimination, gas exchange, and growth of sugarcane cultivars under salinity. Plant Physiol 104:521–526
Miller A, Tsai CH, Hemphill D, Endres M, Rodermel S, Spalding M (1997) Elevated CO2 effects during leaf ontogeny: a new perspective on acclimation. Plant Physiol 115:1195–1200
Mistriotis A, Bot GPA, Picuno P, Scarascia-Mugnozza G (1997) Analysis of the efficiency of greenhouse ventilation using computational fluid dynamics. Agric For Meteorol 85(3):217–228
Mitchell K. L., Bagdigian R. M., Carrasquillo R. L., Carter D. L., Franks G. D., Holder D. W., Hutchens C. F., Ogle K. U., Perry J. L., & Ray C. D. (1994). Technical assessment of MIR-1 life support hardware for the international space station. NASA Technical Memorandum NASA TM-108441, Marshall Space Flight Center, Huntsville
Monson, R. K., & Rawsthorne, S. (2000). CO2 assimilation in C3–C4 intermediate plants. In Photosynthesis pp.533–550. Springer, The Netherlands
Morgan JA, Hunt HW, Monz CA, LeCain DR (1994) Consequences of growth at two carbon dioxide concentrations and two temperatures for leaf gas exchange in Pascopyrum smithii (C3) and Bouteloua gracilis (C4). Plant Cell Environ 17(9):1023–1033
Osborne CP, Sack L (2012) Evolution of C4 plants: a new hypothesis for an interaction of CO2 and water relations mediated by plant hydraulics. Philos Trans R Soc B 367(1588):583–600
Patterson DT, Bunce JA, Alberte RS, Van Volkenburgh E (1977) Photosynthesis in relation to leaf characteristics of cotton from controlled and field environments. Plant Physiol 59(3):384–387
Pérez-López U, Miranda-Apodaca J, Muñoz-Rueda A, Mena-Petite A (2013) Lettuce production and antioxidant capacity are differentially modified by salt stress and light intensity under ambient and elevated CO2. J Plant Physiol 170:1517–1525
Reddy AR, Rasineni GK, Raghavendra AS (2010) The impact of global elevated CO2 concentration on photosynthesis and plant productivity. Curr Sci 99(1):46–57
Robredo A, Pérez-López U, de la Maza HS, González-Moro B, Lacuesta M, Mena-Petite A, Muñoz-Rueda A (2007) Elevated CO2 alleviates the impact of drought on barley improving water status by lowering stomatal conductance and delaying its effects on photosynthesis. Environ Exp Bot 59(3):252–263
Sage RF (2002) How terrestrial organisms sense, signal and respond to carbon dioxide. Integr Comp Biol 42:469–480
Sage RF, Sage TL, Kocacinar F (2012) Photorespiration and the evolution of C4 photosynthesis. Ann Rev Plant Biol 63:19–47
Saliendra NZ, Meinzer FC, Perry M, Thom M (1996) Associations between partitioning of carboxylase activity and bundle sheath leakiness to CO2, carbon isotope discrimination, photosynthesis, and growth in sugarcane. J Exp Bot 47:907–914
Saxe H, Ellsworth DS, Heath J (1998) Tree and forest functioning in an enriched CO2 atmosphere. N Phytol 139(3):395–436
Shangguan Z, Shao M, Dyckmans J (2000) Effects of nitrogen nutrition and water deficit on net photosynthetic rate and chlorophyll fluorescence in winter wheat. J Plant Physiol 156(1):46–51
Silva EN, Ferreira-Silva SL, Fontenele ADV, Ribeiro RV, Viégas RA, Silveira JAG (2010) Photosynthetic changes and protective mechanisms against oxidative damage subjected to isolated and combined drought and heat stresses in Jatropha curcas plants. J Plant Physiol 167:1157–1164
Singh, A., & Agrawal, M. (2014). Effects of ambient and elevated CO2 on growth, chlorophyll fluorescence, photosynthetic pigments, antioxidants, and secondary metabolites of Catharanthus roseus (L.) G Don. grown under three different soil N levels. Environ Sci Pollut R, 1-11
Sperry JS (2003) Evolution of water transport and xylem structure. Int J Plant Sci 164(S3):S115–S127
Terashima I, Miyazawa SI, Hanba YT (2001) Why are sun leaves thicker than shade leaves?—Consideration based on analyses of CO2 diffusion in the leaf. J Plant Res 114(1):93–105
Tyree MT, Zimmermann MH (2002) Xylem structure and the ascent of sap. Springer, Berlin
Wheeler RM, Mackowiak CL, Yorio NC, Sager JC (1999) Effects of CO2 on stomatal conductance: do stomata open at very high CO2 concentrations? Ann Bot London 83(3):243
Wheeler RM, Stutte GW, Subbarao GV, Yorio NC (2001) Plant growth and human life support for space travel. Handb Plant Crop Physiol 2:925–941
Wheeler RM, Mackowiak CL, Stutte GS, Yorio NC, Ruffe LM, Sager JC, Prince RP, Knott WM (2008) Crop productivities and radiation use efficiencies for bioregenerative life support. Adv Space Res 41:706–713
Zhao X, Mao Z, Xu J (2010) Gas exchange, chlorophyll and growth responses of Betula Platyphylla seedlings to elevated CO2 and nitrogen. Int J Biol 2:143–149
Acknowledgments
We wish to thank the Lunar Palace 1 research and maintenance staff for their support. This work was supported by the Ministry of Science and Technology of China (No. 2012DFR30570).
Author information
Authors and Affiliations
Corresponding author
Additional information
Guest Editor: Congming Lu.
Rights and permissions
About this article
Cite this article
Wang, M., Xie, B., Fu, Y. et al. Effects of different elevated CO2 concentrations on chlorophyll contents, gas exchange, water use efficiency, and PSII activity on C3 and C4 cereal crops in a closed artificial ecosystem. Photosynth Res 126, 351–362 (2015). https://doi.org/10.1007/s11120-015-0134-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11120-015-0134-9