Abstract
The resolution of Photosystem II (PS II) crystals has been improved using isolated PS II from the thermophilic cyanobacterium Thermosynechococcus vulcanus. The new 1.9 Å resolution data have provided detailed information on the structure of the water-oxidizing complex (Umena et al. Nature 473: 55–61, 2011). The atomic level structure of the manganese–calcium cluster is important for understanding the mechanism of water oxidation and to design an efficient catalyst for water oxidation in artificial photosynthetic systems. Here, we have briefly reviewed our knowledge of the structure and function of the cluster.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Photosynthesis is a process in which light energy is converted to chemical energy and used to produce organic compounds. It is believed that more than 3 billion years ago, organisms developed the capacity to efficiently capture solar energy and use it to power the synthesis of organic molecules using photosynthesis. The photosynthetic process set into motion an unprecedented explosion in biological activity, allowing life to prosper and diversify on an enormous scale, as witnessed by the fossil record and by the extent and diversity of living organisms on our planet today. Indeed, it was the process of photosynthesis over eons of time which has provided us with the oil, gas, and coal needed to power our technologies, heat our homes and produce the wide range of chemicals and materials that support everyday life (Allakhverdiev 2011, 2012). It is also clear that three of the great challenges facing humanity in the twenty-first century are energy supply, climate change, and global food security. Although global energy demand is expected to continue to increase, the availability of low cost energy will continue to diminish. Coupled with increasing concerns about climate change due to CO2 release from the combustion of fossil fuels, there is now an urgent need to develop novel clean, environmentally friendly and renewable fuels that can support energy systems to replace fossil fuels. The fuel must be made from cheap and “endless” resources that are available everywhere. Taking into account the above considerations, the goal of developing biomimetic artificial photosynthetic systems is to utilize solar energy for conversion into chemical energy through a series of electron transfer events. A promising approach for the design of such systems will be to adhere to the same chemical design principles found in natural photosynthesis (Najafpour 2006; Allakhverdiev 2011, 2012; Najafpour and Allakhverdiev 2012; Najafpour 2012).
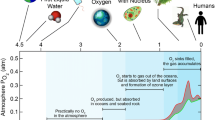
A key step in the evolution of photosynthesis occurred when cyanobacteria evolved about 2.5 billion years ago leading to the accumulation of oxygen in the atmosphere (Wydrzynski and Satoh 2005; Govindjee 2011; Allakhverdiev 2011, 2012). The presence of oxygen made the existence of heterotrophs such as humans possible (Payne et al. 2011). There are two well-known basic parts to photosynthesis: the so-called light reactions and the subsequent series of reactions that “fix” CO2 into carbohydrate. In the light reactions, water is oxidized and reducing power (NADPH) and ATP are produced. Biological water oxidation involves three distinct steps: (i) trapping of light by chlorophyll pigments and rapid energy transfer to the reaction centre (P680) of Photosystem II (PS II), resulting in its oxidized form (P680+); (ii) rapid electron transfer to P680+ from an oxidizable protein side-chain intermediate (tyrosine 161 of the D1 PS II reaction center protein), and (iii) electron transfer to tyrosine 161 from the manganese–calcium cluster which is the catalytic complex for water oxidation. The water-oxidizing complex (WOC), also referred to as the oxygen-evolving complex (OEC), in PS II is the heart of the water-oxidizing machinery of photosynthesis (McEvoy and Brudvig 2006; Najafpour 2006; Najafpour and Allakhverdiev 2012; Najafpour et al. 2012a, b). Following the light reactions the so-called dark reactions that do not depend directly on light are activated in the Calvin–Benson cycle. This series of reactions takes CO2 and energy from the NADPH and ATP that were produced in the light reactions to form the carbohydrates, and subsequently the lipids and proteins that are required for life (Golbeck 2006).
Today we have considerable knowledge of the working of natural photosynthesis and its photosystems, including the water oxidation reaction. However, many questions and details remain unanswered. To fully understand the photosynthetic reactions is not only a satisfying intellectual pursuit, but is also an important goal as we strive to improve agricultural yields and develop new solar technologies for the splitting of water and for generating fuels (Najafpour 2006; Allakhverdiev 2011, 2012; Najafpour and Allakhverdiev 2012; Najafpour et al. 2012a, b). In this mini review we present a snapshot of our current view on the structure and function of the WOC in nature and in biomimetic systems.
The importance of the water-oxidizing complex
For biological water oxidation, Nature uses a manganese–calcium catalytic cluster that oxidizes water with high yield and with a turnover of up to 100–400 molecules of O2 released per second (Dismukes et al. 2009; Duan et al. 2012). Water oxidation is not only important in natural photosynthesis but also it is important in artificial photosynthetic systems. As an example, the production of hydrogen gas, an ideal fuel for the future, from hydrogen ions (Eq. 2) via water splitting (Eqs. 1, 3) has been proposed as the holy grail of chemistry (Bard and Fox 1995). But to evolve hydrogen in a sustainable biomimetic system, it is necessary first to synthesize a “super catalyst” for water oxidation, which is the most challenging half reaction of water splitting (Bard and Fox 1995; Bockris 1977).

Water oxidation as a source for “cheap electrons” (Bockris 1977) (Eq. 1) provides electrons not only for proton reduction but also for other reduction reactions which are equally important in artificial photosynthesis (Scheme 1). Solar power is the most abundant source of renewable energy and the photosynthetic machinery provides a blue print for the use of this energy to power the thermodynamically and chemically demanding reaction of water oxidation. At the heart of the reaction is the Mn4O5Ca cluster of the WOC that is housed in a protein environment that controls reaction coordinates, proton movement and water access (see the latest 1.9 Å resolution structure of PS II by Umena et al. (2011)). PS II is a dimeric, multi-subunit, transmembrane protein complex of ~650 kDa molecular weight, found in the thylakoid membranes of plant and algal chloroplasts and in cyanobacteria (Najafpour and Govindjee 2011). The Mn4O 5 Ca cluster has a dimension of about ~0.5 × 0.25 × 0.25 nm. Thus, the WOC may be considered as a nano-sized manganese–calcium oxide in a protein environment.
Structure of the water-oxidizing complex
The importance of manganese in oxygenic photosynthesis was already implicit from the work of Pirson in (1937). A famous model for the WOC was the dimer-of-dimers model where the Mn complex was assumed to be composed of two Mn “dimers” each being a di-μ-oxo bridged Mn2 unit (Sauer et al. 1992). However, according to EPR data, David Britt’s group at the University of California—Davis, concluded that their electron spin echo electron nuclear double resonance experiments were difficult to reconcile with the dimer-of-dimers model and they proposed the so-called dangler model (Peloquin et al. 2000). The first papers dealing with the detailed structure of PS II were from the research groups of Horst Tobias Witt and Wolfram Saenger, in Berlin (Zouni et al. 2001) followed by the work of Nobuo Kamiya and Jian-Ren Shen in Japan (Kamiya and Shen 2003). In 2004, a detailed study was published from the research groups of James Barber and So Iwata at Imperial College, London, that provided a rather complete structure of PS II (Ferreira et al. 2004). At this point, the positioning of most of the subunits was unveiled. Importantly, for the first time, James Barber, So Iwata and colleagues provided the location of Ca in the WOC and introduced the cluster as a Mn3Ca-cubane structure with the 4th Mn attached a bit away from the cubane core (Ferreira et al. 2004). Then, Yano et al. (2006) proposed a series of chemically related models for which the calculated EXAFS spectra matched the polarized EXAFS data obtained for PS II crystals. In other words, they performed an extended set of hypothetical models of the Mn4Ca complex of PS II and evaluated the calculated EXAFS spectra for the three experimental spectra detected for excitation along the a, b, and c axes (Yano et al. 2006). In 2011, the research groups of Jian-Ren Shen and Nobuo Kamiya significantly improved the resolution of the PS II crystals obtained from the thermophilic cyanobacterium Thermosynechococcus vulcanus (T. vulcanus) down to atomic resolution (Umena et al. 2011; Kawakami et al. 2011). They showed that the WOC contains four manganese ions, one calcium ion, and five oxygen atoms (Umena et al. 2011; Kawakami et al. 2011) (Fig. 1). However, all X-ray studies on PS II crystals have been plagued by X-ray-induced damage to the Mn4O 5 Ca cluster, so that structures now in the literature are of something other than the native S1 state (Yano et al. 2005) (for a detailed discussion of the S states of the WOC see Renger (2012) and the Mechanism of biological water oxidation, section below). For example, the Mn–O distances in the crystallographic model of Umena et al. (2011) are long compared with typical distances in synthetic complexes (Grundmeier and Dau 2012). Regarding the recent model, Grundmeier and Dau (2012) show that the average oxidation state of Mn in the crystal should be +2.5, corresponding on average to a Mn(II,II,III,III) system rather than a pure S1 state with Mn(III,III,IV,IV). This observation implies that at least a fraction of the manganese ions in the enzyme are reduced.
Top the manganese–calcium cluster (circled in red) may be considered as a nano-sized manganese–calcium oxide in a protein environment (Adapted from Kawakami et al. 2011). Bottom the Mn4O 5 Ca cluster in PS II that has dimensions of about ~0.5 × 0.25 × 0.25 nm
An important issue in the new structure proposed by Umena et al. (2011) was the presence of four terminal water ligands: two of which coordinated to Ca and two to the “dangling” Mn (Mn(4)). The structure is thus a manganese–calcium cluster that can be described as a Mn4O5Ca(H2O)4 complex. In the structure, the calcium and three manganese ions occupy four corners and four oxygen atoms form the other four corners of the cubane-like structure. It is important to note that regarding the Ca–O and Mn–O bond lengths, the cubane-like structure is not an ideal and symmetric one. In this structure, there are six ligands around Mn(1): three μ3-O as hard ligands and two carboxylates and one imidazole group as borderline ligands that could together stabilize the oxidation number of III or IV for this manganese ion. The six ligands around Mn(2) are three μ3-O and three bridging COO− (Umena et al. 2011; Kawakami et al. 2011) (Fig. 2).
Ligand structure of the metal cluster determined at 1.9 Å resolution. Residues from D1 are colored in green, and that from CP43 are colored in cyan. (Adapted from Kawakami et al. 2011)
These ligands (Fig. 2) could stabilize the oxidation state of III or IV for this Mn(2) ion. This ion is connected to calcium and two other manganese ions with a bridging carboxylate and three oxo groups. The ligands around Mn(3) are three μ3-O, one μ2-O and two bridging COO− groups. Four hard μ-O ligands could stabilize manganese (IV) rather than manganese (III). The ligands around Mn(4) are one μ4-O, one μ2-O, two bridging COO− groups and two H2O molecules (Umena et al. 2011; Kawakami et al. 2011). These two water molecules are very important and one of them may serve as one of substrates for water oxidation (Umena et al. 2011; Kawakami et al. 2011). The ligands around Mn(4) could help to stabilize the oxidation number of (III) for this Mn ion, but de-porotonation of water molecules could also participate in the stabilization of the oxidation state of (IV) as well as higher oxidation states. However, this simple analysis shows that the cluster with four or even three manganese ions with oxidation states of IV may not be stable. Analysis of various experimental, theoretical and computational results shows that higher oxidation states of the cluster in S2, S3, or S4 with four or even three manganese ions with oxidation states of IV are unstable and decay to lower oxidation states (McEvoy and Brudvig 2006; Grundmeier and Dau 2012).
Another important ion in the WOC is calcium. The structure reported by the research team of Jian-Ren Shen and Nobuo Kamiya shows calcium has seven ligands, three μ3-O bridges, two bridging COO− groups and two H2O molecules (Umena et al. 2011; Kawakami et al. 2011). Calcium, as a co-factor in the WOC, was found by Ghanotakis et al. (1984) and shown to be essential in oxygen evolution. Among many metal (II) ions, strontium (II) is the only cation that can functionally substitute for calcium in the WOC (see Boussac et al. 2004). Lee et al. (2007) provided direct support for the proposal that calcium plays a structural role in the early S-state transitions. They found the role can also be fulfilled by other cations with similar ionic radius. However, Lohmiller et al. (2012) using X- and Q-band EPR, and 55Mn electron nuclear double resonance (ENDOR), showed that upon Ca2+ removal from the WOC, its electronic structure remains essentially unaltered. It was also proposed that Ca2+ could be important for the proton-coupled electron transfer between Yz and the Mn cluster or, alternatively, may act as an initial binding site for substrate water (Lohmiller et al. 2012).
The imidazole nitrogen of D1-His 337 is hydrogen-bonded to the μ3-oxo bridge connecting Mn(1), Mn(2), and Mn(3). Petrie et al. (2012) proposed that such interactions may account for the lengthening of the Mn–Mn distances observed in the most recent (1.9 Å) crystal structure of PS II compared to earlier, lower-resolution (2.9 Å or greater) X-ray-derived structures and EXAFS studies on functional PS II. They used density functional theory (DFT) calculations to test the influence on Mn–Mn distances on H-bonding interactions mediated by the proximate His 337 residue and their calculations showed that the H-bonding interaction of Mn4O5Ca with the His 337 residue leads to expansion of the “close” Mn–Mn distances (Petrie et al. 2012). The role of this hydrogen bond may be as a stabilizer for the WOC (Umena et al. 2011; Kawakami et al. 2011) (Fig. 3).
Arginine 357 in the second coordination sphere of the WOC is an important residue that may play an important role in maintaining the structure of the metal cluster, either in stabilizing the cubane structure or by providing partial positive charges to compensate for the negative charges induced by the oxo bridges and carboxylate ligands of the WOC. One of nitrogens of CP43-Arg 357 is hydrogen-bonded to two μ-O ligands of the manganese–calcium cluster, whereas the other is hydrogen-bonded to the carboxylate oxygen of D1-Asp 170 and to that of D1-Ala 344 (see Fig. 3). The structure shows that the distances between the nitrogen atoms of the arginine side chain and Ca2+ are 4.2 and 4.4 Å, respectively. Also, the distances between the nitrogen atoms of the arginine side chain and Mn(4) are 4.7 and 6.0 Å, respectively. The side chain of arginine may stabilize the structure of the WOC as it is hydrogen-bonded to two μ-O bridges and one carboxylate group bridging between Ca2+ and Mn(2).
Two chloride ions (Cl1 and Cl2) are in the recent structure of the WOC (Fig. 4). Both chloride ions are surrounded by water molecules and amino acids. Calculations combined with molecular dynamics proposed that chloride depletion forms a salt-bridge between D1-Asp 61 and D2-Lys 317, the two acido-basic side chains that contribute to the high-affinity binding site for chloride. This salt bridge would modify the pKa of D1-Asp 61 and would prevent the efficient proton release associated with the accumulation of oxidizing power within the catalytic center. The Cl1 site would thus set the conformation appropriate for the efficient conduction of protons (Rivalta et al. 2011).
A hydrogen-bond network connecting the Mn4CaO5-cluster and chloride ions (Adapted from Kawakami et al. 2011)
After photons are captured and excitation energy is transferred to the reaction center chlorophyll P680, a primary charge separation occurs: oxidized P680 (P +680 ) and reduced pheophytin (Phe−) are formed (Wydrzynski and Satoh 2005). Then, P +680 is reduced by electron transfer from the tyrosine (Tyrosine 161) residue (Yz) to form a tyrosine radical ((Y ·z ) (Hammarstrom and Styring 2011). A hydrogen bond is observed between Yz and the ε-nitrogen of a histidine (D1-His 190) (Fig. 5). The D1-His 190 was further hydrogen-bonded to other amino acids or water molecules to form a hydrogen-bond network suggested as an exit channel for protons (Umena et al. 2011; Kawakami et al. 2011). Yz is connected to a water molecule at a distance of 2.46 Å, which in turn is connected to another water molecule that is bound to the Ca2+ of the WOC. Recently, Hammarstrom and Styring (2011) reviewed the possible roles of Yz in the WOC of PS II. Firstly, Yz is a rapid electron donor to P680 +. Secondly, the presence of Yz allows the WOC to be positioned at an appropriate distance from P680; it is argued that a closer proximity of the WOC and P680 could lead to non-productive quenching of excited P680 by reverse electron transfer (Hammarstrom and Styring 2011; Hammarstrom et al. 2001). Thirdly, Yz may possibly be directly involved in the water oxidation chemistry. The proton may transfer back and forth along the hydrogen bond between Yz and the neighbouring D1-His 190. Proton transfer to D1-His 190 may possibly control electrostatics such that oxidation of Yz triggers de-protonation of the WOC of PS II (Hammarstrom and Styring 2011).
Three different channels for oxygen, water and protons could be found in PS II (Barber 2008; Barber and Murray 2008; Ho 2008; Ho and Styring 2008; Guskov et al. 2009). Electrostatic, structural, and orientational grounds were proposed as functional assignments of these channels. Investigations of water movement in PS II introduced a novel perspective to the study of the supply of water to the WOC and showed that functional PS II is characterized by a branched water supply structure with multiple control points.
Manganese-stabilizing protein
Nowadays it is well known that higher plants, algae and cyanobacteria all perform oxygenic photosynthesis, and the basic structure of their photosynthetic machinery is highly conserved. The PS II complex is composed of the core proteins, D1 and D2, which bind all the redox-active components involved in electron transfer through PS II. In addition to the D1 and D2 proteins, PS II contains the inner chlorophyll-binding antenna proteins CP43 and CP47, and the Cyt b 559 proteins PsbE and PsbF. Moreover, several low-molecular-mass proteins are required for proper function and assembly of the PS II dimer (Komenda et al. 2012). Although the ultimate function of the WOC of PS II is very similar in the eukaryotic organisms and the prokaryotic cyanobacteria, the detailed characteristics of the individual WOC proteins differ between these organisms. Another difference in PS II between the eukaryotic and prokaryotic organisms concerns the light-harvesting machinery (see for review Aro et al. 1993; Allakhverdiev and Murata 2004; Nishiyama et al. 2006; 2011; Murata et al. 2007, 2012; Mulo et al. 2012). The WOC is also shielded from the thylakoid lumen by several extrinsic polypeptides attached to the intrinsic subunits of PS II (Bricker et al. 2012).
Photosynthetic organisms growing under variable environmental conditions are often exposed to different types of stress like harmful irradiation (UV-B or high intensity visible light), heat, cold, high salt, and also infection by pathogens (viruses, bacteria) and PS II can become unstable (Aro et al. 1993; Keren et al. 1997; Allakhverdiev and Murata 2004; Jo et al. 2010; Nishiyama et al. 2006, 2011; Wang et al. 2011; Zulfugarov et al. 2011; Murata et al. 2007, 2012; Mulo et al. 2012). The largest extrinsic protein is the manganese-stabilizing protein (PsbO) that is present in all O2-evolving photosynthetic organisms (Miyao and Murata 1984; Popelkova et al. 2011). PsbO is essential for stabilization and retention of the Mn and Cl− cofactors (Bricker 1992; Eaton-Rye et al. 2003; Bricker et al. 2012). The results by Popelkova et al. (2011) showed that the protein affects the ability of calcium to protect the Mn cluster against inhibition by reduction in the dark. It was reported with in vitro experiments that after depletion of PsbO from PS II using CaCl2 or urea, water oxidation is reduced to only ∼20 % of the control value (Ono and Inoue 1983; Miyao and Murata 1984; Bricker 1992; Bricker et al. 2012). Miyao and Murata (1984) showed that incubation of PsbO-depleted PS II at low Cl− concentrations causes the loss of two of four Mn ions from the WOC, but this loss can be prevented by the addition of a high concentration of Cl−. Similarly, several ionic species such as metal cations, ruthenium red, or polyamines deplete PsbO from the WOC and destabilize the Mn4O 5 Ca cluster but this depletion can be prevented by an increased concentration of Ca2+ and/or Cl− (Beauchemin et al. 2007; Boisvert et al., 2007; Gauthier and Carpentier 2008; Hamdani and Carpentier 2009). Popelkova et al. (2006) showed by introducing specific Arg mutations in PsbO that the protein is important for retention of Cl− in the WOC. Ädelroth et al. (1995) showed removal of PsbO either eliminates the Ca2+ binding site in PS II or removes a barrier that prevents rapid exchange of Ca2+ between the WOC and the surrounding medium (for review see Bricker et al. 2012).
Mechanism of biological water oxidation
Kok cycle
In 1969, Pierre Joliot showed that flash illumination produced an oscillating pattern of oxygen evolution that has a maximum yield on every fourth flash (Joliot et al. 1969; Kok et al. 1970; Mar and Govindjee 1972; Joliot and Kok 1975; Joliot 2005; Govindjee et al. 2010; Grundmeier and Dau 2012). This observation was a major advance toward understanding the mechanism of water oxidation as oxidation of two water molecules to produce one oxygen molecule requires the removal of four electrons. Based on the pattern of oxygen evolution, it was proposed that in a cycle of water oxidation, a succession of oxidizing equivalents is stored on each separate and independent WOC and when four oxidizing equivalents have accumulated one by one, oxygen is spontaneously evolved (see Kok et al. 1970). Each oxidation state of the WOC is known as an “S-state”, with S0 being the most reduced state and S4 the most oxidized state in the catalytic cycle (Fig. 6) (Kok et al. 1970). The S4 → S0 transition is light independent and in this state oxygen is evolved. All other S-state transitions are induced by the photochemical oxidation of the PS II reaction center (P680) (Kok et al. 1970).
Classical S-state cycle of photosynthetic water oxidation. Absorption of a photon causes charge separation at the reaction center P680 of PS II that leads to the formation of Y ·z (oxidized tyrosine-161 on the D1-protein) within less than one μs. Reduction of Y ·z by electron transfer (ET) from the manganese complex results in Si → Si+1 transition; typical time constants of the ET step are indicated in the diagram. There are several similar S-state cycle schemes. Here, we show plausible oxidation-states of the four Mn ions in the different S-states (a). The extended S-state cycle including not only four oxidation but also four deprotonation steps is also shown (b) (Adapted from Grundmeier and Dau 2012)
There is general acceptance that S0 → S1 and S1 → S2 are metal-centered oxidations but the S2 → S3 or the S3 → S4 transitions are still controversial as to whether a metal-centered or a ligand-centered oxidation occurs (for a review see Grundmeier and Dau 2012). In the S4 → S0 transition rapid oxidation of two substrate water molecules occurs (McEvoy and Brudvig 2006). Based on the experimental evidence accumulated thus far, the four Mn ions in the S2-state are believed to be Mn(III)2Mn(IV)2, but a lower valence combination may also be possible. Regarding the Kok cycle, recent results show proton transfer from the WOC into the lumen occurs with a 1:0:1:2 pattern within a strictly alternate sequence of electron and proton transfer steps (Dau and Haumann 2008). The S1 → S2 is the only oxidation step that is not accompanied by a concomitant proton transfer.
A detailed physico-chemical mechanism of water oxidation by the WOC of PS II is still not resolved and remains a major challenge in bioinorganic chemistry. There are several proposed mechanisms for water oxidation in nature. Babcock and co-workers proposed that two different Mn ions are successively deprotonated and oxidized through hydrogen-atom abstraction by tyrosine 161 until the O–O bond is formed between the two terminally coordinated oxides or oxyl radicals (Fig. 7a) (Hoganson and Babcock 1997). However, it is apparent from the current 1.9 Å structure from T. vulcanus that the distance between the tyrosine 161 and manganese ions in the WOC is too large for direct abstraction of a hydrogen atom from a water species coordinated to these ions. This means that two cornerstones of the hydrogen-atom abstraction model are no longer tenable in its current form but it has inspired others to propose new models (Hammarstrom and Styring 2011; Rutherford and Boussac 2004).
The O–O bond formation (Fig. 7b) between the oxygens of the two water molecules coordinated to one manganese ion is another idea. However, Thomas Wydrzynski and colleagues in Canberra, Australia, have shown that there are two binding sites for the two substrate water molecules that can exchange their positions within the WOC only slowly (e.g., Hillier and Wydrzynski 2008). If two water-oxygens coordinated to Mn4 were immiscible, the route of O–O bond formation shown in Fig. 7b would be in agreement with the water-exchange data.
The O–O bond formation between two bridging oxygens (Fig. 7c) has been proposed by some groups, but μ-O pairs are too inert for bond formation in the WOC. A few mechanisms for water oxidation are based on O–O bond formation by the nucleophilic attack of a water or hydroxide that either is positioned by coordination to the Ca ion or stems from outer-sphere water (Fig. 7d–f). Holgar Dau and co-workers proposed that the O–O bond could be formed by nucleophilic attack of an outer-sphere water to a Mn = O that may be facilitated by the transfer of a proton from an outer-sphere substrate water to a bridging oxygen (Fig. 7e) (Dau et al. 2001). Using DFT calculations, Siegbahn (2009) has proposed that the O–O bond is formed between an oxygen radical and an oxygen atom bridged between two manganese ions and the calcium ion (Fig. 7g). Another proposed mechanism involves oxygen evolution by reaction of two bridging oxygens between one manganese ion and the calcium of the Mn4O 5 Ca cluster. It was proposed that the O–O bond could be formed by attack of an outer-sphere water to a water molecule attached to calcium that may be facilitated by Tyr-161 (Pecoraro et al. 1998; Limburg et al. 1999).
Water oxidation in artificial photosynthesis
One of the most important goals of artificial photosynthesis is to make high-energy chemicals to store sunlight (Pace 2005; Allakhverdiev 2011, 2012; Allakhverdiev et al. 2009, 2010; Najafpour 2011a; Najafpour and Allakhverdiev 2012). To obtain sustainable hydrogen production from water spilitting, synthesizing a “super catalyst” for water oxidation is necessary (Kanan and Nocera 2008). As discussed in previous sections, there is an efficient system for water oxidation in cyanobacteria, algae and plants. To design an efficient water-oxidizing complex for artificial photosynthesis, one way could be learning and using wisely the knowledge about water oxidation and the WOC in the natural system (Hou 2010; Hou and Mauzerall 2011; Najafpour and Govindjee 2011; Najafpour and Allakhverdiev 2012; Najafpour et al. 2012a). A nano-sized compound could be defined as a particle with size in the range of 1–100 nm (102–107 atoms) from zero (0-D) to three dimensions (3-D). The manganese–calcium oxide has dimensions of about ~0.5 × 0.25 × 0.25 nm and could be considered as a nano-sized complex. Nanocompounds can exhibit unique physiochemical properties as compared with bulk ones. Two types of size effects may be distinguished with nanoscale material as compared with bulk compounds. The first effects rely on the increased surface-to-volume ratio, and the second, true-size effects which involve changes of local material properties (Jamnik and Maier 2003; Guo et al. 2002).
Nanoscale particles could have completely different redox potentials as compared with bulk material. Navrotsky et al. (2010) have reported that nanophase transition metal oxides show large thermodynamically driven shifts in oxidation–reduction equilibria. This effect could change the redox potential of nano-sized manganese and also water-oxidizing activity of nanosized manganese oxide as compared to bulk manganese oxides (Navrotsky et al. 2010). Nanosized manganese oxides are promising compounds for water oxidation because they are low-cost, environmentally friendly, and also are used by Nature to oxidize water (Harriman et al. 1987; Jiao and Frei 2010a, b; Brimblecombe et al. 2010; Hocking et al. 2011; Boppana and Jiao 2011; Najafpour 2006, 2011a, b, c; Najafpour and Govindjee 2011; Najafpour et al. 2011; Najafpour 2012; Najafpour and Allakhverdiev 2012; Najafpour et al. 2012a, b, c).
Hundreds of amino acids in PS II are also very important in water oxidation but only a small fraction of the residues, 3–4 residues on the average, are directly coordinated to the Mn4O 5 Ca cluster (Umena et al. 2011; Kawakami et al. 2011). The roles for these residues that have direct interaction with the Mn4O 5 Ca cluster could be in the regulation of charges as well as in the coordination of water molecules at appropriate metal sites, and in the stability of the cluster. The PS II manganese-stabilizing protein is a highly conserved extrinsic component of the WOC (Shutova et al. 2005). Its deletion from PS II causes a dramatic lowering of the rate of oxygen evolution (Shutova et al. 2005). The manganese-stabilizing protein has also been suggested to be important in linking the active site of the WOC with the lumen and to be involved in a proton transfer network. A simple inorganic manganese–calcium core without coordinated amino acids shows much less activity than the WOC in PS II. Thus, the amino acids around the Mn cluster are very important indeed and the design of a super anode for water oxidation in artificial photosynthesis needs a deep understanding of the roles of these amino acids in water oxidation (Debus 2008). Thus, nano-sized manganese oxide attached to groups that could facilitate proton transfer, regulation of charges, and help in coordinating water molecules at appropriate sites could be an important strategy in artificial systems.
Water splitting needs not only a highly efficient water-oxidizing catalyst but also a chemically and mechanically stable catalyst. There has been much research focused on synthesizing efficient catalysts but not on their long-term stability. It is clear that biological organisms are unstable systems in an aerobic atmosphere and under environmental stresses and in such conditions PS II activity is vulnerable to inactivation. However, Nature, by employing self-repair mechanisms, has been able to solve the problem of PS II stability by extending the lifetime of sustained water oxidation (Aro et al. 1993; Keren et al. 1997; Allakhverdiev and Murata 2004; Nishiyama et al. 2006, 2011; Murata et al. 2007, 2012; Mulo et al. 2012). Incorporating a self-repair mechanism could be important in artificial model complexes. As discussed by Lutterman et al. (2009), the compounds that catalyse four-electron transfer reactions are prone to structural rearrangement, and instability, during turnover. Thus, the designs of catalysts that repair themselves are very important for energy science.
References
Ädelroth P, Lindberg K, Andreasson LE (1995) Studies of Ca2+ binding in spinach photosystem II using 45Ca2+. Biochemistry 34:9021–9027
Allakhverdiev SI (2011) Recent progress in the studies of structure and function of photosystem II. J Photochem Photobiol B: Biol 104:1–8
Allakhverdiev SI (2012) Photosynthetic and biomimetic hydrogen production. Int J Hydrogen Energy 37:8744–8752
Allakhverdiev SI, Murata N (2004) Environmental stress inhibits the synthesis de novo of proteins involved in the photodamage-repair cycle of photosystem II in Synechocystis sp. PCC 6803. Biochim Biophys Acta 1657:23–32
Allakhverdiev SI, Kreslavski VD, Thavasi V, Zharmukhamedov SK, Klimov VV, Nagata T, Nishihara H, Ramakrishna S (2009) Hydrogen photoproduction by use of photosynthetic organisms and biomimetic systems. Photochem Photobiol Sci 8:148–156
Allakhverdiev SI, Thavasi V, Kreslavski VD, Zharmukhamedov SK, Klimov VV, Ramakrishna S, Los DA, Mimuro M, Nishihara H, Carpentier R (2010) Photosynthetic hydrogen production. J Photochem Photobiol, C 11:87–99
Aro EM, Virgin I, Andersson B (1993) Photoinhibition of photosystem II inactivation, protein damage and turnover. Biochim Biophys Acta 1143:113–134
Barber J (2008) Crystal structure of the oxygen-evolving complex of photosystem II. Inorg Chem 47:1700–1710
Barber J, Murray JW (2008) Revealing the structure of the Mn-cluster of photosystem II by X-ray crystallography. Coord Chem Rev 252:233–243
Bard AJ, Fox MA (1995) Artificial photosynthesis: solar splitting of water to hydrogen and oxygen. Acc Chem Res 28:141–145
Beauchemin R, Gauthier A, Harnois J, Boisvert S, Govindachary S, Carpentier R (2007) Spermine and spermidine inhibition of photosystem II: disassembly of the oxygen evolving complex and consequent perturbation in electron donation from Tyrz to P680+ and the quinone acceptors Q −A to QB. Biochim Biophys Acta 1767:905–912
Bockris JOM (1977) Energy-the solar hydrogen alternative. Wiley, New York
Boisvert S, Joly D, Leclerc S, Govindachary S, Harnois J, Carpentier R (2007) Inhibition of the oxygen-evolving complex of photosystem II and depletion of extrinsic polypeptides by nickel. Biometals 20:879–889
Boppana VBR, Jiao F (2011) Nanostructured MnO2: an efficient and robust water oxidation catalyst. Chem Commun 47:8973–8975
Boussac A, Rappaport F, Carrier P, Verbavatz JM, Gobin R, Kirilovsky D, Rutherford AW, Sugiura M (2004) Biosynthetic Ca2+/Sr2+ exchange in the photosystem II oxygen evolving enzyme of Thermosynechococcus elongatus. J Biol Chem 279:22809–22819
Bricker TM (1992) Oxygen evolution in the absence of the 33-kilodalton manganese-stabilizing protein. Biochemistry 31:4623–4628
Bricker TM, Roose JL, Fagerlund RD, Frankel LK, Eaton-Rye JJ (2012) The extrinsic proteins of photosystem II. Biochim Biophys Acta 1817:121–142
Brimblecombe R, Koo A, Dismukes GC, Swiegers GF, Spiccia L (2010) Solar-driven water oxidation by a bio-inspired manganese molecular catalyst. J Am Chem Soc 132:2892–2894
Duan L, Bozoglian F, Mandal S, Stewart B, Privalov T, Llobet A, Sun L (2012) A molecular ruthenium catalyst with water-oxidation activity comparable to that of photosystem II. Nature Chem 4:418–423
Dau H, Haumann M (2008) The manganese complex of photosystem II in its reaction cycle-basic framework and possible realization at the atomic level. Coord Chem Rev 252:273–295
Dau H, Iuzzolino L, Dittmer J (2001) The tetra-manganese complex of photosystem II during its redox cycle: X-ray absorption results and mechanistic implications. Biochim Biophys Acta 1503:24–39
Debus RJ (2008) Protein ligation of the photosynthetic oxygen-evolving center. Coord Chem Rev 252:244–258
Dismukes GC, Brimblecombe R, Felton GAN, Pryadun RS, Sheats JE, Spiccia L, Swiegers GF (2009) Development of bioinspired Mn4O4-cubane water oxidation catalysts: lessons from photosynthesis. Acc Chem Res 42:1935–1943
Eaton-Rye JJ, Shand JA, Nicoll WS (2003) pH-Dependent photoautotrophic growth of specific photosystem II mutants lacking lumenal extrinsic polypeptides in Synechocystis PCC 6803. FEBS Lett 543:148–153
Ferreira KN, Iverson TM, Maghlaoui K, Barber J, Iwata S (2004) Architecture of the photosynthetic oxygen evolving centre. Science 303:1831–1838
Gauthier A, Carpentier R (2008) Disorganization of the Mn4Ca complex of photosystem II by ruthenium red: a thermoluminescence study. Luminescence 24:108–114
Ghanotakis DF, Babcock GT, Yocum CF (1984) Calcium reconstitutes high rates of oxygen evolution in polypeptide depleted photosystem II preparations. FEBS Lett 167:127–130
Golbeck JH (2006) Advances in photosynthesis and respiration photosystem I: the light-driven plastocyanin: ferredoxin oxidoreductase, vol 24. Springer, Dordrecht
Govindjee Shevela D (2011) Adventures with cyanobacteria: a personal perspective. Front Plant Sci 2:1–17
Govindjee, Kern JF, Messinger J, Whitmarsh J (2010) Photosystem II. In: Encyclopedia of life sciences (ELS). Wiley, Chichester. doi:10.1002/9780470015902.a0000669
Grundmeier A, Dau H (2012) Structural models of the manganese complex of photosystem II and mechanistic implications. Biochim Biophys Acta 1817:88–105
Guo X, Sigle W, Fleig J, Maier J (2002) Role of space charge in the grain boundary blocking effect in doped zirconia. Solid State Ionics 154–155:555–561
Guskov A, Kern J, Gabdulkhakov A, Broser M, Zouni A, Saenger W (2009) Cyanobacterial photosystem II at 2.9-Å resolution and the role of quinones, lipids, channels and chloride. Nat Struct Mol Biol 16:334–342
Hamdani S, Carpentier R (2009) Interaction of methylamine with extrinsic and intrinsic subunits of photosystem II. Biochim Biophys Acta 1787:1223–1229
Hammarstrom L, Styring S (2011) Proton-coupled electron transfer of tyrosines in photosystem II and model systems for artificial photosynthesis: the role of a redox-active link between catalyst and photosensitizer. Energy Environ Sci 4:2379–2388
Hammarstrom L, Sun L, Akermark B, Styring S (2001) A biomimetic approach to artificial photosynthesis: Ru(II)–polypyridine photo-sensitisers linked to tyrosine and manganese electron donors. Spectrochim Acta, A 37:2145–2160
Harriman A, Richoux M, Christensen PA, Mosseri S, Neta P (1987) Redox reactions with colloidal metal o xides. Comparison of radiation-generated and chemically generated RuO2·2H2O. J Chem Soc, Faraday Trans 1(83):3001–3014
Hillier W, Wydrzynski T (2008) 18O-Water exchange in photosystem II: substrate binding and intermediates of the water-splitting reaction. Coord Chem Rev 252:306–317
Ho FM (2008) Uncovering channels in photosystem II by computer modelling: current progress, future prospects, and lessons from analogous systems. Photosynth Res 98:503–522
Ho FM, Styring S (2008) Access channels and methanol binding site to the CaMn4 cluster in photosystem II based on solvent accessibility simulations, with implications for substrate water access. Biochim Biophys Acta 1777:140–153
Hocking RK, Brimblecombe R, Chang L, Singh A, Cheah MH, Glover C, Casey WH, Spiccia L (2011) Water-oxidation catalysis by manganese in a geochemical-like cycle. Nat Chem 3:461–465
Hoganson CW, Babcock GT (1997) A metalloradical mechanism for the generation of oxygen from water in photosynthesis. Science 277:1953–1956
Hou HJ (2010) Structural and mechanistic aspects of Mn-oxo and co-based compounds in water oxidation catalysis and potential applications in solar fuel production. J Integr Plant Biol 52:704–711
Hou HJ, Mauzerall D (2011) Listening to PS II: enthalpy, entropy, and volume changes. J Photochem Photobiol, B 104:357–365
Jamnik J, Maier J (2003) Nanocrystallinity effects in lithium battery materials. Aspects of nano-ionics. Phys Chem Chem Phys 5:5215–5220
Jiao F, Frei H (2010a) Nanostructure manganese oxide clusters supported on mesoporous silica as efficient oxygen-evolving catalysts. Chem Commun 46:2920–2922
Jiao F, Frei H (2010b) Nanostructured cobalt and manganese oxide clusters as efficient water oxidation catalysts. Energy Environ Sci 3:1018–1027
Jo IS, Han DU, Cho YJ, Lee EJ (2010) Effects of light, temperature, and water depth on growth of a rare aquatic plant, Ranunculus kadzusensis. J Plant Biol 54:384–395
Joliot P (2005) Period-four oscillations of the flash-induced oxygen formation in photosynthesis. Photosynth Res 20:371–378
Joliot P, Kok B (1975) Oxygen evolution in photosynthesis. In: Govindjee (ed) Bioenergetics of photosynthesis. Academic Press, New York, pp 387–412
Joliot P, Barbieri G, Chabaud R (1969) Un nouveau modele des centresphotochimiques du systeme II. Photochem Photobiol 10:309–329
Kamiya N, Shen JR (2003) Crystal structure of oxygen-evolving photosystem II from Thermosynechococcus vulcanus at 3.7 Å resolution. Proc Natl Acad Sci USA 100:98–103
Kanan MW, Nocera DG (2008) In situ formation of an oxygen-evolving catalyst in neutral water containing phosphate and Co2+. Science 321:1072–1075
Kawakami K, Umena Y, Kamiya N, Shen JR (2011) Structure of the catalytic, inorganic core of oxygen-evolving photosystem II at 1.9 Å resolution. J Photochem Photobiol, B 104:9–18
Keren N, Berg A, van Kan PJM, Levanon H, Ohad I (1997) Mechanism of photosystem II photoinactivation and D1 protein degradation at low light: the role of back electron flow. Proc Natl Acad Sci USA 94:1579–1584
Kok B, Forbush B, McGloin M (1970) Cooperation of charges in photosynthetic O2 evolution: I. A linear four-step mechanism. Photochem Photobiol 11:457–475
Komenda J, Sobotka R, Nixon PJ (2012) Assembling and maintaining the photosystem II complex in chloroplasts and cyanobacteria. Curr Opin Plant Biol 15:245–251
Lee C, Lakshmi KV, Brudvig GW (2007) Probing the functional role of Ca2+ in the oxygen-evolving complex of photosystem II by metal ion inhibition. Biochemistry 46:3211–3223
Limburg J, Szalai A, Brudvig GW (1999) A mechanistic and structural model for the formation and reactivity of a Mn(V) = O species in photosynthetic water oxidation. J Chem Soc, Dalton Trans 9:1353–1362
Lohmiller T, Cox N, Su JH, Messinger J, Lubitz W (2012) The basic properties of the electronic structure of the oxygen-evolving complex of photosystem II are not perturbed by Ca2+ removal. J Biol Chem 287:24721–24733
Lutterman DA, Surendranath Y, Nocera DG (2009) A self-healing oxygen-evolving catalyst. J Am Chem Soc 131:3838–3839
Mar T, Govindjee (1972) Kinetic models of oxygen evolution. J Theoret Biol 36:427–446
McEvoy J, Brudvig GW (2006) Water-splitting chemistry of photosystem II. Chem Rev 106:4455–4483
Miyao M, Murata N (1984) Role of the 33 kDa polypeptide in preserving Mn in the photosynthetic oxygen-evolution system and its replacement by chloride ions. FEBS Lett 170:350–354
Mulo P, Sakurai I, Aro EM (2012) Strategies for psbA gene expression in cyanobacteria, green algae and higher plants: from transcription to PSII repair. Biochim Biophys Acta 1817:247–257
Murata N, Takahashi S, Nishiyama Y, Allakhverdiev SI (2007) Photoinhibition of photosystem II under environmental stress. Biochim Biophys Acta 1767:414–421
Murata N, Allakhverdiev SI, Nishiyama Y (2012) The mechanism of photoinhibition in vivo: re-evaluation of the roles of catalase, α-tocopherol, non-photochemical quenching, and electron transport. Biochim Biophys Acta 1817:1127–1133
Najafpour MM (2006) Current molecular mechanisms of photosynthetic oxygen evolution. Plant Biosyst 140:163–170
Najafpour MM (2011a) Calcium manganese oxides as structural and functional models for active site in oxygen evolving complex in photosystem II: Lessons from simple models. J Photochem Photobiol B 104:111–117
Najafpour MM (2011b) Amorphous manganese-calcium oxides as a possible evolutionary origin for the CaMn4 cluster in photosystem II. Orig Life Evol Biosph 41:237–247
Najafpour MM (2011c) A soluble form of nano-sized colloidal manganese (IV) oxide as an efficient catalyst for water oxidation. Dalton Trans 40:3805–3807
Najafpour MM (ed) (2012) Artificial photosynthesis. Tech Publications, Rijeka. ISBN 979-953-307-665-1
Najafpour MM, Govindjee (2011) Oxygen evolving complex in photosystem II: better than excellent. Dalton Trans 40:9076–9084
Najafpour MM, Allakhverdiev SI (2012) Manganese compounds as water oxidizing catalysts for hydrogen production via water splitting: from manganese complexes to nano-sized manganese oxides. Int J Hydrogen Energy 37:8753–8764
Najafpour MM, Nayeri S, Pashaei B (2011) Nano-size amorphous calcium–manganese oxide as an efficient and biomimetic water oxidizing catalyst for artificial photosynthesis: back to manganese. Dalton Trans 40:9374–9378
Najafpour MM, Nemati Moghaddam A, Allakhverdiev SI, Govindjee (2012a) Biological water oxidation: lessons from nature. Biochim Biophys Acta 1817:1110–1121
Najafpour MM, Pashaei B, Nayeri S (2012b) Nano-sized layered aluminium or zinc–manganese oxides as efficient water oxidizing catalysts. Dalton Trans 41:7134–7140
Najafpour MM, Rahimi F, Amini M, Nayeri S, Bagherzadeh M (2012c) A very simple method to synthetize nano-sized manganese oxide: an efficient catalyst for water oxidation and epoxidation of olefins. Dalton Trans. doi:10.1039/C2DT30553D
Navrotsky A, Lilova C, Ma K, Birkner N (2010) Nanophase transition metal oxides show large thermodynamically driven shifts in oxidation-reduction equilibria. Science 330:199–201
Nishiyama Y, Allakhverdiev SI, Murata N (2006) A new paradigm for the action of reactive oxygen species in the photoinhibition of photosystem II. Biochim Biophys Acta 1757:742–749
Nishiyama Y, Allakhverdiev SI, Murata N (2011) Protein synthesis is the primary target of reactive oxygen species in the photoinhibition of photosystem II. Physiol Plant 142:35–46
Ono TA, Inoue Y (1983) Mn-preserving extraction of 33-, 23-, and 16 kDa proteins from O2-evolving PS II particles by divalent salt-washing. FEBS Lett 164:255–260
Pace R (2005) An integrated artificial photosynthesis model. In: Collings AF, Critchley C (eds) Artificial photosynthesis: from basic biology to industrial application, 1st edn. Wiley, Weinheim, pp 13–34
Payne JL, McClain CR, Boyer AG, Brown JH, Finnegan S, Kowalewski M, Krause RA Jr, Lyons SK, McShea DW, Novack-Gottshall PM, Smith FA, Spaeth P, Stempien JA, Wang SC (2011) The evolutionary consequences of oxygenic photosynthesis: a body size perspective. Photosynth Res 107:7–10
Pecoraro VL, Baldwin MJ, Caudle MT, Hsieh WY, Law NA (1998) A proposal for water oxidation in photosystem II. Pure Appl Chem 70:925–929
Peloquin JM, Campbell KA, Randall QW, Evanchik MA, Pecoraro VL, Armstrong WH, Britt RD (2000) 55Mn ENDOR of the S2-state multiline EPR signal of photosystem II: implications on the structure of the tetranuclear Mn cluster. J Am Chem Soc 22:10926–10942
Petrie S, Gatt P, Stranger R, Pace RJ (2012) The interaction of His337 with the Mn4Ca cluster of photosystem II. Phys Chem Chem Phys 14:4651–4657
Pirson A (1937) A study of the nutrition and metabolism of Fontinalis and Chlorella. Z Bot 31:193–267
Popelkova H, Betts SD, Lydakis-Simantiris N, Im MM, Swenson E, Yocum CF (2006) Mutagenesis of basic residues R151 and R161 in manganese-stabilizing protein of photosystem II causes inefficient binding of chloride to the oxygen evolving complex. Biochemistry 45:3107–3115
Popelkova H, Boswell N, Yocum C (2011) Probing the topography of the photosystem II oxygen evolving complex: PsbO is required for efficient calcium protection of the manganese cluster against dark-inhibition by an artificial reductant. Photosynth Res 110:111–121
Renger G (2012) Photosynthetic water splitting: apparatus and mechanism. In: Eaton-Rye JJ, Tripathy BC, Sharkey TD (eds) Photosynthesis: plastid biology, energy conversion and carbon assimilation, advances in photosynthesis and respiration, vol 34. Springer, Dordrecht, pp 359–411
Rivalta I, Amin M, Luber S, Vassiliev S, Pokhrel R, Umena Y, Kawakami K, Shen JR, Kamiya N, Bruce D, Brudvig GW, Gunner MR, Batista VS (2011) Structural–functional role of chloride in photosystem II. Biochemistry 50:6312–6315
Rutherford AW, Boussac A (2004) Water photolysis in biology. Science 303:1782–1784
Sauer K, Yachandra VK, Britt RD, Klein MP (1992) The photosynthetic water oxidation complex studied by EPR and X-ray absorption spectroscopy. In: Pecoraro VL (ed) Manganese redox enzymes. VCH, New York
Shutova T, Nikitina J, Deikus G, Andersson B, Klimov V, Samuelsson G (2005) Structural dynamics of the manganese-stabilizing protein effect of pH, calcium, and manganese. Biochemistry 44:15182–15192
Siegbahn PE (2009) Structures and energetics for O2 formation in photosystem II. Acc Chem Res 42:1871–1880
Umena Y, Kawakami K, Shen JR, Kamiya N (2011) Crystal structure of oxygen-evolving photosystem II at a resolution of 1.9 Ǻ. Nature 473:55–60
Wang Y, Kim SG, Kim ST, Agrawal GK, Rakwal R, Kang KY (2011) Biotic stress-responsive rice proteome: an overview. J Plant Biol 54:219–226
Wydrzynski TJ, Satoh K (eds) (2005) Photosystem II: the light-driven water: plastoquinone oxidoreductase, advances in photosynthesis and respiration, vol 22. Springer, Dordrecht
Yano J, Kern J, Irrgang KD, Latimer MJ, Bergmann U, Glatzel P, Pushkar Y, Biesiadka J, Loll B, Sauer K, Messinger J, Zouni A, Yachandra VK (2005) X-ray damage to the Mn4Ca complex in single crystals of photosystem II: a case study for metalloprotein crystallography. Proc Natl Acad Sci USA 102:12047–12052
Yano J, Kern J, Sauer K, Latimer MJ, Pushkar Y, Biesiadka J, Loll B, Saenger W, Messinger J, Zouni A, Yachandra VK (2006) Where water is oxidized to dioxygen: structure of the photosynthetic Mn4Ca cluster. Science 314:821–825
Zouni A, Witt HT, Kern J, Fromme P, Krauss N, Saenger W (2001) Crystal structure of photosystem II from Synechococcus elongatus at 3.8 Å resolution. Nature 409:739–743
Zulfugarov I, Tovuu A, Kim J-H, Lee C-H (2011) Detection of reactive oxygen species in higher plants. J Plant Biol 54:351–357
Acknowledgments
M. M. Najafpour and A. Nemati Moghaddam are grateful to the Institute for Advanced Studies in Basic Sciences for financial support. This study was also supported by Grants from the Russian Foundation for Basic Research (Nos: 11-04-01389a, 11-04-92690a, and 12-04-92101a), by BMBF (No: 8125) Bilateral Cooperation between Germany and Russia, and by Brain Pool Program of the Ministry of Education Science and Technology (MEST) and the Korean Federation of Science and Technology (KOFST) to SIA. C–H Lee is grateful for the support by the National Research Foundation (NRF) of Korea grant funded by MEST (No. 2012-0004968).
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Najafpour, M.M., Moghaddam, A.N., Yang, Y.N. et al. Biological water-oxidizing complex: a nano-sized manganese–calcium oxide in a protein environment. Photosynth Res 114, 1–13 (2012). https://doi.org/10.1007/s11120-012-9778-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11120-012-9778-x