Abstract
To investigate the effects of elevated atmospheric CO2 concentrations ([CO2]) on autumnal phenology and end of season photosynthesis of different bud-break leaves of trees, we fumigated 2-year-old red maple seedlings with 800, 600, and 400 μL L−1 [CO2] in nine continuous stirred tank reactor (CSTR) chambers. Leaves were subdivided into first (B1), second (B2), and third bud-break (B3) leaves. The results indicated that (1) autumnal leaf senescence, including the beginning date, end date, and duration of leaf abscission of all three bud-break leaf groups, was not affected by elevated [CO2]; (2) elevated [CO2] increased leaf photosynthesis of B1, B2, and B3 leaves throughout the whole of the growing season; (3) elevated [CO2] significantly increased whole plant photosynthesis only for B2 leaves, accounting for 41.2–54.7% of the whole plant photosynthesis, due to the larger whole leaf area of B2. In conclusion, enhanced seasonal carbon gain in response to atmospheric CO2 enrichment is the result of strong stimulation of photosynthesis throughout the growing season, especially for B2 leaves but not by extending or shortening the growing season in autumn.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
Elevated atmospheric [CO2] has been shown to affect the growth rhythm of forest trees by altering the timing of spring bud-break (Bigras and Bertrand 2006), flowering (Springer and Ward 2007), and autumnal leaf senescence (Taylor et al. 2008; Tallis et al. 2010; Warren et al. 2011). The timing of autumnal leaf senescence has a significant impact on plant carbon gain, which is directly affected by how many leaves are maintained on the plant and their photosynthetic rates, especially during the late season. However, results from previous experiments, performed with a variety of methods ranging from controlled indoor growth chambers to open-top chambers to free-air CO2 enrichment (FACE) experiments in the field, showed a large variability of specific forest trees going into the autumnal phenophase in response to elevated [CO2]. The response ranged from advances (Warren et al. 2011; Quercus in Asshoff et al. 2006; Sigurdsson 2001; McConnaughay et al. 1996) to delays (Taylor et al. 2008; Tallis et al. 2010; Carpinus and Fagus in Asshoff et al. 2006; Rae et al. 2006; Li et al. 2000) to no effects (Norby et al. 2003a; Norby et al. 2003b; Herrick and Thomas 2003; Li et al. 2018). From these studies, the ecological consequences of elevated [CO2] on phenological changes and productivity seem highly uncertain. There are few reports of the effects of elevated [CO2] on autumnal senescence of leaves of different ages, especially in conditions where nutrients, light, and water are not limiting.
Elevated [CO2] initially increases net primary production by enhancing leaf photosynthesis in most plant communities (Ainsworth and Long 2005; Overdieck 2016). Although the response of leaf-level photosynthesis to CO2 enrichment has been well documented (Ainsworth and Long 2005; Overdieck 2016; Michael et al. 2017), we found that most leaf-level photosynthesis was determined by only one instantaneous middle growing season measurement (Norby et al. 1999). One aspect of this stimulation has been given little attention, and that is whether elevated [CO2] changes leaf photosynthesis during the end of the growing season, especially in different aged leaves. The response of photosynthesis to elevated [CO2] as a function of leaf age and CO2-induced changes in leaf longevity could affect seasonal integrated carbon gain. For the age effect, studies suggest that deciduous broad-leaved trees, after full photosynthetic capacity is attained and maintained following leaf full expansion and maturation, invariably experience a strong decline in leaf photosynthesis with the onset of senescence (Koike 1990; Reich et al. 1999). For CO2-induced changes in leaf longevity, it has been found that due to the different responses to carbon demand under CO2 enrichment, some species inclined to early senescence in autumn while some leaf senescence was delayed in autumn in favor of a longer growing season (Taylor et al. 2008; Warren et al. 2011). Late leaves of overstory sweetgum (Liquidambar styraciflua) had significantly greater photosynthesis from late September to late October under elevated [CO2] than older leaves, while in early October and November, there were no significant differences (Herrick and Thomas 2003). Leaf phenology and photosynthetic function define how much carbon a plant fixes during the season. A change in either of these factors will substantially affect carbon gain as CO2 continues to increase. It is important to understand late-season net photosynthesis at the leaf level and whole plant scale to integrate the response to elevated [CO2] across an entire growing season. Few studies directly incorporate estimates of the response of different bud-break leaves of seedlings to elevated [CO2]. Based on the above, we assume that net photosynthesis cannot be determined by only a single instantaneous middle growing season measurement without considering phenology, leaf age, and consecutive measurements of late-season net photosynthesis. It is therefore of concern to evaluate whether changes in the length of the growing season that affect changes in leaf senescence could explain some of the inconsistencies between leaf/plant level photosynthesis and annual growth.
Red maple is one of the most common and widespread deciduous trees of eastern and central North America and is adaptable to a very wide range of site conditions. In this experiment, we examine whether elevated [CO2] alters the timing of leaf drop during autumnal senescence when soil nitrogen and water are not limiting, and whether elevated [CO2] alters photosynthetic carbon gain during autumnal senescence in leaves and the whole plant as a function of leaf age. We hypothesize that atmospheric CO2 enrichment prolongs leaf span and thus delays the timing of leaf abscission of red maple, so photosynthesis also lasts longer until delayed leaf senescence finishes. Red maple trees in our experiment have shoots that are produced three times during the growing season. Thus, we followed demography and late-season gas exchange by dividing all leaves into first, second, and third bud-break leaves.
2 Materials and Methods
2.1 Experimental System and Design
The experiment was performed in nine continuously stirred tank reactor (CSTR) chambers, which were located inside a glass greenhouse at the University of Massachusetts, Amherst. The irradiance inside was around 85–95% of that outside. Details of the operation of the CSTRs and CO2 control and measurement system were previously described (Li et al. 2018; Elagöz et al. 2006; Manning and Krupa 1992). Light, water, and nutrient were not limiting factors in the experiment. The time course of the irradiance was adjusted to be similar to the natural environment outside the greenhouse. Three [CO2] treatments, 800 (A800), 600 (A600), and 400 (A400) μL L−1 were set randomly among the nine chambers with three replications each. [CO2] enrichment was administered with pure CO2 continuously for 24 h, with the [CO2] fluctuation range being ± 10 μL L−1 around the target [CO2].
2.2 Plant Material and Management
Two-year-old red maple seedlings grew from field seeds obtained from a nursery of North Amherst, MA. Twenty-seven seedlings, uniform in height and basal diameter, were selected and divided into nine groups of three pots each (bottom diameter 18 cm, top diameter 25 cm, and height 24 cm). Readings began in early April, 2014 when first bud-break leaves were observed and marked. All seedlings with their pots were transplanted to the greenhouse on 3 June. The growing medium used was SunGro MetroMix 300 (SunGro Horticulture, MA, USA). Before being placed into CSTRs, the seedlings were acclimated to the greenhouse environment from 3 June to 25 June (23 days) until all seedlings grew well. The seedlings were then moved with their pots into the CSTRs on 26 June. All seedlings were watered every other day and fertilized with a water-soluble fertilizer (16–17–18; Peters Professional; Scotts, OH, USA) (3.9 g L−1) weekly. A micronutrient soluble trace (B + Cu + Fe + Mn + Mo + Zn, Micromax TE Mix, Micromax, SD, USA) was applied once on 8 August. Leaves were separated and marked into three groups: first bud-break (B1) leaves were produced mainly in late April, second bud-break leaves (B2) were produced generally in the period 3–20 June, and then after a bud-break suspension for almost 10 days, third bud-break leaves (B3) were produced until 10 July. The position of all seedlings inside the chamber was moved every week to mitigate the possibility of extraneous effects. Readings were carried out from 26 June to 28 November, 2014 (156 days).
2.3 Leaf Dynamics Measurements
The red maple trees produced leaves continuously from late April to early July. We consider natural leaf senescence as significant declines in photosynthetic capacity, with the color of the leaves also changing during senescence (Kikuzawa and Lechowicz 2011). The senescing leaves were easily identified as they displayed yellowish colors and were easily removed by very gentle shaking. Leaf numbers at B1, B2, and B3 were counted twice every month prior to October, when leaf senescence began and the frequency was changed to once every other day until total abscission.
Leaf area was calculated by the formula (Wargo 1978):
where L and W were the longest length (cm) and widest width (cm) of the maple leaf, respectively. The whole plant leaf area was calculated as the sum of the total first, second, and third bud-break leaf areas, which were calculated in turn as the products of individual leaf number and leaf area in the first, second, and third bud-break leaves, respectively. We measured six selected marked leaves (the same leaves used for photosynthesis measurements) from three bud-break cohorts every time.
2.4 Chlorophyll Index
Chlorophyll index was measured weekly from 5 September until complete senescence with a SPAD-502 meter (Konica Minolta, Tokyo, Japan) in the same marked leaves used for determining both leaf area and photosynthesis. Readings on each of the selected leaves were taken in triplicate at each measurement date. The non-destructive SPAD measurements are considered to be a good surrogate for leaf chlorophyll concentration (Hoel and Solhaug 1998) and have previously been used in other atmospheric CO2 enrichment studies (Herrick and Thomas 2003).
2.5 Leaf and Whole Plant Light-Saturated Net Photosynthetic Rate
Leaf light-saturated net photosynthetic rate (PNsat) was measured using a portable Li-Cor 6400 photosynthesis system with a 6400-02B LED light source chamber (Li-Cor Inc., Lincoln NE, USA). The system controlled saturating PPDF at 1000 μmol m−2 s−1 (light saturation point), and the [CO2] inside the leaf chamber was set to be similar to the CO2 treatments through a supply from a CO2 cylinder. Each of two fully expanded sun leaves from the first, second, and third bud-break leaves per seedling were selected individually and marked for the measurements of leaf light-saturated net photosynthetic rate. The CO2 and irradiance-acclimated time for a leaf was around 10–15 min until the cuvette photosynthetic rate parameters kept stable. To avoid the “noon-sleep” phenomenon, all measurements were conducted from 09:00 to 11:30 and 14:00 to 15:00. The whole plant light-saturated photosynthetic rate (PNsatw) in B1, B2, and B3 was determined by the sum of products of PNsat and whole plant leaf area (i.e., the products of leaf number and individual leaf area) from B1, B2, and B3, respectively.
2.6 Data Analysis
The effects of [CO2] on leaf phenology (i.e., beginning, end, and duration of leaf abscission) were analyzed by one-way ANOVA with the chamber as the experimental unit (three chambers at each [CO2]) and the three [CO2] as the levels of the treatment factor. The remaining response variables (e.g., photosynthetic rate and leaf number) were analyzed using repeated measures analysis of variance with [CO2] and leaf age (if present) as between-subject variables and the measurement time as the within-subject variable. Multiple comparisons among the first, second, and third bud-break leaves were performed with the Bonferroni test when the CO2 or age effect was significant, considered to occur when P < 0.05. Before the statistical analyses, data were tested for normality with the Kolmogorov–Smirnov test. All data analyses were carried out with SPSS statistics software (Version 18.0, SPSS Inc., Chicago, IL, USA).
3 Results
3.1 Autumnal Leaf Phenology
We found that the timing of the start, end, and duration of the autumnal leaf abscission of the first (B1), second (B2), and third (B3) bud-break leaves was not influenced by [CO2] but they were significantly different due to leaf age (Tables 1 and 2). The average starting date of abscission of B1 leaves was 48 and 59 days earlier than B2 and B3 leaves, respectively, while the end date of abscission of B1 leaves was 9 and 13 days earlier than B2 and B3 leaves, respectively. Thus, the average duration of abscission in B1 leaves was 39 and 46 days longer than B2 and B3 leaves, respectively. No interactions between elevated [CO2] and leaf age were detected (Table 2).
3.2 Chlorophyll Index, Leaf Number, and Whole Plant Leaf Area
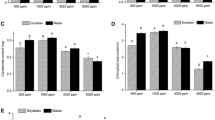
CO2 concentration and leaf age both significantly affected chlorophyll index as measured by SPAD (Table 2), but the effect of these treatments throughout the growing season is not straightforward, given the different leaf phenologies. For all leaf age groups, chlorophyll index declined continually throughout the measurement period (Fig. 1). First and second bud-break leaves did not display a significant effect due to [CO2] (P > 0.05), although there was a trend towards lower SPAD values resulting from elevated [CO2] (A600 and A800) compared with A400. For B3 leaves, [CO2] significantly affected the chlorophyll index (P = 0.03), showing a 17.3% and 22.3% decline due to the A600 and A800 treatments at its greatest extent (day 232, 20 August), respectively.
Chlorophyll index (assessed as SPAD values) of the first (a), second (b), third bud-break (c) leaves of red maple (Acer rubrum) under three CO2 concentration treatments, 400 (A400), 600 (A600), and 800 (A800) μL L−1. Values signify mean ± SD. Uppercase letters in the graph indicate significant differences among the three bud-break leaf groups (P < 0.05)
Unlike chlorophyll index, differing [CO2] did not affect leaf number and whole plant leaf area (Table 2). However, leaf age did affect those response variables (Fig. 2, Table 2), as B3 leaves were more abundant while B2 grew a larger whole plant leaf area (Fig. 2). Significant synergistic interactions of [CO2] and leaf age were found on whole plant leaf area (Table 2). For instance, whole plant leaf area of B2 increased by factors of 8.01, 4.12, and 1.61 on average due to A800, A600, and A400 treatments, respectively, compared with B1 (Fig. 2).
Attached leaf numbers and whole plant leaf area of first bud-break (a, d), second bud-break (b, e), third bud-break (c, f) of red maple (Acer rubrum) under three CO2 concentration treatments, 400 (A400), 600 (A600), and 800 (A800) μL L−1. Values signify mean ± SD. Uppercase letters in the graph indicate significant differences among the three bud-break leaf groups (P < 0.05)
3.3 Leaf and Whole Plant Light-Saturated Net Photosynthetic Rate
Leaf light-saturated net photosynthesis (PNsat) was significantly affected both by [CO2] and leaf age (Table 2, Fig. 3). B3 leaves generally had higher PNsat than B1 and B2 leaves. Although PNsat variably declined strongly as they aged and with the onset of senescence, elevated [CO2] consistently enhanced leaf PNsat of B1, B2, and B3 leaves until late in the growing season (Fig. 3). At their greatest extent (prior to day 268, 25 Sept.), PNsat in A800 showed a 105.6%, 59.8%, and 74.5% increase, respectively, compared with A400 in B3, B2, and B1 leaves, while A600 showed a 43.8%, 37.6%, and 28.6% increase. After day 268 (25 Sept.), when leaf abscission started, these differences among the effects of A800, A600, and A400 tended to be maintained until the end of the observation period. No significant interaction between [CO2] and leaf age was observed for PNsat (Table 2).
Effects of CO2 concentration, 400 (A400), 600 (A600), and 800 (A800) μL L−1, on PNsat of the first (a), second (b), and third bud-break leaves (c) of Acer rubrum. Values signify mean ± SD. Uppercase letters in the graph indicate significant differences among the three bud-break leaf groups (P < 0.05)
Although whole plant light-saturated net photosynthetic rate (PNsatw) was significantly affected both by CO2 concentration and leaf age, as was PNsat, there was a significant interaction between the two factors (Table 2). Elevated [CO2] only significantly enhanced PNsatw of B2 leaves but not B1 and B3 leaves (Fig. 4). For the B2 leaves, A800 and A600 enhanced PNsatw by 189.9% and 78.4% compared with A400 (Fig. 4). For age effects, PNsatw of B2 was 44.3% and 108% higher, respectively, than PNsatw of B3 and B1.
Whole plant PNsatw of the first bud-break (a), second bud-break (b), and third bud-break leaves (c) of Acer rubrum under three CO2 concentration treatments, 400 (A400), 600 (A600), and 800 (A800) μL L−1. Values signify mean ± SD. Uppercase letters in the graph indicate significant differences among the three bud-break leaf groups (P < 0.05)
We also determined the contribution of the three bud-break leaf groups to PNsatw. On average, B1 contributed 14–22%, B2 41–55%, and B3 29–40% (Table 3), there being no direct effect of [CO2] or interaction with leaf age (Table 2).
4 Discussion
The timing of autumnal leaf senescence has a significant impact on ecosystem productivity (Moors 2010; Polgar and Primack 2011). The present results indicated that autumnal phenology (including the beginning date, ending date, or the duration of leaf abscission) of Acer rubrum was not affected by elevated [CO2], results of which are similar to those of Norby et al. (2003b), who found that CO2 enrichment (+ 300 μL L−1) had no effect on the timing of autumnal leaf abscission of Acer rubrum after 4 years of growth in open-top chambers with seedlings planted into soil. This result for Acer rubrum is also in accordance with our previous study (Li et al. 2018) on sugar maple (A. saccharum Marsh), but different from the outcome for American linden (Tilia americana L.) (Li et al. 2019a). The experiments with the three species were conducted at the same time with the same treatments. We attribute its lack of response to elevated [CO2] to the nature of its sensitivity to the photoperiod (day length relative to night length) (Körner and Basler 2010) and temperature (Norby et al. 2003a, 2003b) of this early-successional species (Miao and Bazzazs 1993). This characteristic is controlled genetically and cannot be easily changed by transplantation or variable environmental factors (Borchert et al. 2005). The life span of single leaves including the timing of abscission, the differences of the duration of abscission in the B1, B2, and B3 leaf groups can also be explained by a cost-benefit theory, which suggests that leaf longevity is the result of a trade-off among photosynthetic rates, construction costs, and maintenance costs as the leaf tries to maximize total biomass production (Kikuzawa and Lechowicz 2011). We also found that abscission duration in B1 lasted longer, which may lead to higher N resorption efficiency in autumn (Li et al. 2019b).
Chlorophyll has an important role in photosynthesis and a high correlation with N contents (Sharwood et al. 2017). Interestingly, PNsat in B1, B2, and B3 were consistently enhanced by elevated [CO2] until the end of the growing season (Fig. 3) while chlorophyll index significantly declined under elevated [CO2] (Fig. 1). It is possible that less nitrogen is invested in the light-harvesting complex in chloroplasts (Overdieck 2016) but more nitrogen is allocated to RuBP (ribulose-1,5-bisphosphate) regeneration and Pi (inorganic phosphate) regeneration (Moore et al. 1999; Urban et al. 2012). Thus, the nitrogen use efficiency of plants in photosynthesis increases and could also maintain sufficient photosynthetic capacity during the growing season.
Elevated [CO2] stimulates photosynthesis of many plant species when rates are measured during the middle portion of the growing season but less is known about the situation during the end of the growing season (Ainsworth and Long 2005). We found that during the period that leaf senescence occurred, one growing season elevated [CO2] consistently stimulated photosynthesis of B1, B2, and B3 leaves until the end of experiment, unlike the findings that elevated [CO2] reduced photosynthetic rates midway through the senescent period (McConnaughay et al. 1996). The magnitude of the photosynthetic response to elevated [CO2] was variable and was maintained at the end of the growing season as the leaves senesced. Before day 269 (26 Sept.), PNsat with treatment A800 showed a 59.8–105.6% increase compared with A400, while A600 gave a 28.6–43.8% increase compared with A400 (Fig. 3), which was comparable with other similar studies, e.g., on Acer rubrum (red maple) (Norby et al. 1999; Norby et al. 2010), Liriodendron tulipifera (yellow-poplar), and Quercus alba (white oak) (Gunderson et al. 1993). This enhancement of photosynthesis by elevated [CO2] in late season is in accordance with Herrick and Thomas (2003), who found that whole-shoot net photosynthetic rates of overstory sweetgum (Liquidambar styraciflua) were enhanced by atmospheric CO2 enrichment throughout the season until early November. These results indicate that atmospheric CO2 enrichment stimulates seasonally integrated C gain of red maple trees by increasing photosynthesis consistently until the end of, but not by changing the length of, the growing season for leaf phenology. PNsat and PNsatw displayed inconsistencies due to significant differences in whole plant leaf area of B2 leaves (Fig. 2), where in mid-season, more appropriate temperature and light led to larger whole plant leaf area than for B1 and B3 leaves. It is therefore important when estimating whole plant photosynthesis to divide leaves into different bud-break stages before making individual leaf photosynthesis measurements, as the effect of elevated [CO2] on whole plant photosynthesis is expressed mainly through the B2 leaves.
Although in this experiment we investigated conditions where water, nutrient, and light were not limiting, and we employed large pots as space permitted, it has to be noted that autumnal leaf phenology was confounded and controlled by a complex suite of environmental factors besides elevated [CO2], such as soil nutrient conditions, plant culture, temperature, duration of the experiment, and photoperiod, of which temperature and photoperiod are the most important. Thus, our results may only apply when light, soil nutrition, and soil water are not limiting. Complementary long-term experiments are clearly needed.
5 Conclusions
We found that elevated [CO2] did not alter the abscission timing of leaves in first, second, and third bud-break leaves, and thus had no effect on the length of the growing season in autumn. Elevated [CO2] increased net photosynthesis in all leaves throughout the season and the second bud-break leaves contributed more to whole plant photosynthesis due to their larger leaf area. Thus, enhanced carbon gain in response to atmospheric CO2 enrichment in Acer rubrum will be the result of strong stimulation of photosynthesis throughout the growing season specifically on second bud-break leaves, including the portion of the season during which leaves senesce, but not by extending or shortening the growing season in autumn.
Abbreviations
- P Nsat :
-
leaf light-saturated net photosynthetic rate
- P Nsatw :
-
whole plant light-saturated photosynthetic rate
References
Ainsworth, E. A., & Long, S. P. (2005). What have we learned from 15 years of free-air CO2 enrichment (FACE)? A meta-analytic review of the responses of photosynthesis, canopy properties and plant production to rising CO2. New Phytologist, 165, 351–372. https://doi.org/10.1111/j.1469-8137.2004.01224.x.
Asshoff, R., Zotz, G., & Koerner, C. (2006). Growth and phenology of mature temperate forest trees in elevated CO2. Global Change Biology, 12, 848–861. https://doi.org/10.1111/j.1365-2486.2006.01133.x.
Bigras, F. J., & Bertrand, A. (2006). Responses of picea mariana to elevated CO2 concentration during growth, cold hardening and dehardening: phenology, cold tolerance, photosynthesis and growth. Tree Physiology, 26, 875–888. https://doi.org/10.1093/treephys/26.7.875.
Borchert, R., Robertson, K., Schwartz, M. D., & Williams-Linera, G. (2005). Phenology of temperate trees in tropical climates. International Journal of Biometeorology, 50(1), 57–65. https://doi.org/10.1007/s00484-005-0261-7.
Elagöz, V., Han, S. S., & Manning, W. J. (2006). Acquired changes in stomatal characteristics in response to ozone during plant growth and leaf development of bush beans (Phaseolus vulgaris L.) indicate phenotypic plasticity. Environmental Pollution, 140, 395–405. https://doi.org/10.1016/j.envpol.2005.08.024.
Gunderson, C. A., Norby, R. J., & Wullschleger, S. D. (1993). Foliar gas exchange responses of two deciduous hardwoods during 3 years of growth in elevated CO2: no loss of photosynthetic enhancement. Plant, Cell & Environment, 16(7), 797–807. https://doi.org/10.1111/j.1365-3040.1993.tb00501.x.
Herrick, J. D., & Thomas, R. B. (2003). Leaf senescence and late-season net photosynthesis of sun and shade leaves of overstory sweetgum (Liquidambar styraciflua) grown in elevated and ambient carbon dioxide concentrations. Tree Physiology, 23, 109–118. https://doi.org/10.1093/treephys/23.2.109.
Hoel, B. O., & Solhaug, K. A. (1998). Effect of irradiance on chlorophyll estimation with the Minolta SPAD-502 leaf chlorophyll meter. Annals of Botany, 82, 389–392. https://doi.org/10.1006/anbo.1998.0683.
Kikuzawa, K., & Lechowicz, M. J. (2011). “Ecology of leaf longevity”in Ecological research monographs, 1st ed. (Tokyo: Springer). https://doi.org/10.1007/978-4-431-53918-6.
Koike, T. (1990). Autumn coloring, photosynthetic performance and leaf development of deciduous broad-leaved trees in relation to forest succession. Tree Physiology, 7, 21–32. https://doi.org/10.1093/treephys/7.1-2-3-4.21.
Körner, C., & Basler, D. (2010). Phenology under global warming. Science, 327, 1461–1462.
Li, L., Xiaoke, W., & Manning, W. J. (2019a). Effects of elevated CO2 on leaf senescence, leaf nitrogen resorption, and late-season photosynthesis in Tilia americana L. Frontiers in Plant Science, 10, 1217. https://doi.org/10.3389/fpls.2019.01217.
Li, J. H., Dijkstra, P., Hymus, G. J., Wheeler, R. M., & Drake, B. G. (2000). Leaf senescence of Quercus myrtifolia as affected by long-term CO2 enrichment in its native environment. Global Change Biology, 6, 727–733. https://doi.org/10.1046/j.1365-2486.2000.00347.x.
Li, L., Manning, W. J., & Xiaoke, W. (2018). Autumnal leaf abscission of sugar maple is not delayed by atmospheric CO2 enrichment. Photosynthetica, 56, 1134–1139. https://doi.org/10.1007/s11099-018-0802-z.
Li, L., Manning, W. J., & Xiaoke, W. (2019b). Elevated CO2 increases root mass and leaf nitrogen resorption in red maple (Acer rubrum L.) for more N demand. Forests, 10, 420.
Manning, W. J., & Krupa, S. V. (1992). Experimental methodology for studying the effects of ozone on crops and trees. In R. B. Flagler (Ed.), Surface level ozone exposures and their effects on vegetation (pp. 93–156). Lewis Publishers, Inc: Chelsea.
McConnaughay, K. D. M., Bassow, S. L., Berntson, G. M., & Bazzaz, A. F. (1996). Leaf senescence and decline of end of season gas exchange in five temperate deciduous tree species grown in elevated CO2 concentrations. Global. Global Change Biology, 2, 25–33. https://doi.org/10.1111/j.1365-2486.1996.tb00046.x.
Miao, F. A., & Bazzazs, L. (1993). Successional status, seed size, and responses of tree seedlings to CO2, light, and nutrients. Ecology, 74(1), 104–112.
Michael, T., Dananjali, G., Naoki, H., Anke, M., & Saman, S. (2017). Effects of elevated carbon dioxide on photosynthesis and carbon partitioning: a perspective on root sugar sensing and hormonal crosstalk. Frontiers in Physiology, 8, 578. https://doi.org/10.3389/fphys.2017.00578.
Moore, B. D., Cheng, S. H., Sims, D., & Seeman, J. R. (1999). The biochemical and molecular basis for photosynthetic acclimation to elevated atmospheric CO2. Plant, Cell & Environment, 22, 567–582. https://doi.org/10.1046/j.1365-3040.1999.00432.x.
Moors, E. J. (2010). Influence of spring and autumn phenological transitions on forest ecosystem productivity. Philosophical Transactions of the Royal Society of London, 365, 3227–3246. https://doi.org/10.1098/rstb.2010.0102.
Norby, R. J., Wullschleger, S. D., Johnson, D. W., & Ceulemans, R. (1999). Tree responses to rising CO2 in field experiments: implications for the future forest. Plant, Cell & Environment, 22, 683–714. https://doi.org/10.1046/j.1365-3040.1999.00391.x.
Norby, R. J., Sholtis, J. D., Gunderson, C. A., & Jawdy, S. S. (2003a). Leaf dynamics of a deciduous forest canopy: no response to elevated CO2. Oecologia (Berlin), 136, 574–584. https://doi.org/10.1007/s00442-003-1296-2.
Norby, R. J., Hartz-Rubin, J. S., & Verbrugge, M. J. (2003b). Phenological responses in maple to experimental atmospheric warming and CO2 enrichment. Global Change Biology, 9, 1792–1801. https://doi.org/10.1111/j.1365-2486.2003.00714.x.
Norby, R. J., Warren, J. M., Iversen, C. M., Medlyn, B. E., & Mcmurtrie, R. E. (2010). CO2 enhancement of forest productivity constrained by limited nitrogen availability. PNAS, 107, 19368–19373. https://doi.org/10.1073/pnas.1006463107.
Overdieck, D. (2016). CO2, temperature, and trees (pp. 84–Singapore, 85). Springer Singapore.
Polgar, C. A., & Primack, R. B. (2011). Leaf-out phenology of temperate woody plants: from trees to ecosystems. New Phytologist, 191, 926–941. https://doi.org/10.1111/j.1469-8137.2011.03803.x.
Rae, A. M., Ferris, R., Tallis, M. J., & Taylor, G. (2006). Elucidating genomic regions determining enhanced leaf growth and delayed senescence in elevated CO2. Plant, Cell & Environment, 29, 1730. https://doi.org/10.1111/j.1365-3040.2006.01545.x.
Reich, P. B., Ellsworth, D. S., Walters, M. B., Vose, J. M., Grewham, C., Volin, J. C., et al. (1999). Generality of leaf trait relationships: a test across six biomes. Ecology, 80, 1955–1969. https://doi.org/10.2307/176671.
Sharwood, R. E., Crous, K. Y., Whitney, S. M., Ellsworth, D. S., Ghannoum, O. (2017). Linking photosynthesis and leaf allocation under future elevated CO2, and climate warming in Eeucalyptus globulus. Journal of Experimental Botany, erw484. https://doi.org/10.1093/jxb/erw484.
Sigurdsson, B. (2001). Elevated CO2 and nutrient status modified leaf phenology and growth rhythm of young Populus trichocarpa trees in a 3-year field study. Trees, 15, 403–413. https://doi.org/10.1007/s004680100121.
Springer, C. J., & Ward, J. K. (2007). Flowering time and elevated atmospheric CO2. New Phytologist, 176, 243–255. https://doi.org/10.1111/j.1469-8137.2007.02196.x.
Tallis, M. J., Lin, Y., Rogers, A., Zhang, J., Street, N. R., Miglietta, F., et al. (2010). The transcriptome of populus in elevated CO2 reveals increased anthocyanin biosynthesis during delayed autumnal senescence. New Phytologist, 186, 415–428. https://doi.org/10.2307/27797564.
Taylor, G., Tallis, M. J., Giardina, C. P., Percy, K. E., Miglietta, F., Gupta, P. S., et al. (2008). Future atmospheric CO2 leads to delayed autumnal senescence. Global Change Biology, 14, 264–275. https://doi.org/10.1111/j.1365-2486.2007.01473.x.
Urban, O., Hrstka, M., Zitová, M., Holisová, P., Sprtová, M., Klem, K., et al. (2012). Effect of season, needle age and elevated CO2 concentration on photosynthesis and rubisco acclimation in picea abies. Plant Physiology and Biochemistry, 58, 135–141. https://doi.org/10.1016/j.plaphy.2012.06.023.
Wargo, P. M. (1978). Correlations of leaf area with length and width measurements of leaves of black oak, white oak, and sugar maple. Northeastern Forest Experiment Station: Forest Service Research Note.
Warren, J. M., Norby, R. J., & Wullschleger, S. D. (2011). Elevated CO2 enhances leaf senescence during extreme drought in a temperate forest. Tree Physiology, 31, 117–130. https://doi.org/10.1093/treephys/tpr002.
Acknowledgments
We appreciate Professor Michelle DaCosta in UMass, Amherst, for loaning us the LI-Cor 6400 photosynthesis system and giving us many kind suggestions during the experiment, Professors David Ratkowsky (Tasmanian Institute of Agriculture) and Bo Larson (University of Copenhagen) helped greatly with English polishing and suggestions for revision. We thank anonymous reviewers for their time and efforts in improving this paper.
Funding
This work was funded by the National Natural Science for Youth Foundation of China (31700439) and China Postdoctoral Foundation (2018M631595).
Author information
Authors and Affiliations
Contributions
Conceptualization, William Manning; methodology, William Manning and Li Li; formal analysis, Li Li; investigation, Li Li; data curation, Li Li; writing–original draft preparation, Li Li; writing–review and editing, Wang Xiaoke; project administration, William Manning; funding acquisition, William Manning and Li Li.
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Li, L., Manning, W. & Wang, X. Effects of Elevated CO2 Concentrations on Leaf Senescence and Late-Season Net Photosynthesis of Red Maple (Acer rubrum). Water Air Soil Pollut 231, 467 (2020). https://doi.org/10.1007/s11270-020-04828-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11270-020-04828-z