Abstract
Fucoxanthin–chlorophyll proteins (FCP) are the major light-harvesting proteins of diatom algae, a major contributor to marine carbon fixation. FCP complexes from representatives of centric (Cyclotella meneghiniana) and pennate (Phaeodactylum tricornutum) diatoms were prepared by sucrose gradient centrifugation and studied by means of electron microscopy followed by single particle analysis. The oligomeric FCP from a centric diatom were observed to take the form of unusual chain-like or circular shapes, a very unique supramolecular assembly for such antennas. The existence of the often disputed oligomeric form of FCP in pennate diatoms has been confirmed. Contrary to the centric diatom FCP, pennate diatom FCP oligomers are very similar to oligomeric antennas from related heterokont (Stramenopila) algae. Evolutionary aspects of the presence of novel light-harvesting protein arrangement in centric diatoms are discussed.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Diatoms (Bacillariophyceae) are a ubiquitous group of eukaryotic algae that contribute significantly to marine biomass production (Field et al. 1998; Falkowski et al. 2004). Phylogenetically, diatoms belong to a group of algae with secondary plastids of red algal origin—Stramenopila. They are classified into two groups distinguished by the symmetry of their silica cell walls—radially symmetric centric diatoms and bilaterally symmetric pennate diatoms. This division is generally reflected in their lifestyles where centric diatoms are mostly planktonic whereas pennate diatoms are benthic (Falciatore and Bowler 2002).
While the overall features of the photosynthetic membranes of diatoms are similar to those of higher plants, there are important differences. First of all, the diatom photosynthetic membranes do not form grana and stroma lamellae but rather assemble into stacks of three thylakoids. Second, the photosystems are not segregated but only randomly distributed across the membrane (Pyszniak and Gibbs 1992).
The major peripheral light-harvesting complexes of diatoms are fucoxanthin–chlorophyll proteins (FCPs). Besides fucoxanthin, the main light-harvesting carotenoid, they contain diadinoxanthin and chlorophylls (Chls) a and c. Diatom FCPs are encoded by a family of nuclear genes phylogenetically related to the Lhcr genes of the red algal ancestor of diatom chloroplasts (Hoffman et al. 2011). Both FCPs and Lhcr are members of a huge gene family of chlorophyll binding proteins (cab family, Dittami et al. 2010). The FCP proteins are structurally similar to other members of cab family (Eppard and Rhiel 1998). They contain three transmembrane helices binding a number of cofactor molecules though the carotenoid to chlorophyll ratio is higher in FCP than in LHC I and II proteins—four carotenoids per five Chls (Papagiannakis et al. 2005; Premvardhan et al. 2010).
In higher plants, light-harvesting proteins of the cab family are functionally specialized into PSI-specific (Wientjes et al. 2009), PSII-specific (Boekema et al. 1999) and mobile antenna complexes (Wientjes et al. 2013). Higher plant LHCIs assemble in a belt on one side of photosystem I (Ben-Shem et al. 2003) whereas LHCIIs form trimeric and higher oligomeric complexes (Dekker et al. 1999; Barros and Kühlbrandt 2009). In red algae, Lhcr proteins assemble a crescent on one side of PSI, in analogy to the situation in higher plants (Gardian et al. 2007; Busch et al. 2010).
So far, no PSII-specific peripheral antenna proteins have been described in Stramenopile algae (Lepetit et al. 2012) and it is assumed that the main antenna pool is shared between the two photosystems (Lepetit et al. 2012). In addition, PSI light harvesting is supplemented by complexes arranged in a manner similar to LHCI/LHCr complexes of higher plants and red algae (Ikeda et al. 2013). In Stramenopile algae, trimeric complexes of antenna proteins appear to be the main building block as have been described in phaeophytes (Katoh et al. 1989; Fujii et al. 2012), xanthophytes (Gardian et al. 2011) and diatoms (Joshi-Deo et al. 2010; Grouneva et al. 2011). These trimers have been described to assemble into higher oligomers in all mentioned groups of algae (Katoh and Ehara 1990; Büchel 2003) as well as in Chromera velia, which has closely related antenna proteins (Tichy et al. 2013).
The diatom genes encoding the main FCP antenna proteins form two clusters split along the phylogenetic line between centric and pennate diatoms (Gundermann et al. 2013). In centric diatoms, the oligomeric complexes of FCPs are well known (Büchel 2003) whereas in pennate diatoms no conclusion has been reached so far. Lepetit et al. (2007) reported on weakly bound oligomeric FCP complex in Phaeodactylum but many others found little evidence of such complex (Guglielmi et al. 2005; Joshi-Deo et al. 2010; Grouneva et al. 2011; Gundermann et al. 2013; Nagao et al. 2013).
In this work, we report projection structural maps of oligomeric FCP complexes isolated from representatives of centric (Cyclotella meneghiniana) and pennate (Phaeodactylum tricornutum) diatoms. We have found that the arrangements of these complexes are markedly different. Circular dichroism spectroscopy also supported the structural difference between centric and pennate diatom FCPs.
Materials and methods
Culture and growth conditions
Cyclotella meneghiniana (SAG 1020-1a) and P. tricornutum (SAG 1090-1a) were batch cultivated in 5 l flasks at room temperature in ASW f/2 medium. The light intensity was ~50 µmol photons m−2 s−1. Cells were harvested by centrifugation at 5,000×g for 5 min, washed with distilled water, and resuspended in a buffer containing 10 mM MES (pH 6.5), 2 mM KCl, 5 mM EDTA, 1 M Sorbitol.
Thylakoid membrane isolation
Cells were broken by several passages through an EmulsiFlex-C5 High Pressure cell disrupter (Avestin Inc., Canada) at a pressure of 100–120 MPa, while keeping the apparatus refrigerated on ice in the dark. All following isolations steps were carried out at 4 °C and dim light conditions. Unbroken cells were removed by centrifugation for 10 min at 1,000×g. The supernatant was then centrifuged for 30 min at 60,000×g to pellet thylakoid membranes. Membranes were resuspended in MES buffer (MES (pH 6.5), 2 mM KCl, 5 mM EDTA) and solubilised with 0,5 % n-dodecyl-β-d-maltoside at chlorophyll concentration of 1 mg (Chl) ml−1 for 20 min (detergent:Chl 10:1). The unsolubilized material was removed by centrifugation for 20 min at 40,000×g, and the supernatant was loaded onto a fresh 0–1.2 M continuous sucrose density gradient prepared by freezing and thawing the centrifuge tubes filled with a buffer containing MES (pH 6.5), 2 mM KCl, 5 mM EDTA, 0.01 % DM, 0.6 M sucrose. The following centrifugation was carried out using a SW-40 swing-out rotor (Beckman Coulter) at 150,000×g for 16 h, the zone with light-harvesting antennae was collected. The antenna zone was further exposed to a gel filtration using Sephadex G-75 (Amersham Biosciences, Sweden), to reduce the amount of sucrose and improve contrast in TEM.
Protein composition and spectroscopic analysis
The protein composition was determined by SDS-PAGE using a precast 12 % polyacrylamide SDS gel (C.B.S. Scientific) and visualized with Coomassie Brilliant Blue or by silver staining. Apparent molecular weights were estimated by co-electrophoresis of a low molecular weight protein standard (Fermentas).
Chlorophyll concentration was determined according to Ogawa and Vernon (1971). Low temperature fluorescence emission spectra were measured at liquid nitrogen temperature using a Fluorolog-2 spectrofluorometer (Jobin–Yvon, Edison, NJ, USA) with an excitation wavelength of 435 nm and a chlorophyll concentration of 10 μg (Chl a ml−1). Circular dichroism (CD) spectra were recorded at 4 °C with a Jasco J-715 spectropolarimeter with a slit width of 2 nm and optical path length of 1 cm.
Transmission electron microscopy (TEM) and image analysis
Freshly prepared FCP complexes and photosynthetic membrane of C. meneghiniana were immediately used for TEM. The specimen was placed on glow discharged carbon-coated copper grids and negatively stained with 2 % uranyl acetate. TEM was performed with a JEOL JEM–2100F transmission electron microscope (JEOL, Japan) using 200 kV at 20,000× magnification. TEM images were recorded by a bottom-mount Gatan CCD Orius SC1000 camera, corresponding to a pixel size of 3.4 Å.
Image analyses were carried out using Spider and Web software package (Frank et al. 1996). Manually selected projections were rotationally and translationally aligned, and treated by multivariate statistical analysis in combination with classification procedure (van Heel and Frank 1981; Harauz et al. 1988). Classes from each of the subsets were used for refinement of alignments and subsequent classifications. For the final sum, the best of the class members were summed using a cross-correlation coefficient of the alignment procedure as a quality parameter (van Heel and Frank 1981).
Results and discussion
In this work, we obtained purified FCP complexes from C. meneghiniana and P. tricornutum for electron microscopy analysis. Solubilised thylakoid membranes of both organisms were separated into 4 zones by sucrose density gradient centrifugation (Fig. 1a).
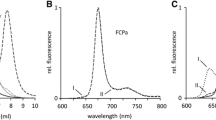
a Sucrose density gradient of solubilized thylakoids of P. tricornutum (left) and C. meneghiniana (right). The second (brown) zone of both gradients contains the most of FCP. b SDS-PAGE analysis of FCP zones from P. tricornutum (left) and C. meneghiniana (right) shows prominent bands at about 17–19 kDa, which are characteristic for polypeptides of FCP. c 77 K fluorescence emission spectra of FCP zones from P. tricornutum (dashed line) and C. meneghiniana (solid line). The excitation wavelength was 435 nm. Spectra are normalized to their maxima
For studies of arrangement of FCPs, we were interested in the second (brown) zones of both gradients as they contain the most of FCPs as indicated by SDS-PAGE. The SDS-PAGE gels (Fig. 1b) show prominent bands at about 17–19 kDa, corresponding to polypeptides of FCP antennas (Büchel 2003; Gundermann and Büchel 2008). In C. meneghiniana, the protein composition of trimers and oligomers is known to differ (Büchel 2003)—the trimers are primarily assembled from the lighter (~18 kDa) proteins whereas the higher oligomers are primarily made up of the heavier (~19 kDa) proteins. The intensity of the two bands in our preparation (Fig. 1b) was about equal so the zone was most probably a mixture of both trimers and oligomers as was the case in Büchel (2003)
The protein composition of oligomeric FCP from P. tricornutum described by Lepetit et al. (2007) did not differ from the FCP trimers. However, given the position of the FCP gradient band in our experiment compare to that of Lepetit et al. (2007) and also to our C. meneghiniana gradient, we expect it probably consisted mostly of trimeric FCP. It should be noted here that our prime concern was minimizing the number of purification steps in order to increase the probability of observing near-native oligomeric complexes. The heterogeneity of FCP oligomerization in collected fractions was of secondary importance because in the analysis of TEM images only the oligomeric particles were readily picked and processed.
The 77 K fluorescence emission spectra of FCP zones (Fig. 1c) of the two diatoms clearly differ. In P. tricornutum, the Chl a emission peaks at 680 nm in good correspondence with spectra reported by Lepetit et al. (2007) while in C. meneghiniana the emission peaks at 685 nm. Given that both of the samples are supposedly very closely related FCP isolations, this is a significant difference. The bathochromic shift of emission in C. meneghiniana might be indicative of higher aggregation or oligomerization status of its LHC proteins (Pascal et al. 2005; Miloslavina et al. 2008) which is also suggested by slightly higher width of the emission peak and different positions of the respective bands in sucrose density gradients. A comparison of 77 K emission spectra of C. meneghiniana FCP zone with those of Gundermann and Büchel (2012) shows good correspondence with their FCPb (oligomeric) complexes contrary to the FCPa (trimeric) complexes. However, our SDS-PAGE and CD spectroscopy data (see below) indicate a mixture of oligomeric states in this zone.
The circular dichroism (CD) spectra of the two antenna isolations (Fig. 2) differed markedly mainly in the Qy region of chlorophyll a, in agreement with published data (Büchel 2003; Lepetit et al. 2007). The two negative features of the CD of C. meneghiniana, at 663 and 680 nm, are characteristic for FCPs isolated from this diatom. The CD of trimers shown in Büchel (2003) has equal intensity of these two peaks whereas the CD of oligomers has only the higher energy peak. Our CD spectrum has about 1:2 ratio of the 680:663 peaks which indicates that the FCP band of the sucrose density gradient is a mixture of FCP trimers and oligomers, in line with the results of SDS-PAGE (Fig. 1 b). The CD spectrum of the FCP band of P. tricornutum does not allow distinguishing between trimeric or oligomeric complexes (Lepetit et al. 2007). While subtle, the differences in CD spectra of FCPs of closely related organisms provide an independent line of evidence of structural differences of these proteins.
Purified FCP complexes of both organisms were negatively stained with 2 % uranyl acetate, visualized by TEM and processed by image analysis. Typical TEM images of FCP zone from C. meneghiniana and P. tricornutum are shown in Fig. 3. It is apparent that both FCP sucrose density gradient zones contained at least two types of particles, presumably small FCP complexes and bigger oligomeric forms of FCPs.
For single particle analysis, top-view projections of the oligomeric particles (in diameter bigger that 10 nm) were manually selected. 8,000 particles were selected from the P. tricornutum FCP zone and 5,200 particles from C. meneghiniana FCP zone. After the classification steps, the particles were separated into 18 and 28 classes for P. tricornutum and C. meneghiniana, respectively. The most representative classes of particles are shown in Fig. 4.
The most representative class averages obtained by classification of 5,200 particles from C. meneghiniana (up) and 8,000 particles from P. tricornutum (down). The numbers of averaged particles are 280 (a), 312 (b), 277 (c) for C. meneghiniana and 582 (d), 528 (e), 478 (f) for P. tricornutum. The scale bar represents 5 nm
There were striking differences in the shapes and sizes of the FCP oligomers from the two organisms. The FCP oligomers isolated from P. tricornutum form approximately elliptical structure with major and minor axis of about 15 and 11 nm, respectively. This structure is very similar to the phylogenetically related oligomeric antennas of Xanthonema XLH (Gardian et al. 2011) and Chromera CLH (Tichy et al. 2013). Extrapolating from these results and also the particles of a brown alga FCP (Katoh and Ehara 1990), one might be tempted to conclude that all Stramenopile algae share the same peripheral antenna quaternary structure. Yet, the C. meneghiniana FCPs are very different from all these despite the fact that they are, in terms of their amino acid sequences, the most similar to P. tricornutum FCPs.
The FCP oligomers from C. meneghiniana assemble flexible chain-like structures, often enclosed to form a ring of varied shape. The size of these complexes (about 17 nm in diameter) is bigger than in P. tricornutum. The apparent flexibility of these structures caused an increase in the number of particle classes revealed during the single particle analysis. The higher noise in the averaged projections is also stemming from the lower number of particles per class of C. meneghiniana FCP oligomers.
The high variability of the shape of observed FCP oligomers from C. meneghiniana raises the question of what is the native shape of these complexes. One attractive possibility is that the antenna could encircle a photosystem in analogy to bacterial LH1 antennas (Walz et al. 1998) but there are no data supporting such speculation. Direct observation of the diatom photosynthetic membrane would be very informative in this matter (Kouril et al. 2013). The localization of FCP proteins in Cyclotella was studied by immunogold electron microscopy of freeze-fractured thylakoid membranes (Westermann and Rhiel 2005), but the method does not provide enough resolution to visualize shapes of the complexes.
To resolve this issue, we tried to visualize C. meneghiniana photosynthetic membrane by the TEM negative staining method. It was possible to observe many particles with similar thickness as our FCP oligomeric complexes but essentially linear arrangement (Fig. 5, Supplementary Fig. 1). Due to the abundance of such particles in the photosynthetic membrane and their shape, we interpret these particles as the native FCP antenna oligomers. The ring arrangement displayed in Fig. 4b, c is then most probably an artifact associated with the high affinity of FCPs to themselves. Unfortunately, no photosystem particles could be unambiguously assigned by this method.
Electron microscopy image of negatively stained photosynthetic membrane of C. meneghiniana. The insets (right) show three raw particles from the single particle analysis of oligomeric FCP antennas of C. meneghiniana. The scale bar represents 50 nm. Additional images are presented in supplementary Fig. 1
The presence and abundance of oligomeric forms in our P. tricornutum FCP isolation is perhaps surprising given the literature consensus (reviewed by Gundermann et al. 2013) and also our own data presented in Fig. 1. A likely explanation is that the oligomeric form of pennate FCP is very fragile, and we used a very crude isolation method. It is possible that the oligomeric FCP will not survive multi-step purification where the sucrose gradient is followed by other procedures, such as ion exchange chromatography or gel filtration.
The presence of unique light-harvesting protein arrangement in C. meneghiniana is puzzling. Recent diatom evolution studies using molecular data (Sims et al. 2006; Kooistra et al. 2007) split diatoms into three subgroups—radial centric diatoms, multipolar centric diatoms, and pennate diatoms—where the latter two groups form together a single clade that includes both C. meneghiniana (multipolar centric) and P. tricornutum (pennate). To our knowledge, no radial centric diatom FCPs have been studied in detail, the best known member is probably Coscinodiscus (Lang and Kroth 2001). In light of the results presented here, it will be interesting to know if the arrangement of antenna observed in C. meneghiniana FCP is unique to this species or whether it is characteristic of a larger phylogenetic group. Also, it remains to be seen if this particular geometry of supramolecular aggregates represents any advantage in terms of regulation of light-harvesting efficiency, and might thus be an adaptation to the living condition of the organism. This is an important question given the significance of diatoms as marine primary producers.
Abbreviations
- Chl:
-
Chlorophyll
- MES:
-
2-(N-morpholino)ethanesulfonic acid
- DM:
-
n-Dodecyl-β-d-maltoside
- TEM:
-
Transmission electron microscopy
- FCP:
-
Fucoxanthin–chlorophyll proteins
- LHC:
-
Light-harvesting complex
- PSI:
-
Photosystem I
- PSII:
-
Photosystem II
- SDS-PAGE:
-
Polyacrylamide gel electrophoresis in the presence of sodium dodecylsulfate
- CD:
-
Circular dichroism
References
Barros T, Kühlbrandt W (2009) Crystallisation, structure and function of plant light-harvesting Complex II. Biochim Biophys Acta 1787:753–772
Ben-Shem A, Frolow F, Nelson N (2003) Crystal structure of plant photosystem I. Nature 426:630–635
Boekema EJ, van Roon H, Calkoen F, Bassi R, Dekker JP (1999) Multiple types of association of photosystem II and its light-harvesting antenna in partially solubilized photosystem II membranes. Biochemistry 38:2233–2239
Büchel C (2003) Fucoxanthin–chlorophyll proteins in diatoms: 18 and 19 kDa subunits assemble into different oligomeric states. Biochemistry 42:13027–13034
Busch A, Nield J, Hippler M (2010) The composition and structure of photosystem I-associated antenna from Cyanidioschyzon merolae. Plant J 62:886–897
Dekker JP, van Roon H, Boekema EJ (1999) Heptameric association of light-harvesting complex II trimers in partially solubilized photosystem II membranes. FEBS Lett 449:211–214
Dittami SM, Michel G, Collén J, Boyen C, Tonon T (2010) Chlorophyll-binding proteins revisited—a multigenic family of light-harvesting and stress proteins from a brown algal perspective. BMC Evol Biol 10:365
Eppard M, Rhiel E (1998) The genes encoding light-harvesting subunits of Cyclotella cryptica (Bacillariophyceae) constitute a complex and heterogeneous family. Mol Gen Genet 260:335–345
Falciatore A, Bowler C (2002) Revealing the molecular secrets of marine diatoms. Annu Rev Plant Biol 53:109–130
Falkowski PG, Katz ME, Knoll AH, Quigg A, Raven JA, Schofield O, Taylor FJR (2004) The evolution of modern eukaryotic phytoplankton. Science 305:354–360
Field CB, Behrenfeld MJ, Randerson JT, Falkowski PG (1998) Primary production of the biosphere: integrating terrestial and oceanic components. Science 281:237–240
Frank J, Radermacher M, Penczek P, Zhu J, Li YH, Ladjadj M, Leith A (1996) SPIDER and WEB: processing and visualization of images in 3D electron microscopy and related fields. J Struct Biol 116:190–199
Fujii R, Kita M, Iinuma Y, Oka N, Takaesu Y, Taira T, Iha M, Cogdell RJ, Hashimoto H (2012) Isolation and purification of the major photosynthetic antenna, Fucoxanthin–Chl a/c protein, from cultured discoid germilings of the brown Alga, Cladosiphon okamuranus TOKIDA (Okinawa Mozuku). Photosynth Res 111:157–163
Gardian Z, Bumba L, Schrofel A, Herbstova M, Nebesarova J, Vacha F (2007) Organisation of Photosystem I and Photosystem II in red alga Cyanidium caldarium: encounter of cyanobacterial and higher plant concepts. Biochim Biophys Acta 1767:725–731
Gardian Z, Tichy J, Vacha F (2011) Structure of PSI, PSII and antennae complexes from yellow-green alga Xanthonema debile. Photosynth Res 108:25–32
Grouneva I, Rokka A, Aro EM (2011) The thylakoid membrane proteome of two marine diatoms outlines both diatom-specific and species-specific features of the photosynthetic machinery. J Proteome Res 10:5338–5353
Guglielmi G, Lavaud J, Rousseau B, Etienne AL, Houmard J, Ruban AV (2005) The light-harvesting antenna of the diatom Phaeodactylum tricornutum. Evidence for a diadinoxanthin-binding subcomplex. FEBS J 272:4339–4348
Gundermann K, Büchel C (2008) The fluorescence yield of the trimeric fucoxanthin–chlorophyll-protein FCPa in the diatom Cyclotella meneghiniana is dependent on the amount of bound diatoxanthin. Photosynth Res 95:229–235
Gundermann K, Büchel C (2012) Factors determining the fluorescence yield of fucoxanthin–chlorophyll complexes (FCP) involved in non-photochemical quenching in diatoms. Biochim Biophys Acta 1817:1044–1052
Gundermann K, Schmidt M, Weisheit W, Mittag M, Büchel C (2013) Identification of several sub-populations in the pool of light harvesting proteins in the pennate diatom Phaeodactylum tricornutum. Biochim Biophys Acta 1827:303–310
Harauz G, Boekema EJ, van Heel M (1988) Statistical image analysis of electron micrographs of ribosomal subunits. Methods Enzymol 164:35–49
Hoffman GE, Sanchez Puerta MV, Delwiche CF (2011) Evolution of light-harvesting complex proteins from Chl c-containing algae. BMC Evol Biol 11:101
Ikeda Y, Yamagishi A, Komura M, Suzuki T, Dohmae N, Shibata Y, Itoh S, Koike H, Satoh K (2013) Two types of fucoxanthin–chlorophyll-binding proteins I tightly bound to the photosystem I core complex in marine centric diatoms. Biochim Biophys Acta 1827:529–539
Joshi-Deo J, Schmidt M, Gruber A, Weisheit W, Mittag M, Kroth PG, Büchel C (2010) Characterization of a trimeric light-harvesting complex in the diatom Phaeodactylum tricornutum built of FcpA and FcpE proteins. J Exp Bot 61:3079–3087
Katoh T, Ehara T (1990) Supramolecular assembly of fucoxanthin–chlorophyll-protein complexes isolated from a brown alga, Petalonia fascia. Electron microscopic studies. Plant Cell Physiol 31:439–447
Katoh T, Mimuro M, Takaichi S (1989) Light-harvesting particles isolated from a brown alga, Dictyota dichotoma—a supramolecular assembly of fucoxanthin–chlorophyll-protein complexes. Biochim Biophys Acta 976:233–240
Kooistra WHCF, Gersonde R, Medlin LK, Mann DG (2007) The origin and evolution of the diatoms: their adaptation to a planktonic existence. In: Falkowski PG, Knoll AH (eds) Evolution of primary producers in the sea. Academic Press-Elsevier, Amsterdam, pp 207–249
Kouril R, Wientjes E, Bultema JB, Croce R, Boekema EJ (2013) High-light vs. low-light: effect of light acclimation on photosystem II composition and organization in Arabidopsis thaliana. Biochim Biophys Acta 1827:411–419
Lang M, Kroth PG (2001) Diatom fucoxanthin chlorophyll a/c-binding protein (FCP) and land plant light-harvesting proteins use a similar pathway for thylakoid membrane insertion. J Biol Chem 276:7985–7991
Lepetit B, Volke D, Szabó M, Hoffmann R, Garab G, Wilhelm C, Goss R (2007) Spectroscopic and molecular characterization of the oligomeric antenna of the diatom Phaeodactylum tricornutum. Biochemistry 46:9813–9822
Lepetit B, Goss R, Jakob T, Wilhelm C (2012) Molecular dynamics of the diatom thylakoid membrane under different light conditions. Photosynth Res 111:245–257
Miloslavina Y, Wehner A, Lambrev PH, Wientjes E, Reus M, Garab G, Croce R, Holzwarth AR (2008) Far-red fluorescence: a direct spectroscopic marker for LHCII oligomer formation in non-photochemical quenching. FEBS Lett 582:3625–3631
Nagao R, Takahashi S, Suzuki T, Dohmae N, Nakazato K, Tomo T (2013) Comparison of oligomeric states and polypeptide compositions of fucoxanthin chlorophyll a/c-binding protein complexes among various diatom species. Photosynth Res 117:281–288
Ogawa T, Vernon LP (1971) Increased content of cytochromes 554 and 562 in Anabaena variabilis cells grown in the presence of diphenylamine. Biochim Biophys Acta 226:88–97
Papagiannakis E, HM van Stokkum I, Fey H, Büchel C, van Grondelle R (2005) Spectroscopic characterization of the excitation energy transfer in the fucoxanthin–chlorophyll protein of diatoms. Photosynth Res 86:241–250
Pascal AA, Liu Z, Broess K, van Oort B, van Amerongen H, Wang C, Horton P, Robert B, Chang W, Ruban A (2005) Molecular basis of photoprotection and control of photosynthetic light-harvesting. Nature 436:134–137
Premvardhan L, Robert B, Beer A, Büchel C (2010) Pigment organization in fucoxanthin chlorophyll a/c(2) proteins (FCP) based on resonance Raman spectroscopy and sequence analysis. Biochim Biophys Acta 1797:1647–1656
Pyszniak A, Gibbs SP (1992) Immunocytochemical localization of photosystem I and the fucoxanthin–chlorophyll a/c light-harvesting complex in the diatom Phaeodactylum tricornutum. Protoplasma 166:208–217
Sims PA, Mann DG, Medlin LK (2006) Evolution of the diatoms: insights from fossil, biological and molecular data. Phycologia 45:361–402
Tichy J, Gardian Z, Bina D, Konik P, Litvin R, Herbstova M, Pain A, Vacha F (2013) Light harvesting complexes of Chromera velia, photosynthetic relative of apicomplexan parasites. Biochim Biophys Acta 1827:723–729
van Heel M, Frank J (1981) Use of multivariate statistics in analyzing the images of biological macromolecules. Ultramicroscopy 6:187–194
Walz T, Jamieson SJ, Bowers CM, Bullough PA, Hunter CN (1998) Projection structures of three photosynthetic complexes from Rhodobacter sphaeroides: LH2 at 6 A, LH1 and RC-LH1 at 25 A. J Mol Biol 282:833–845
Westermann M, Rhiel E (2005) Localisation of fucoxanthin chlorophyll a/c-binding polypeptides of the centric diatom Cyclotella cryptica by immuno-electron microscopy. Protoplasma 225:217–223
Wientjes E, Oostergetel GT, Jansson S, Boekema EJ, Croce R (2009) The role of Lhca complexes in the supramolecular organization of higher plant photosystem I. J Biol Chem 284:7803–7810
Wientjes E, Drop B, Kouril R, Boekema EJ, Croce R (2013) During state 1 to state 2 transition in Arabidopsis thaliana the Photosystem II supercomplex gets phosphorylated but does not disassemble. J Biol Chem 288(46):32821–32826
Acknowledgments
The authors would like to acknowledge institutional support RVO:60077344 as well as financial support of the Czech Science Foundation projects P205/11/1164 (Z. G.) and P501/12/G055 (R. L., D. B. and F. V.).
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Gardian, Z., Litvín, R., Bína, D. et al. Supramolecular organization of fucoxanthin–chlorophyll proteins in centric and pennate diatoms. Photosynth Res 121, 79–86 (2014). https://doi.org/10.1007/s11120-014-9998-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11120-014-9998-3