Abstract
Over 40 years ago, Joliot et al. (Photochem Photobiol 10:309–329, 1969) designed and employed an elegant and highly sensitive electrochemical technique capable of measuring O2 evolved by photosystem II (PSII) in response to trains of single turn-over light flashes. The measurement and analysis of flash-induced oxygen evolution patterns (FIOPs) has since proven to be a powerful method for probing the turnover efficiency of PSII. Stemler et al. (Proc Natl Acad Sci USA 71(12):4679–4683, 1974), in Govindjee’s lab, were the first to study the effect of “bicarbonate” on FIOPs by adding the competitive inhibitor acetate. Here, we extend this earlier work by performing FIOPs experiments at various, strictly controlled inorganic carbon (Ci) levels without addition of any inhibitors. For this, we placed a Joliot-type bare platinum electrode inside a N2-filled glove-box (containing 10–20 ppm CO2) and reduced the Ci concentration simply by washing the samples in Ci-depleted media. FIOPs of spinach thylakoids were recorded either at 20-times reduced levels of Ci or at ambient Ci conditions (390 ppm CO2). Numerical analysis of the FIOPs within an extended Kok model reveals that under Ci-depleted conditions the miss probability is discernibly larger (by 2–3 %) than at ambient conditions, and that the addition of 5 mM HCO3 − to the Ci-depleted thylakoids largely restores the original miss parameter. Since a “mild” Ci-depletion procedure was employed, we discuss our data with respect to a possible function of free or weakly bound HCO3 − at the water-splitting side of PSII.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Oxygenic photosynthesis is a fundamental biological process by which higher plants, algae, and cyanobacteria reduce atmospheric CO2 to energy-rich carbohydrates using the electrons extracted from water during oxidative water-splitting. Photosynthetic water-splitting takes place in the oxygen-evolving complex (OEC) of photosystem II (PSII), a large pigment-binding protein complex found in all oxygen-evolving organisms (Wydrzynski and Satoh 2005; Govindjee et al. 2010; Kouril et al. 2012). The OEC contains a μ-oxo bridged tetra-manganese calcium (Mn4CaO5) cluster (Yano et al. 2006; Umena et al. 2011) that is capable of step-wise oxidation of water into four protons, four electrons, and molecular oxygen (Messinger and Renger 2008; Dau et al. 2012; Cox and Messinger 2013). The energy for this is provided by light-induced charge separation within the chlorophyll-containing reaction center of PSII (Renger and Holzwarth 2005; Renger 2010).
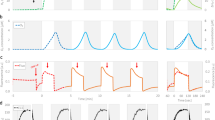
The current understanding of the mechanism of photosynthetic water oxidation originates from the classic measurements of flash-induced oxygen evolution patterns (FIOPs) performed by Joliot et al. (1969). By illuminating dark-adapted algae and chloroplasts with a train of short saturating (single turn-over) light flashes and by employing a specially designed highly sensitive rapid-response polarograph for O2 detection, Joliot et al. (1969) observed that: (i) no O2 was evolved in response to the 1st flash; (ii) a small O2 yield was induced by the 2nd flash; (iii) the most pronounced maximum of O2 yield occurred after the 3rd flash; (iv) subsequent flashes induced an oscillating pattern of O2 evolution with a maximum on every 4th flash (i.e., in the 7th, 11th, 15th,…); and (v) this period-four oscillation gradually disappeared after several cycles. Figure 1a illustrates this notable FIOP in its original version as obtained by Joliot et al. (1969) (for historical review, see Joliot 2003). On the basis of these observations, Kok and coworkers developed a kinetic model (also known as “Kok model” or “Kok cycle”) demonstrating a four-electron chemistry of water oxidation and O2 production by the OEC (see Kok et al. 1970 and, for an early discussion of this and other models, see Mar and Govindjee 1972). According to the Kok model, the OEC cycles through five redox intermediates during water oxidation, which were termed S i states (i = 0,…,4), where i signifies the number of stored oxidizing equivalents (see Fig. 1b). Upon accumulation of four oxidizing equivalents, the highly reactive S4 state decays into the S0 state while two H2O molecules are oxidized to O2.
Classical flash-induced oxygen evolution pattern (FIOP) as was first obtained by Joliot et al. (1969) in dark-adapted spinach chloroplasts upon illumination with a series of single saturating light flashes (separated with dark time of 300 ms) at pH 7.9 and 20 °C (a), and the Kok cycle that describes this phenomenon and represent the reaction scheme of photosynthetic water oxidation and oxygen evolution (b). a is modified and adapted from Joliot et al. (1969). In b, light-induced S-state transitions of the Kok cycle are shown by black arrows and the number of light flashes required for a certain transitions are indicated by numbers in circles on the arrows, assuming that in the dark, the Mn4CaO5 cluster (shown in the center of the cycle) is mostly in the S1 state. Gray arrow between S4 and S0 states indicates the transition that does not require light. Note that one water molecule is entering the cycle on the S2 → S3 transition, and another on the S4 → S0 transition. For the original version of the Kok cycle, see Kok et al. (1970) and Forbush et al. (1971). The structural model of the Mn4CaO5 cluster is as derived from the recent PSII crystal structure at a 1.9 Å resolution (Umena et al. 2011). For explanation of all other symbols in the Kok cycle, see text
In well dark-adapted samples, almost all PSII centers are in the “dark-stable“ S1 state (that is why the first maximum of O2 evolution is observed upon the 3rd flash). This is due to the deactivation of the metastable S0, S2, and S3 states to S1 via different pathways that involve a redox active tyrosine residue (YD) and the reduced acceptor side quinones (Diner 1977; Vermaas et al. 1984; Nugent et al. 1987; Styring and Rutherford 1987; Vass et al. 1990a; Messinger and Renger 1993; Isgandarova et al. 2003). In order to explain the observed disappearance of the O2 oscillation patterns with increasing number of flashes, the S i state turnovers are suggested to be coupled to the so-called “miss” and “double hit” probabilities (Kok et al. 1970; Forbush et al. 1971). The miss probability (α) accounts for the failed advancement of the S i cycle to the next S state (i.e., S i → S i transitions) upon flash excitation. The “misses” were suggested to be dependent on all redox equilibria and kinetics of both the donor and acceptor side of PSII, as well as on pH and temperature; thus, it represents a very sensitive probe for the changes in the turnover efficiency of PSII under various conditions and sample treatments (see reviews by Shinkarev 2005; Messinger and Renger 2008; Renger and Hanssum 2009, and references therein). The classical Kok model assumed that the miss parameter is S i state and flash number independent. However, there is experimental data indicating that α-values are dependent on the redox state S i of the OEC (Renger and Hanssum 1988; Shinkarev and Wraight 1993; de Wijn and van Gorkom 2002; Isgandarova et al. 2003; Han et al. 2012; Suzuki et al. 2012). Since under most circumstances, the kinetics of electron transfer on the acceptor side are limiting for the S-state advancement (Fromme et al. 1987; Ananyev and Dismukes 2005; Shevela and Messinger 2012) the double hits (i.e., S i → S i+2 transitions) reflect mostly events on the electron-acceptor side (namely on QA and QB, the primary and secondary plastoquinone electron acceptors of PSII). The magnitude of the double hits is affected by the FWHM-flash profile (Kok et al. 1970; Jursinic 1981; Messinger et al. 1993). In addition to miss and double hit parameters our extended Kok models include other factors that can affect FIOPs (Hillier and Messinger 2005; Messinger and Renger 2008). Among them, for instance, there is a correction factor that accounts for a change of the number of active PSII centers during the flash train (Kebekus et al. 1995; Messinger et al. 1997), and back reactions of S2 and S3 states with YD during the dark time between flashes (Vass et al. 1990a; Messinger and Renger 1993; Isgandarova et al. 2003).
Despite numerous reports about the requirement of hydrogen carbonate (“bicarbonate”; HCO3 −) and other inorganic carbon (Ci) species (CO2, CO3 2–) for optimal stability and/or activity of the OEC of PSII, its role—if any—in photosynthetic water-splitting chemistry remains highly controversial. The first report by Warburg and Krippahl (1958) on the “bicarbonate effect” on the electron flow in PSII triggered many subsequent studies by other research groups (for recent reviews, see McConnell et al. 2012; Shevela et al. 2012). Since then, two sites of HCO3 − action were explored within PSII. In 1975, a pioneering work by Wydrzynski and Govindjee (1975) provided a strong evidence for the participation of HCO3 − ions in the electron transfer on the acceptor side of PSII (Wydrzynski and Govindjee 1975). Numerous subsequent experiments showed that this electron-acceptor side HCO3 − facilitates the reduction of QB and participates in protonation reactions near the QB-binding site (Van Rensen et al. 1999; Van Rensen 2002; McConnell et al. 2012; Shevela et al. 2012). Another site for “bicarbonate” action is thought to be the electron-donor side of PSII, where HCO3 − ions may be required for optimal functionality and stability of the OEC, and for the photo-induced assembly of the inorganic core of the OEC (Stemler 2002; Van Rensen and Klimov 2005; Dasgupta et al. 2008). HCO3 − binding to the non-heme Fe between two quinones QA and QB on the acceptor side of PSII was recently confirmed by X-ray crystallography studies of PSII (Ferreira et al. 2004; Guskov et al. 2010; Umena et al. 2011). In contrast, the site of interaction of HCO3 − on the donor side is not obvious. Based on recent X-ray crystallography (Umena et al. 2011), mass spectrometry (Shevela et al. 2008b; Ulas et al. 2008), and FTIR spectroscopy (Aoyama et al. 2008) studies, HCO3 − does not bind tightly at or near the Mn4CaO5 cluster. At the same time, the possibility for a weakly bound, mobile, and rapidly exchanging HCO3 − within the OEC cannot be excluded. Thus, for instance, HCO3 − was recently proposed to act as an acceptor of protons produced during water-splitting (see Fig. 1b) (Villarejo et al. 2002; Ananyev et al. 2005; Shutova et al. 2008; Pobeguts et al. 2010; Ulas and Brudvig 2010).
In case of the participation of mobile or weakly bound HCO3 − ions in the water-oxidizing reactions of the OEC, their removal should impair the efficiency of the S i state transition. In this regard, important information on the turnover efficiency of PSII before and after the Ci-depletions can be obtained from the FIOPs measurements. The first results revealing the effect of the Ci-depletion on FIOPs were obtained in Govindjee’s group by Stemler et al. (1974). In that report, authors observed strong damping of FIOPs in HCO3 −-depleted chloroplasts, indicating impaired turnover efficiency of PSII upon removal of HCO3 −. In that and numerous subsequent FIOPs experiments effect of HCO3 − on PSII was studied by addition of chemical analogs of HCO3 −, such as acetate or formate (combined with low pH treatments) to photosynthetic samples (Stemler and Govindjee 1973; Stemler et al. 1974; Stemler 1980, 1982; Jursinic and Stemler 1982, 1984; Vermaas et al. 1984; Mende and Wiessner 1985; Stemler and Lavergne 1997; Shevela et al. 2007). However, since later the above-mentioned anions were shown to remove a tightly bound HCO3 − from the acceptor side of PSII (Govindjee et al. 1991, 1997; Shevela et al. 2008a, b; Ulas et al. 2008), such Ci depletion procedure is not suitable for elucidation of the possible participation of weakly bound HCO3 − in the water-splitting reactions of PSII. Therefore, for studies of the donor side “bicarbonate effects,” a milder Ci-depletion method was developed, in which dilution and washing of the samples in Ci-depleted media is used to remove Ci from the samples, but not affecting HCO3 − bound on the acceptor side of PSII (Klimov et al. 1995a, b). Because of the chemical characteristics of the CO2/HCO3 – system, expressed by Eq. 1:
the control and constant monitoring of the residual Ci levels in sample suspension after the “mild” CO2/HCO3 −-depletion procedure and during the FIOPs measurements must be an integral part of all relevant experiments. This is evident from a recent report, where we clearly demonstrated that after a thorough removal of Ci from the buffer solution by bubbling with CO2-free argon, CO2 diffused back into the depleted solution within tens of seconds during the transfer of the sample onto the Joliot-type electrode (Shevela et al. 2007). Thus, while our Ci-depletion procedure led to ~50-fold reduction of Ci level in the freshly-depleted medium, the actual level of Ci in sample suspension during the FIOPs measurements (even after short sample handling) rose to ~fivefold below ambient. Such underestimation of the actual Ci level during the experiment may be one of the reasons for the discrepancies in interpretation of the “bicarbonate effect” on the PSII donor side in the literature (Stemler 2002; Van Rensen and Klimov 2005; McConnell et al. 2012; Shevela et al. 2012).
In this study, we have performed FIOPs experiments using a Joliot-type electrode at ambient and at 20-times reduced levels of Ci. To achieve and maintain such low level of Ci in both gas spaces around the electrode and aqueous phase around the sample, the FIOPs measurements were done inside a glove-box filled with N2 and in Ci-depleted buffers. In our present FIOPs experiments, we have fully controlled the levels of the residual Ci upon CO2/HCO3 −-depletion procedure. Thus, the concentration of CO2 in the gas phase above the sample was continuously monitored with an infrared CO2 analyzer, while Ci levels in sample suspensions were detected by membrane-inlet mass spectrometry (MIMS).
Materials and methods
Sample preparation
Thylakoids were prepared from fresh leaves of Spinacia oleracea based on methods described earlier (Winget et al. 1965) with slight modifications (Messinger et al. 1997). The rates of O2 evolution for such thylakoid preparations were ~270 μmol (O2) × mg (Chl)−1 × h−1. After isolation steps the thylakoids were frozen in small aliquots in liquid nitrogen and then stored at −80 °C until used. Shortly before measurements, the samples were thawed in the dark on ice and diluted to desired concentrations with “SNM buffer” (400 mM sucrose, 35 mM NaCl, and 20–40 mM MES/KOH) adjusted to pH 6.3.
CO2/HCO3 −-depletion and monitoring of Ci levels
Depletion of Ci from media and spinach thylakoids was achieved as described earlier (Shevela et al. 2007) with some modifications: initially, Ci was removed from the SNM buffer by means of 60 min flushing with N2 depleted of CO2 by passage through a 20-cm layer of ascarite (5–20 mesh particle size; Sigma-Aldrich, Seelze, Germany). This resulted in our Ci-depleted (C −i ) media. To avoid contamination of C −i media with atmospheric CO2, the depletion procedure and all following sample handling and measuring steps were performed inside a glove-box (“Belle”) that was filled with a slight overpressure of N2. The level of CO2 inside the glove-box was constantly monitored by The Qubit Systems IRGA CO2 Analyzer (Model No. S151). To reduce the amount of CO2 in the N2 flow, the incoming house N2 (liquid N2 blow off) was passed through a 20-cm ascarite-column (see above) before it entered the glove-box. The final CO2 concentration inside the glove-box after such depletion procedure was in the range of 10–20 ppm (C −i conditions), while the CO2 level off the air was about 390 ppm (C +i conditions).
Thylakoids were depleted of Ci by 20–25-fold dilution with the C −i medium and subsequent dark-incubations on ice for 10−20 min. Thereafter, the samples were collected by centrifugation and washed at least twice in the C −i medium resulting in the C −i samples. Prior to the FIOPs measurements, the sample pellet was diluted with the C −i medium to a Chl concentration of 1 mg ml−1.
FIOPs measurements
After long-term (several months) dark low-temperature sample storage, the preparations are enriched (≥80 %) in the reduced form of tyrosine D (YD) (Messinger and Renger 1990; Vass et al. 1990b). In order to populate thylakoids with oxidized form of YD (Y oxD ), all samples were pre-flashed with one saturating flash and subsequently incubated in darkness for 5 min shortly before the FIOPs measurements.
FIOPs measurements were carried out within a Belle glove-box either under the C +i conditions, when the samples were incubated and measured in the C +i medium (that contained a mixture of CO2 and HCO3 −) with open glove-box, or under the C −i conditions, in which the samples were incubated and measured in the C −i medium under Ci-depleted N2 atmosphere as described above.
The FIOPs were obtained in the absence of exogenous electron acceptors with a home-built Joliot-type bare Pt-electrode (Joliot 1972; Messinger 1993). The measurements were performed at an electrode temperature of 20 °C (±0.3 °C). To insure complete sedimentation and temperature equilibration, the sample aliquots (10 μl) were kept for 3 min on the Pt-electrode prior to starting the measurements. The polarization voltage (−750 mV) was switched on 40 s before exposure of the samples to a train of saturating Xenon flashes (given by Perkin Elmer, LS-1130-4) at a frequency of 2 Hz. The amplified amperometric signals were recorded with a personal computer at a sampling rate of 3 ms/point. For the C −i conditions, only freshly prepared C −i buffer (SNM, pH 6.3) was used as a flow medium. For details on a setup of a Joliot-type electrode, see Messinger (1993) and Renger and Hanssum (2009).
FIOPs analysis
The FIOPs were analyzed using a spread sheet program that is based on an extended Kok model (see Fig. 1b) which was described earlier (Isgandarova et al. 2003; Nöring et al. 2008). In addition to the normal Kok parameters, the program included a correction factor (d) for a change of the number of active O2-evolving centers during the flash train (Messinger et al. 1997). Since no agreement has yet been reached about the precise S i state dependence of the miss parameter, all FIOPs were analyzed employing the equal miss and two possible S i state-dependent miss fitting approaches. For these latter approaches, the S i state-dependent miss parameters (α12, α23, α30, α01; see Fig. 1b) were allowed to vary (for further details on the S i state-dependent miss factors, see de Wijn and van Gorkom 2002; Shinkarev 2005; Messinger and Renger 2008; Renger and Hanssum 2009; Han et al. 2012; and Suzuki et al. 2012). However, using all these miss parameters as free variables leads to an underdetermined fit due to the limited number of independent data points in a single FIOP. Therefore, in one case the α-values for the transitions S0 → S1 and S1 → S2 were fixed to “0” (α01 = α12 = 0) since they are assumed to be significantly smaller than those of S2 → S3 and S3 → S0 + O2 + nH+ transitions, which were used as free running parameters, but fixed to be equal, i.e., α23 = α30. This fit approach, which was successfully applied by us previously (Isgandarova et al. 2003), is an extreme form of the parameters suggested by de Wijn and van Gorkom (2002) and Suzuki et al. (2012). In the second unequal miss fit approach, the FIOPs were analyzed assuming high miss probabilities for the S0 → S1, S1 → S2, and S2 → S3 transitions, and almost none for the S3 → S0 transition, as was proposed recently by Han et al. (2012). In such fits, the α01, α12, and α23 values were set as free, but equal, running parameters (i.e., α01 = α12 = α23), while the α30 was fixed to “0”. The comparison of the three approaches should therefore allow evaluating if the fit results are stable toward various possible models for the fitting. The values of 100 % S1 state and 0 % YD populations were fixed for all fits, since we employed pre-flashed samples and because fits in which the dark S-state populations were varied did not yield any significant S2 or S0 populations.
Membrane-inlet mass spectrometry measurements
The content of Ci (HCO3 −/CO2) in the C −i and C +i working buffers (pH 6.3) was measured by membrane-inlet mass spectrometry (MIMS) (Konermann et al. 2008; Beckmann et al. 2009) according to the procedure described previously (Shevela et al. 2007). MIMS measurements were performed by an isotope ratio mass spectrometer (ThermoFinniganPlus XP) that was connected via a cooling trap (dry ice+C2H5OH; ~200 K) to a home-built membrane-inlet cell very similar to that described earlier (Messinger et al. 1995). The working volume of the chamber (150 μl) was separated from a high vacuum (3 × 10−8 bar) of the mass spectrometer via an inlet (Ø 10 mm) that was covered by a 25 μm-thick silicon membrane (Mempro MEM-213) resting on a porous Teflon support (Small Parts Inc.) and permeable only for gasses. Before the measurements, the SNM buffer (pH 6.3) in the measuring chamber was degassed for 20–30 min until an only slightly sloping baseline was reached. After that, 5 μl aliquots of buffer solutions were injected into the chamber. To avoid CO2 contamination from air when handling samples, all transfers into membrane-inlet chamber were made within 20 s with gas-tight syringes that were pre-flushed with argon. The reaction mixture was constantly kept at 20 °C and stirred with a magnetic stir bar. CO2 was monitored online at m/z 44. CO2 levels in the media were obtained by determining the area under the m/z 44 signal using OriginPro software.
Results
Monitoring the Ci levels in C −i and C +i buffers
Monitoring the actual levels of Ci in working media under each C −i and C +i experimental conditions is highly important when studying effects of Ci on PSII. Figure 2 displays the levels of CO2 in the C +i buffers (trace 1) and in C −i buffer (trace 2) at pH 6.3 and 20 °C, as monitored by MIMS. A comparison of these two traces shows that our Ci depletion procedure (see “Materials and methods” section) significantly reduces the level of CO2 in the medium. It is also evident from trace 3 in Fig. 2 that the level of CO2 in the C −i buffer remains almost at the same level even after 2-h-storage under CO2-depleted N2 atmosphere inside the glove-box. Analysis of the area of CO2 signals shows that our set-up allowed measurements of FIOPs at about 20-fold reduced Ci levels in the media as compared to the level of Ci under ambient air conditions. The Ci depletion factor of 20 in sample solution correlates very well with the factor of reduction of CO2 concentration in the gas phase above the sample (from 390 ppm CO2 in air to the maximal concentration of 20 ppm CO2 in CO2-depleted N2 atmosphere).
MIMS measurements of the Ci content in working media used in this study. 5 μl aliquots each were injected into MNS buffer (pH 6.3) that was thoroughly degassed in the 150-μl MIMS cell at 20 °C. The time of injection is marked by arrows. Samples: MNS buffer (pH 6.3) before (trace 1) and after (trace 2) Ci-depletion by N2-gas-stream that was also used for filling in atmosphere inside the glove-box (for details see “Materials and methods” section). Trace 3 is the same as trace 2, but after 2-h exposure of the Ci-depleted buffer to the N2-gas atmosphere inside the glove-box
FIOPs experiments
Figure 3 displays normalized FIOPs (symbols) of spinach S1Y oxD thylakoids that were obtained at pH 6.3 at two Ci levels. The FIOP shown in Fig. 3a was obtained in the C +i sample under ambient conditions (390 ppm CO2 in air). It exhibits a typical period-four oscillation with maxima at the 3rd, 7th, 11th, and 15th flashes (see insert for original polarographic signal). For quantification of the obtained FIOPs we analyzed them by three different approaches within the “extended” Kok model (see Fig. 1b and “Materials and methods” section). The results are summarized in Table 1.
Normalized FIOPs of dark-adapted thylakoids (S1Y oxD ) from spinach measured under various levels of Ci at pH 6.3. The FIOPs were obtained either in the C +i samples under ambient Ci level (a) or in the C −i samples under Ci-depleted conditions (b). c represents FIOP that was monitored in the C −+i samples that were obtained after addition of 5 mM NaHCO3 to the C −i samples. Solid lines correspond to the average fits b shown in Table 1, while the symbols represent the average of experimental values of the collected FIOPs data, and error bars show the standard deviations for these values (n ≥ 3). For a better comparison, dashed gray lines and symbols in b and c depict FIOPs of the C +i sample (the same as in a). Normalization of the FIOPs was performed by dividing each yield of O2 per flash by the average of the O2-yields induced by flashes 3–6 (one cycle). The insets show examples of original FIOPs that were recorded with a flash frequency of 2 Hz at 20 °C. No artificial electron acceptors were used
In the most simple and widely used fit approach (fits a 1 –a 3 ), the misses (α) and double hits (β) were assumed to be equal in each S i transition. In such a case, the Kok parameters (α = 9.1 % and β = 2.8 %) in the C +i samples are typical for spinach thylakoids illuminated with Xenon flashes (Hillier and Messinger 2005; Messinger and Renger 2008). However, there are convincing arguments that the miss parameter is S i state dependent. The reason for using S i state independent miss parameter is that a single FIOP does not contain enough independent data points to uniquely extract the S i state dependence of the miss factor. EPR and FTIR spectroscopy were recently employed to study the S i state dependence of the miss parameter, but two quite different results were obtained (Han et al. 2012; Suzuki et al. 2012). To insure that any possible differences in the miss parameter between C +i and C −i samples are not fit model dependent, we employ here two simplified extreme cases of the above suggestions to fit our data.
A good improvement of the fit quality (Fq) can be achieved with a fit approach, in which the α-values for the transitions S0 → S1 and S1 → S2 are set to be “zero,” while those for the transitions S2 → S3 and S3 → (S4) → S0 are free running parameters that are forced to be equal (Isgandarova et al. 2003; Shevela et al. 2006a; Nöring et al. 2008) (also see “Materials and methods” section). This approach is an extreme case of the S state dependence described by Suzuki et al. (2012) based on FTIR data. The results derived from such approach are shown as fits b 1–b 3 in Table 1 and are depicted for each FIOP as solid lines in Fig. 3. With this fit approach, the α-value in the C +i sample was found to be 15.5 % for the two terminal S i state transitions, and double hits had a value of 2.5 %. Based on the obtained Fq values for fits a and b (smaller values indicate a better fit), we conclude that although all both fit approaches allow a good description of the data, the unequal miss fit approach b provides a slightly better description of the data. As the third fit approach (see fits c 1 –c 3 in Table 1), we employ a simplified version of the S i state dependence of the miss parameter reported by Han et al. (2012). These authors found that at 20 °C the miss parameter is between 10 and 16 % for the S0 → S1, S1 → S2, and S2 → S3 transitions, and very small (3 %) for the S3 → S0 transition. To minimize the number of free variables we therefore forced α01, α12 and α23 to be equal and varied them together, while α30 was fixed to 0 %. The results of this fit approach confirm (with worse Fq values) the above-described analysis performed by fits a and b.
To evaluate the effect of Ci depletion, the above data obtained in C +i samples (Fig. 3a) were compared with normalized FIOPs of the C −i samples (see Fig. 3b) that were obtained under Ci-depleted conditions (i.e., 10–20 ppm CO2 in a glove-box atmosphere). A close comparison of the data reveals that FIOPs of the C −i samples have reproducibly a higher damping as compared to that observed in the C +i thylakoids (for a better visualization, gray symbols and dashed line in Fig. 3b represent FIOP of the C +i sample, i.e., the same as in Fig. 3a). Detailed FIOPs analysis confirms these qualitative observations, independent of which fit approach was used (Table 1). According to all three fit approaches, the average miss parameter in the C −i samples reproducibly increases by about 2–3 % as compared to the α-values found in the C +i samples (p < 0.01 according to the two-tailed t test with an assumption of unequal variance). In order to check the reversibility of the observed effect, we measured the FIOPs shortly (within ~3 min) after addition of 5 mM HCO3 − to the C −i samples (C −+i samples). The FIOP shown in Fig. 3c and its fits (Table 1) demonstrate that such addition largely reverses the increase of the miss parameter (the two-tailed, unpaired t test for the C −i and C −+i samples; p < 0.01). Interestingly, the double hits were found to be almost identical in the C −i and C +i samples, and only insignificantly decreased in the C −+i samples. While the data presented in the manuscript were obtained with one specific thylakoid preparation, we performed test experiments with other preparations and consistently found a similar increase of the miss parameter after Ci-depletion.
Thus, the above presented FIOPs data and the numerical analysis clearly show that a 20-fold reduction of the Ci level in the sample medium decreases the PSII turnover efficiency (i.e., increases the miss parameter) in spinach thylakoids, while the addition of HCO3 − to Ci-depleted samples largely restores it.
Discussion
The results obtained in this FIOPs study clearly demonstrate a small, but reproducible increase of misses at strongly reduced Ci levels in sample suspension and in the gas phase above the sample as compared to the ambient levels of Ci in the air-exposed conditions. In previous FIOPs studies that employed a similar Ci depletion method for thylakoids, no change of the miss-values were found (Shevela et al. 2006b, 2007) unless formate or acetate were added to replace HCO3 − from PSII (Stemler et al. 1974; Mende and Wiessner 1985; Shevela et al. 2007). This discrepancy can be readily explained by the relatively high residual level of Ci in the C −i samples during the FIOPs measurements in these previous studies. Thus, in our previous report we showed, that during sample transfer onto the Joliot-type electrode and its subsequent polarization (~40 s) required for the FIOPs measurements, the initial 50-fold reduction of the Ci level in the freshly-depleted sample solution rises to about fivefold below ambient at the time of the FIOPs measurements (Shevela et al. 2007). This happens due to the fairly quick diffusion of CO2 from air back to the C −i sample suspension. In the present study, we excluded contamination of CO2 from air by performing Ci-depletion, transfers and measuring procedures inside the glove-box flushed in with N2 that contained maximum 20 ppm CO2. This experimental design allowed us to obtain FIOPs at 4-times lower levels of Ci than in earlier report (20-fold vs. ~fivefold depletion). This clearly demonstrates that, in addition to the Ci-depletion method, the actual levels of CO2/HCO3 − in the investigated samples are highly important and must be monitored in any relevant studies of HCO3 − effects in PSII. In principle, it is possible that the anaerobic conditions during measurements of the C −i thylakoids contribute to the observed increase of the miss parameter. However, HCO3 − addition under anaerobic conditions largely reversed the miss factor back to normal (Fig. 3). We therefore conclude that the possible contribution of O2-free conditions is negligible.
It is known that the misses may reflect the properties of both the donor and the acceptor side of PSII (Messinger et al. 1993; Shinkarev and Wraight 1993). The increased magnitude of the α-values at C −i conditions may therefore either indicate (i) changes in redox equilibria between the donor and the acceptor side of PSII (i.e., redox-potential differences between Q •−B QA/QBQA •−, P680/P680•+, YZ/Y oxZ and the S i states of the OEC) (Renger and Hanssum 1988; Shinkarev and Wraight 1993), (ii) the slower reduction of P680•+ by YZ (Christen et al. 1999), or (iii) slowed proton release from the OEC during water-splitting (Zaharieva et al. 2011). While the misses may reflect the properties of both the donor and the acceptor side of PSII, the double hits reflect mainly the properties of the acceptor side and are related linearly to the rate of electron transfer from QA •– to QB/QB •– (Messinger et al. 1993). Giving the fact that the β-values were found to be almost unaffected (Table 1) by the removal of CO2/HCO3 − in the samples, the changes in misses most likely appears to be restricted to events on the donor side of PSII (i.e., to the OEC).
Evidence for the role of “bicarbonate” to regulate the electron transfer on the acceptor side of PSII has been established in numerous previous studies (for recent reviews, see Van Rensen 2002; Van Rensen and Klimov 2005; McConnell et al. 2012; Shevela et al. 2012). It was shown to be bound to the non-heme iron (NHI) located between QA and QB (Diner and Petrouleas 1990; Hienerwadel and Berthomieu 1995), and this binding has been recently supported by PSII crystal structures (Guskov et al. 2010; Umena et al. 2011). Current model suggests that HCO3 −/CO3 2− facilitates the reduction of QB and participates in its protonation involving the H-network of D1-H215 and other residues (Berthomieu and Hienerwadel 2001; Cox et al. 2009; Cardona et al. 2012; Müh et al. 2012; Shevela et al. 2012; Saito et al. 2013). This acceptor side “bicarbonate” is known to be a so tightly bound ligand to the NHI that it can be efficiently exchanged/removed only by high concentrations of other carboxylic acids, such as formate or acetate (Govindjee et al. 1991, 1997; Aoyama et al. 2008; Shevela et al. 2008a, b; Ulas et al. 2008). In the present study, no formate or acetate was added to the sample during Ci-depletion procedure. It is therefore very unlikely that the observed effect of low CO2/HCO3 − levels in the sample suspension on the misses originates from the electron-acceptor side “bicarbonate”.
El-Shintinawy and Govindjee (1990) found with spinach leaf disks that HCO3 −-depletion resulted in an about 40 % loss of the O2-evolving activity after a short (10 s) infiltration of the leaf disks in Ci-free media that did not contain formate (and acetate) as compared to un-infiltrated (control) samples. Based on the observation that only 15 % of the O2 evolution rate was impaired after the same infiltration procedure, but in the presence of exogenous HCO3 −, the authors concluded that the observed effect was caused not only by the infiltration procedure, but also due to a partial Ci depletion of the leaf disks (El-Shintinawy and Govindjee 1990). These results are in a good agreement with the effects of formate/acetate-free Ci-depletion on miss parameter found in our study. It should be mentioned, that similar depletion procedure via infiltration of the leaf disks in the presence of formate induced the “bicarbonate effect,” and thereby gave an indication for two sites of action for the HCO3 − (one beyond QA, and another one was speculated to be between the primary electron acceptor, pheophytin, and QA) (El-Shintinawy and Govindjee 1990).
In contrast to the acceptor side effect, the role of HCO3 − ions in the process of water oxidation on the electron-donor side of PSII is not well understood (Stemler 2002; Van Rensen and Klimov 2005; Shevela et al. 2012). Several hypotheses for its functioning within (or in the vicinity of) the OEC were/are currently discussed: (i) exchangeable HCO3 − supplies substrate H2O to the OEC (Warburg 1964; Metzner 1978); (ii) tightly bound non-exchangeable HCO3 − is a structural part of the functional Mn4CaO5 cluster (Allakhverdiev et al. 1997; Klimov et al. 1997; Ferreira et al. 2004); (iii) HCO3 − is a native cofactor during photo-assembly of the Mn4CaO5 cluster, but is not part of the functioning OEC (Baranov et al. 2000, 2004) and (iv) a loosely bound (or non-bound) exchangeable HCO3 − is involved in proton removal during photosynthetic water oxidation (Villarejo et al. 2002; Ananyev et al. 2005). The results obtained by many laboratories have made the two first hypotheses very unlikely (Clausen et al. 2005; Hillier et al. 2006; Siegbahn and Lundberg 2006; Aoyama et al. 2008; Shevela et al. 2008b; Ulas et al. 2008; Guskov et al. 2010; Umena et al. 2011), while the role of HCO3 – during photoactivation of the OEC was confirmed by recent EPR data (Dasgupta et al. 2007, 2008, 2010). Recently, the first indications for participation of HCO3 − in removal of protons from the OEC were reported (Shutova et al. 2008; Ulas and Brudvig 2010). In particular, Shutova et al. (2008) showed that in algae Chlamydomonas reinhardtii, HCO3 − and Cah3 protein (the carbonic anhydrase protein associated with the PSII donor side in C. reinhardtii) have “donor side” effects only on proton release steps, and not on the electron transfer steps. Ulas and Brudvig (2010), using exogenous zwitterion (glycine betaine) as selective inhibitors of proton-transport processes, showed that the decreased catalytic rate of the OEC in Synechocystis PCC 6803 is recovered upon addition of exogenous HCO3 −. Our FIOPs data presented here are in a good agreement with these results and thus with the idea that HCO3 − may be required for the efficient turnover of the OEC by facilitating the proton release away from the OEC during water-splitting. Indeed, de-protonation reactions of the OEC are thought to have a significant impact on the thermodynamics of water oxidation (for reviews, see Krishtalik 2000 and Dau and Haumann 2008). Zaharieva et al. (2011) showed that high proton concentration in the vicinity of Mn4CaO5 cluster can severely inhibit the light-induced S i state transitions of the Kok cycle. Such inhibition should be reflected as increased overall miss factor of the Kok cycle. Therefore, an increase of the miss factor in our Ci-depleted samples may be indicative for less effective de-protonation of the OEC due to a deficit of HCO3 − species. The observation of the partial restoration of the turnover efficiency of the OEC upon the addition of the extrinsic HCO3 − to the C − i samples is completely coherent with this suggestion.
Abbreviations
- α:
-
Miss parameter
- β:
-
Double hit parameter
- Ci :
-
Inorganic carbon (HCO3 −, CO2, CO3 2−)
- C −i :
-
Inorganic carbon depleted
- C +i :
-
Containing ambient level of inorganic carbon
- FIOP:
-
Flash-induced oxygen evolution pattern
- MIMS:
-
Membrane-inlet mass spectrometry
- PSII:
-
Photosystem II
- OEC:
-
Oxygen-evolving complex
- S i states:
-
Oxidation states of the OEC, where i is the number of stored oxidizing equivalents
References
Allakhverdiev SI, Yruela I, Picorel R, Klimov VV (1997) Bicarbonate is an essential constituent of the water-oxidizing complex of photosystem II. Proc Natl Acad Sci USA 94(10):5050–5054
Ananyev G, Dismukes GC (2005) How fast can photosystem II split water? Kinetic performance at high and low frequencies. Photosynth Res 84(1):355–365
Ananyev G, Nguyen T, Putnam-Evans C, Dismukes GC (2005) Mutagenesis of CP43-arginine-357 to serine reveals new evidence for (bi)carbonate functioning in the water oxidizing complex of photosystem II. Photochem Photobiol Sci 4(12):991–998
Aoyama C, Suzuki H, Sugiura M, Noguchi T (2008) Flash-induced FT-IR difference spectroscopy shows no evidence for the structural coupling of bicarbonate to the oxygen-evolving Mn cluster in photosystem II. Biochemistry 47(9):2760–2765
Baranov SV, Ananyev GM, Klimov VV, Dismukes GC (2000) Bicarbonate accelerates assembly of the inorganic core of the water-oxidizing complex in manganese depleted photosystem II: a proposed biogeochemical role for atmospheric carbon dioxide in oxygenic photosynthesis. Biochemistry 39(20):6060–6065
Baranov SV, Tyryshkin AM, Katz D, Dismukes GC, Ananyev GM, Klimov VV (2004) Bicarbonate is a native cofactor for assembly of the manganese cluster of the photosynthetic water oxidizing complex. Kinetics of reconstitution of O2 evolution by photoactivation. Biochemistry 43(7):2070–2079
Beckmann K, Messinger J, Badger MR, Wydrzynski T, Hillier W (2009) On-line mass spectrometry: membrane inlet sampling. Photosynth Res 102:511–522
Berthomieu C, Hienerwadel R (2001) Iron coordination in photosystem II: interaction between bicarbonate and QB pocket studied by Fourier transform infrared spectroscopy. Biochemistry 40:4044–4052
Cardona T, Sedoud A, Cox N, Rutherford AW (2012) Charge separation in Photosystem II: a comparative and evolutionary overview. Biochim Biophys Acta 1817(1):26–43
Christen G, Seeliger A, Renger G (1999) P680+· reduction kinetics and redox transition probability of the water oxidizing complex as a function of pH and H/D isotope exchange in spinach thylakoids. Biochemistry 38(19):6082–6092
Clausen J, Beckmann K, Junge W, Messinger J (2005) Evidence that bicarbonate is not the substrate in photosynthetic oxygen evolution. Plant Physiol 139(3):1444–1450
Cox N, Messinger J (2013) Reflections on substrate water and dioxygen formation. Biochim Biophys Acta. doi:10.1016/j.bbabio.2013.01.013 (in press)
Cox N, Jin L, Jaszewski A, Smith PJ, Krausz E, Rutherford AW, Pace R (2009) The semiquinone-iron complex of photosystem II: structural insights from ESR and theoretical simulation; evidence that the native ligand to the non-heme iron is carbonate. Biophys J 97(7):2024–2033
Dasgupta J, Tyryshkin AM, Dismukes GC (2007) ESEEM spectroscopy reveals carbonate and an N-donor protein-ligand binding to Mn2+ in the photoassembly reaction of the Mn4Ca cluster in photosystem II. Angew Chem Int Ed 46(42):8028–8031
Dasgupta J, Ananyev GM, Dismukes GC (2008) Photoassembly of the water-oxidizing complex in photosystem II. Coord Chem Rev 252(3–4):347–360
Dasgupta J, Tyryshkin AM, Baranov SV, Dismukes GC (2010) Bicarbonate coordinates to Mn3+ during photo-assembly of the catalytic Mn4Ca core of photosynthetic water oxidation: EPR characterization. Appl Magn Reson 37(1–4):137–150
Dau H, Haumann M (2008) The manganese complex of photosystem II in its reaction cycle: basic framework and possible realization at the atomic level. Coord Chem Rev 252(3–4):273–295
Dau H, Zaharieva I, Haumann M (2012) Recent developments in research on water oxidation by photosystem II. Curr Opin Chem Biol 16(1–2):3–10
de Wijn R, van Gorkom HJ (2002) S-state dependence of the miss probability in photosystem II. Photosynth Res 72(2):217–222
Diner BA (1977) Dependence of deactivation reactions of photosystem II on redox state of plastoquinone pool A varied under anaerobic conditions. Equilibria on the acceptor side of photosystem II. Biochim Biophys Acta 460:247–258
Diner BA, Petrouleas V (1990) Formation by NO of nitrosyl adducts of redox components of the photosystem II reaction center. 2. Evidence that HCO3 −/CO2 binds to the acceptor-side non-heme iron. Biochim Biophys Acta 1015(1):141–149
El-Shintinawy F, Govindjee (1990) Bicarbonate effect in leaf discs from spinach. Photosynth Res 24:189–200
Ferreira KN, Iverson TM, Maghlaoui K, Barber J, Iwata S (2004) Architecture of the photosynthetic oxygen-evolving center. Science 303(5665):1831–1838
Forbush B, Kok B, McGloin MP (1971) Cooperation of charges in photosynthetic oxygen evolution. II. Damping of flash yield oscillation, deactivation. Photochem Photobiol 14(3):307–321
Fromme R, Hagemann R, Renger G (1987) Comparative studies of electron transport and atrazine binding in thylakoids and PS II particles from spinach. In: Biggens J (ed) Progress in Photosynthesis Research, vol III. Martinus Nijhoff Publishers, Dordrecht, pp 783–786
Govindjee, Xu C, van Rensen JJS (1997) On the requirement of bound bicarbonate for photosystem II activity. Z Naturforsch 52(1–2):24–32
Govindjee Weger HG, Turpin DH, van Rensen JJS, Devos OJ, Snel JFH (1991) Formate releases carbon dioxide/bicarbonate from thylakoid membranes: measurements by mass spectroscopy and infrared gas analyzer. Naturwissenschaften 78(4):168–170
Govindjee, Kern J, Messinger J, Whitmarsh J (2010) Photosystem II. In: Encyclopedia of Life Sciences (ELS). Wiley, Chichester. doi:10.1002/9780470015902.a0000669.pub2
Guskov A, Gabdulkhakov A, Broser M, Glöckner C, Hellmich J, Kern J, Frank M, Saenger W, Zouni A (2010) Recent progress in the crystallographic studies of photosystem II. ChemPhysChem 11:1160–1171
Han GY, Mamedov F, Styring S (2012) Misses during water oxidation in photosystem II are S state-dependent. J Biol Chem 287(16):13422–13429
Hienerwadel R, Berthomieu C (1995) Bicarbonate binding to the non-heme iron of photosystem II investigated by Fourier transform infrared difference spectroscopy and 13C-labeled bicarbonate. Biochemistry 34:16288–16297
Hillier W, Messinger J (2005) Mechanism of photosynthetic oxygen production. In: Wydrzynski T, Satoh K (eds) Photosystem II. The Light-Driven water:plastoquinone oxidoredutase, vol 22. Advances in photosynthesis and respiration. Springer, Dordrecht, pp 567–608
Hillier W, McConnell I, Badger MR, Boussac A, Klimov VV, Dismukes GC, Wydrzynski T (2006) Quantitative assessment of intrinsic carbonic anhydrase activity and the capacity for bicarbonate oxidation in photosystem II. Biochemistry 45(7):2094–2102
Isgandarova S, Renger G, Messinger J (2003) Functional differences of photosystem II from Synechococcus elongatus and spinach characterized by flash-induced oxygen evolution patterns. Biochemistry 42(30):8929–8938
Joliot P (1972) Modulated light source use with the oxygen electrode. In: San Pietro A (ed) Photosynthesis and nitrogen fixation, vol 24 B. Methods of enzymology. Academic Press, New York, pp 123–134
Joliot P (2003) Period-four oscillations of the flash-induced oxygen formation in photosynthesis. Photosynth Res 76(1–3):65–72
Joliot P, Barbieri G, Chabaud R (1969) Un nouveau modele des centres photochimiques du systeme II. Photochem Photobiol 10:309–329
Jursinic P (1981) Investigation of double turnovers in photosystem II charge separation and oxygen evolution with excitation flashes of different duration. Biochim Biophys Acta 635:38–52
Jursinic P, Stemler A (1982) A seconds range component of the reoxidation of the primary photosystem II acceptor Q: effects of bicarbonate depletion in chloroplasts. Biochim Biophys Acta 681(3):419–428
Jursinic PA, Stemler A (1984) Effects of bicarbonate depletion on secondary acceptors of photosystem II. Biochim Biophys Acta 764(2):170–178
Kebekus U, Messinger J, Renger G (1995) Structural changes in the water oxidizing complex monitored via the pH dependence of the reduction rate of redox state S1 by hydrazine and hydroxylamine in isolated spinach thylakoids. Biochemistry 34:6175–6182
Klimov VV, Allakhverdiev SI, Baranov SV, Feyziev YM (1995a) Effects of bicarbonate and formate on the donor side of photosystem 2. Photosynth Res 46(1–2):219–225
Klimov VV, Allakhverdiev SI, Feyziev YM, Baranov SV (1995b) Bicarbonate requirement for the donor side of photosystem II. FEBS Lett 363(3):251–255
Klimov VV, Hulsebosch RJ, Allakhverdiev SI, Wincencjusz H, van Gorkom HJ, Hoff AJ (1997) Bicarbonate may be required for ligation of manganese in the oxygen-evolving complex of photosystem II. Biochemistry 36(51):16277–16281
Kok B, Forbush B, McGloin M (1970) Cooperation of charges in photosynthetic O2 evolution. Photochem Photobiol 11:457–476
Konermann L, Messinger J, Hillier W (2008) Mass spectrometry based methods for studying kinetics and dynamics in biological systems. In: Amesz J, Hoff AJ (eds) Biophysical techniques in photosynthesis, vol 26., Series advances in photosynthesis and respirationSpringer, Dordrecht, pp 167–190
Kouřil R, Dekker JP, Boekema EJ (2012) Supramolecular organization of photosystem II in green plants. Biochim Biophys Acta 1817(1):2–12
Krishtalik LI (2000) The mechanism of the proton transfer: an outline. Biochim Biophys Acta 1458(1):6–27
Mar T, Govindjee (1972) Kinetic models of oxygen evolution in photosynthesis. J Theoret Biol 36:427–446
McConnell IL, Eaton-Rye JJ, Van Rensen JJS (2012) Regulation of photosystem II electron transport by bicarbonate. In: Eaton-Rye JJ, Tripathy BC, Sharkey TD (eds) Photosynthesis: plastid biology, energy conversion and carbon assimilation. Springer, Dordrecht, pp 475–500
Mende D, Wiessner W (1985) Bicarbonate in vivo requirement of photosystem II in the green alga Chlamydobotrys stellata. J Plant Physiol 118(3):259–266
Messinger J (1993) Untersuchungen über die reaktiven Eigenschaften der verschiedenen Redoxzustände der Wasseroxidase Höherer Pflanzen. TU Berlin, Berlin
Messinger J, Renger G (1990) The reactivity of hydrazine with PS II strongly depends on the redox state of the water oxidizing system. FEBS Lett 277:141–146
Messinger J, Renger G (1993) Generation, oxidation by the oxidized form of the tyrosine of polypeptide D2, and possible electronic configuration of the redox States S0, S−1 and S−2 of the water oxidase in isolated spinach thylakoids. Biochemistry 32(36):9379–9386
Messinger J, Renger G (2008) Photosynthetic water splitting. In: Renger G (ed) Primary processes of photosynthesis, part 2 principles and apparatus, vol 9., Comprehensive series in photochemical and photobiological sciencesRSC Publishing, Cambridge, pp 291–351
Messinger J, Schröder WP, Renger G (1993) Structure-function relations in photosystem II. Effects of temperature and chaotropic agents on the period four oscillation of flash induced oxygen evolution. Biochemistry 32:7658–7668
Messinger J, Badger MR, Wydrzynski T (1995) Detection of one slowly exchanging substrate water molecule in the S3 state of photosystem II. Proc Natl Acad Sci USA 92:3209–3213
Messinger J, Seaton G, Wydrzynski T, Wacker U, Renger G (1997) S−3 state of the water oxidase in photosystem II. Biochemistry 36:6862–6873
Metzner H (1978) Photosynthetic oxygen evolution. Academic Press, London
Müh F, Glöckner C, Hellmich J, Zouni A (2012) Light-induced quinone reduction in photosystem II. Biochim Biophys Acta 1817(1):44–65
Nöring B, Shevela D, Renger G, Messinger J (2008) Effects of methanol on the Si-state transitions in photosynthetic water-splitting. Photosynth Res 98:251–260
Nugent JHA, Demetriou C, Lockett CJ (1987) Electron donation in photosystem II. Biochim Biophys Acta 894(3):534–542
Pobeguts OV, Smolova TN, Timoshevsky DS, Klimov VV (2010) Interaction of bicarbonate with the manganese-stabilizing protein of photosystem II. J Photochem Photobiol B 100:30–37
Renger G (2010) The light reactions of photosynthesis. Curr Sci 98:1305–1319
Renger G, Hanssum B (1988) Studies on the deconvolution of flash induced absorption changes into the difference spectra of individual redox steps within the water oxidizing enzyme system. Photosynth Res 16:243–259
Renger G, Hanssum B (2009) Oxygen detection in biological systems. Photosynth Res 102(2–3):487–498
Renger G, Holzwarth AR (2005) Primary electron transfer. In: Wydrzynski TJ, Satoh K (eds) Photosystem II. The light-driven water: plastoquinone oxidoreductase, vol 22. Advances in photosynthesis and respiration. Springer, Dordrecht, pp 139–175
Saito K, Rutherford AW, Ishikita H (2013) Mechanism of proton-coupled quinone reduction in photosystem II. Proc Natl Acad Sci USA 110(3):954–959
Shevela D, Messinger J (2012) Probing the turnover efficiency of photosystem II membrane fragments with different electron acceptors. Biochim Biophys Acta 1817(8):1208–1212
Shevela D, Nöring B, Eckert HJ, Messinger J, Renger G (2006a) Characterization of the water oxidizing complex of photosystem II of the Chl d-containing cyanobacterium Acaryochloris marina via its reactivity towards endogenous electron donors and acceptors. Phys Chem Chem Phys 8(29):3460–3466
Shevela DN, Khorobrykh AA, Klimov VV (2006b) Effect of bicarbonate on the water-oxidizing complex of photosystem II in the super-reduced S-states. Biochim Biophys Acta 1757(4):253–261
Shevela D, Klimov V, Messinger J (2007) Interactions of photosystem II with bicarbonate, formate and acetate. Photosynth Res 94(2–3):247–264
Shevela D, Klimov V, Messinger J (2008a) Formate-induced release of carbon dioxide/hydrogen carbonate from photosystem II. In: Allen JF, Gantt E, Golbeck JH, Osmond B (eds) Photosynthesis. Energy from the Sun. Springer, Glasgow, pp 497–501
Shevela D, Su JH, Klimov V, Messinger J (2008b) Hydrogen carbonate is not a tightly bound constituent of the water-oxidizing complex in photosystem II. Biochim Biophys Acta 1777(6):532–539
Shevela D, Eaton-Rye JJ, Shen J-R, Govindjee (2012) Photosystem II and the unique role of bicarbonate: a historical perspective. Biochim Biophys Acta 1817(8):1134–1151
Shinkarev V (2005) Flash induced oxygen evolution and other oscillatory processes. In: Wydrzynski T, Satoh K (eds) Photosystem II. The light-driven water: plastoquinone oxidoredutase, vol 22. Advances in photosynthesis and respiration. Springer, Dordrecht, pp 539–565
Shinkarev V, Wraight CA (1993) Oxygen evolution in photosynthesis: from unicycle to bicycle. Proc Natl Acad Sci USA 90:1834–1838
Shutova T, Kenneweg H, Buchta J, Nikitina J, Terentyev V, Chernyshov S, Andersson B, Allakhverdiev SI, Klimov VV, Dau H, Junge W, Samuelsson G (2008) The photosystem II-associated Cah3 in Chlamydomonas enhances the O2 evolution rate by proton removal. EMBO J 27(5):782–791
Siegbahn PEM, Lundberg M (2006) Hydroxide instead of bicarbonate in the structure of the oxygen evolving complex. J Inorg Biochem 100(5–6):1035–1040
Stemler A (1980) Forms of dissolved carbon dioxide required for photosystem II activity in chloroplast membranes. Plant Physiol 65(6):1160–1165
Stemler A (1982) The functional role of bicarbonate in photosynthetic light reaction II. In: Govindjee (ed) Photosynthesis vol II. Academic Press, New York, pp 513–538
Stemler AJ (2002) The bicarbonate effect, oxygen evolution, and the shadow of Otto Warburg. Photosynth Res 73(1–3):177–183
Stemler A, Govindjee (1973) Bicarbonate ion as a critical factor in photosynthetic oxygen evolution. Plant Physiol 52(2):119–123
Stemler AJ, Lavergne J (1997) Evidence that formate destabilizes the S−1 state of the oxygen-evolving mechanism in Photosystem II. Photosynth Res 51(2):83–92
Stemler A, Babcock GT, Govindjee (1974) Effect of bicarbonate on photosynthetic oxygen evolution in flashing light in chloroplast fragments. Proc Natl Acad Sci USA 71(12):4679–4683
Styring S, Rutherford AW (1987) In the oxygen evolving complex of photosystem II the S0 state is oxidized to the S1 State by Y +D (Signal IIslow). Biochemistry 26:2401–2405
Suzuki H, Sugiura M, Noguchi T (2012) Determination of the miss probabilities of individual S-state transitions during photosynthetic water oxidation by monitoring electron flow in photosystem II using FTIR spectroscopy. Biochemistry 51(34):6776–6785
Ulas G, Brudvig GW (2010) Zwitterion modulation of O2-evolving activity of cyanobacterial photosystem II. Biochemistry 49(37):8220–8227
Ulas G, Olack G, Brudvig GW (2008) Evidence against bicarbonate bound in the O2-evolving complex of photosystem II. Biochemistry 47(10):3073–3075
Umena Y, Kawakami K, Shen J-R, Kamiya N (2011) Crystal structure of oxygen-evolving photosystem II at a resolution of 1.9 Å. Nature 473:55–60
Van Rensen JJS (2002) Role of bicarbonate at the acceptor side of photosystem II. Photosynth Res 73(1–3):185–192
Van Rensen JJS, Klimov VV (2005) Bicarbonate interactions. In: Wydrzynski T, Satoh K (eds) Photosystem II. The light-driven water: plastoquinone oxidoreductase, vol 22. Advances in photosynthesis and respiration. Springer, Dordrecht, pp 329–346
Van Rensen JJS, Xu C, Govindjee (1999) Role of bicarbonate in photosystem II, the water-plastoquinone oxido-reductase of plant photosynthesis. Physiol Plant 105:585–592
Vass I, Deak Z, Hideg E (1990a) Charge equilibrium between the water oxidizing complex and the electron donor tyrosine D in photosystem II. Biochim Biophys Acta 1017:63–69
Vass I, Deak Z, Jegerschold C, Styring S (1990b) The accessory electron-donor tyrosine D of photosystem II is slowly reduced in the dark during low-temperature storage of isolated thylakoids. Biochim Biophys Acta 1018(1):41–46
Vermaas WEJ, Renger G, Dohnt G (1984) The reduction of the oxygen evolving system in chloroplasts by thylakoid components. Biochim Biophys Acta 764(2):194–202
Villarejo A, Shutova T, Moskvin O, Forssen M, Klimov VV, Samuelsson G (2002) A photosystem II-associated carbonic anhydrase regulates the efficiency of photosynthetic oxygen evolution. EMBO J 21(8):1930–1938
Warburg O (1964) Prefactory chapter. Annu Rev Biochem 33:1–18
Warburg O, Krippahl G (1958) Hill-Reaktionen. Z Naturforsch B 13(8):509–514
Winget GD, Izawa S, Good NE (1965) Stoichiometry of photophosphorylation. Biochem Biophys Res Commun 21(5):438–441
Wydrzynski T, Govindjee (1975) New site of bicarbonate effect in photosystem II of photosynthesis: evidence from chlorophyll fluorescence transients in spinach-chloroplasts. Biochim Biophys Acta 387(2):403–408
Wydrzynski T, Satoh K (eds) (2005) Photosystem II. The light-driven water: plastoquinone oxidoreductase, vol 22. Advances in photosynthesis and respiration. Springer, Dordrecht
Yano J, Kern J, Sauer K, Latimer MJ, Pushkar Y, Biesiadka J, Loll B, Saenger W, Messinger J, Zouni A, Yachandra VK (2006) Where water is oxidized to dioxygen: structure of the photosynthetic Mn4Ca cluster. Science 314:821–825
Zaharieva I, Wichmann JM, Dau H (2011) Thermodynamic limitations of photosynthetic water oxidation at high proton concentrations. J Biol Chem 286(20):18222–18228
Acknowledgments
This study was supported by the Knut and Alice Wallenberg Foundation, the Kempe Foundation, the Swedish Research Council (VR), the Strong Research Environment Solar Fuels (Umeå University), the Artificial Leaf Project (K&A Wallenberg Foundation) and the Max-Planck Gesellschaft. The authors would like to thank Govindjee, Vyacheslav Klimov, and Alan Stemler for fruitful discussions on the “bicarbonate effect” over the last few years and Ethel Hüttel for sample preparation.
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Shevela, D., Nöring, B., Koroidov, S. et al. Efficiency of photosynthetic water oxidation at ambient and depleted levels of inorganic carbon. Photosynth Res 117, 401–412 (2013). https://doi.org/10.1007/s11120-013-9875-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11120-013-9875-5