Abstract
The high concentration of molecular oxygen in Earth’s atmosphere is arguably the most conspicuous and geologically important signature of life. Earth’s early atmosphere lacked oxygen; accumulation began after the evolution of oxygenic photosynthesis in cyanobacteria around 3.0–2.5 billion years ago (Gya). Concentrations of oxygen have since varied, first reaching near-modern values ~600 million years ago (Mya). These fluctuations have been hypothesized to constrain many biological patterns, among them the evolution of body size. Here, we review the state of knowledge relating oxygen availability to body size. Laboratory studies increasingly illuminate the mechanisms by which organisms can adapt physiologically to the variation in oxygen availability, but the extent to which these findings can be extrapolated to evolutionary timescales remains poorly understood. Experiments confirm that animal size is limited by experimental hypoxia, but show that plant vegetative growth is enhanced due to reduced photorespiration at lower O2:CO2. Field studies of size distributions across extant higher taxa and individual species in the modern provide qualitative support for a correlation between animal and protist size and oxygen availability, but few allow prediction of maximum or mean size from oxygen concentrations in unstudied regions. There is qualitative support for a link between oxygen availability and body size from the fossil record of protists and animals, but there have been few quantitative analyses confirming or refuting this impression. As oxygen transport limits the thickness or volume-to-surface area ratio—rather than mass or volume—predictions of maximum possible size cannot be constructed simply from metabolic rate and oxygen availability. Thus, it remains difficult to confirm that the largest representatives of fossil or living taxa are limited by oxygen transport rather than other factors. Despite the challenges of integrating findings from experiments on model organisms, comparative observations across living species, and fossil specimens spanning millions to billions of years, numerous tractable avenues of research could greatly improve quantitative constraints on the role of oxygen in the macroevolutionary history of organismal size.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The interval of geological time from the Cambrian Period to the Recent (543–0 Mya) is termed the Phanerozoic (Phanero—visible; zoic—animals) because the most obvious contrast between Phanerozoic and Precambrian rocks is the presence of macrofossils. It was not until the 1950s and 1960s that definitive fossils were described from the Gunflint Chert (Barghoorn and Tyler 1963, 1965; Tyler and Barghoorn 1954), a 1.88-billion-year-old shallow-water deposit exposed in Minnesota and Ontario on the northern shore of Lake Superior (Fralick et al. 2002). Additional fossil finds and geochemical data have since extended the record of life back another 2 billion years (Allwood et al. 2006; Mojzsis et al. 1996; Rosing 1999; Schidlowski et al. 1979; Schopf 1993; Schopf and Packer 1987; Tice and Lowe 2004). Precambrian fossils can be spectacularly well preserved (cf. Schopf and Klein 1992) but nearly all are microscopic. Larger macroscopic organisms such as clams, snails, insects, trees, dinosaurs, elephants, and whales are all relative newcomers to our planet.
Why did it take 2 billion years for macroscopic organisms to evolve from their microscopic ancestors and another billion years for them to exceed a meter in maximum dimension? Lack of sufficient oxygen appears to have been an important constraint. Earth’s atmosphere lacked oxygen prior to 2.5 Gya, and near-modern levels were not achieved until about 600 million years ago (Mya) (Canfield 2005; Holland 2006; Sessions et al. 2009), coincident with the first appearance of large life forms. For geometrically and physiologically simple aerobic organisms, diffusion of oxygen into the organism limits the maximum possible size (Alexander 1971; Raff and Raff 1970), consistent with the observed link between oxygenation of the atmosphere and the evolution of larger organisms.
The evolutionary importance of atmospheric oxygen extends far beyond its potential to limit body size; oxygen is required for biosynthetic reactions in all eukaryotes; without it, complex life on Earth would likely be impossible (Berkner and Marshall 1965; Catling et al. 2005; Cloud 1965, 1968, 1972; Knoll 2003; Nursall 1959). Considerable discussion has arisen about the evolutionary importance of oxygen in the origin and diversification of early eukaryotes, plants, and animals (e.g., Berkner and Marshall 1965; Berner et al. 2007; Brown et al. 2004; Calder 1984; Cloud 1968, 1972; Falkowski et al. 2005; Gilbert 1996; Gillooly et al. 2001a, b; Graham et al. 1995; Huey and Ward 2005; Knoll 1992, 2003; Knoll and Holland 1995; Lane 2002; Lenton 2003; McAlester 1970; Nursall 1959; Peters 1983; Raven 1991; Raven et al. 1994; Tappan 1974).
In this review, we discuss the role of varying oxygen levels on the evolution of organism size. Size is of great physiological and ecological significance (e.g., Peters 1983); moreover, it is easily quantified and readily compared across distantly related taxa. Oxygen is by no means the only factor to influence the evolution of body size and, therefore, our review addresses two related questions in sequence. Can oxygen constrain the evolution of body size? If so, was it, in fact, a primary control on the evolution of body size over earth history? We take a synthetic approach by linking theory with data from laboratory, field, and fossil studies across a wide range spatial, temporal, and taxonomic scales. We believe a systematic attempt to integrate these recent findings will do as much to spur additional research as it will to inform readers of the current state of knowledge.
History of atmospheric oxygen
Oxygenic photosynthesis evolved only once in the history of life; consequently, all oxygenic photosynthesis today occurs in cyanobacteria and their descendant chloroplasts within eukaryotic cells (Blankenship et al. 2007; Fehling et al. 2007). Accumulation of oxygen in the atmosphere requires production by photosynthesis in excess of consumption through aerobic respiration and the oxidation of other reduced chemical species such as sulfide or ferrous iron. Sequestration of reduced (organic) carbon through burial in rocks is the primary mechanism by which organic carbon is protected from re-oxidation. Oxygen will accumulates in the atmosphere as long as its production exceeds consumption by reaction with reduced gases from volcanoes or existing pools of reduced chemical species in the crust and atmosphere (Holland 2009; Kump and Barley 2007).
Atmospheric oxygen levels have increased in two major steps through Earth history, but have also varied considerably between these major steps. Atmospheric oxygen was less than 0.001% of present atmospheric level (PAL) prior to 2.4 billion years ago (Gya; reviewed by Sessions et al. 2009; Fig. 1a). Between 2.4 Gya and 800 Mya, oxygen concentrations increased to levels between 1 and 18% of PAL (reviewed by Canfield 2005), with a possible excursion back to even lower values ~2.0 Gya (Frei et al. 2009). Near-modern pO2 first achieved ~600 Mya, late in the Neoproterozoic Era (Berner et al. 2003; Canfield et al. 2007; Fike et al. 2006). Figure 2 illustrates the considerable variation in atmospheric oxygen levels through Phanerozoic time, which peaked near 150% PAL (31% of the atmosphere) late in Carboniferous time and dropped as low as 60% PAL (12% of the atmosphere) early in the Jurassic (Fig. 2; Belcher and McElwain 2008; Bergman et al. 2004; Berner 2004, 2006; Falkowski et al. 2005).
Atmospheric oxygen levels and maximum organismal sizes through geological time. a Likely history of atmospheric oxygen levels, modified from Kump (2008) and Lyons and Reinhard (2009). Dashed lines with arrows indicate upper and lower bounds based on proxy constraints. Smooth gray line indicates a best estimate of the history of pO2. b Sizes of the largest known fossils through geological time, modified from Payne et al. (2009). Red triangles represent prokaryotes. Yellow circles represent protists. Blue squares represent animals. Green diamonds represent vascular plants. The gray square represents Dickinsonia, a taxonomically problematic Ediacaran organism. The gray triangle represents an unnamed Archaean acritarch for which prokaryotic versus eukaryotic affinities remain uncertain (see Javaux et al. 2010)
Phanerozoic history of atmospheric oxygen concentrations, with intervals discussed in the text identified. Modified from Berner (2006). Black line represents preferred model results. Gray lines represent upper and lower bounds determined through sensitivity analysis
Oxygen concentrations in the oceans can be partially decoupled from atmospheric values by oceanographic processes, such as the high rates of aerobic respiration at depths of a few hundred meters or shifts in global climate and associated large-scale patterns of ocean circulation. These processes have occasionally produced widespread marine anoxia during Phanerozoic time (Schlanger and Jenkyns 1976), which may have contributed to episodes of mass extinctions of marine animals (Hallam and Wignall 1997; McAlester 1970).
Several recent reviews provide detailed discussion of the geochemical controls on oxygen accumulation and proxy constraints on secular variation in pO2 (Berner 2004; Berner et al. 2003; Canfield 2005; Holland 2006; Sessions et al. 2009).
Theoretical connections between oxygen and size
Oxygen availability can limit organism size because the rate of uptake is governed by surface area available for diffusion whereas demand is governed by mass (Bonner 1988). Surface area scales with the second power of length whereas mass scales with the third power; thus, demand increases with size more quickly than supply and for any given shape there will be a critical size above which oxygen supply cannot match demand. Derivations for the calculation of maximum size have been presented elsewhere (Alexander 1971; Raff and Raff 1970; Runnegar 1982); below we discuss the resulting equations to illustrate general principles.
For a heterotrophic organism that acquires oxygen purely via diffusion, its maximum thickness is limited by oxygen supply. Assuming the entire volume of the organism consists of tissue with uniform metabolic rate and that the outer surface is not penetrated by air channels of any kind, internal oxygen concentration will drop to zero at some distance from the organism’s surface and thereby prevent further size increase. As detailed by Alexander (1971), the maximum thickness (r max) is proportional to the square root of the environmental oxygen level. Supply is governed by oxygen permeability within the organism (k) and the difference between the external oxygen concentration (p e) and the minimum viable internal oxygen concentration (p min). Oxygen consumption is governed by the mass-specific metabolic rate (m).
Figure 3 illustrates maximum tissue thickness as a function of respiration rate and external pO2. At respiration and diffusion rates observed in extant organisms, thicknesses >1 mm is impossible for cylindrical organisms at oxygen concentrations <10% PAL, and even modern oxygen levels (100% PAL) cannot support thicknesses greater than a few mm (Fig. 3). Penetration of the body by air canals, such as the tracheoles in insects or the aerenchyma in plants, increases surface area and reduces distance of internal tissue from oxygen, thereby allowing organisms to achieve larger sizes. Diffusion-related constraints have been hypothesized to limit observed maximum size in numerous invertebrate animal phyla (Brusca and Brusca 2003).
Maximum allowable tissue thickness as a function of oxygen consumption rate for a cylindrical organism. The thickness of a sheet-like organism would be half that of a cylindrical organism at any given oxygen concentration. A spherical organism could reach sizes 50% larger than a cylindrical organism at equivalent pO2 and oxygen consumption rate. Dashed lines represent values for organisms dependent upon diffusion. Solid lines represent values for organisms with internal circulatory systems. Typical rates of oxygen consumption in living animals are near 0.1 cm3/cm3 tissue/h (Alexander 1971). The calculations presented here assume an oxygen permeability in muscle tissue of 2.4 × 10−3 cm2/atm/h (6.7 × 10−7 cm2/atm/s) (Dutta and Popel 1995) and a distance of 0.003 cm between the outer surface of the organism and its circulatory system (following Alexander 1971)
There are two strategies for achieving sizes larger than the bounds set by diffusion. First, an organism may be filled with metabolically inert material, keeping metabolically active cells within the diffusion-imposed maximum radius. Second, an organism may develop an internal transport system to move oxygen and other metabolically important materials from the site of acquisition (or production) to the sites where they are used. Large size in vascular plants and some animals (e.g., jellyfish) is achieved primarily via the former strategy, whereas large size in bilaterian animals such as vertebrates, mollusks, and arthropods is achieved primarily by the latter. There is no obvious oxygen-imposed limitation on total mass or volume for organisms filled with metabolically inert material, but the thickness of metabolically active tissue remains limited by diffusion.
Size in organisms with internal circulation is limited by the ratio of the metabolically active volume to the surface area available for gas exchange. For a geometrically simple (e.g., spherical or cylindrical) organism with a smooth external wall, one can still conceive of this as a limitation on maximum radius (Alexander 1971). In other words, one can calculate the maximum size of a spherical organism with internal circulation as a function of pO2. For an organism with internal circulation, the maximum radius (or volume to surface area ratio) (R max) is proportional to oxygen permeability though the surface of the organism (k), the difference between external and internal oxygen concentrations (p e – p i), and inversely proportional to the specific metabolic rate (m) and the thickness of the membrane and boundary layer across which oxygen must diffuse to enter the organism (d).
At modern oxygen levels, active circulation alone can increase the maximum radius of a cylindrical organism from 1.5 mm to 3.5 cm, assuming a typical metabolic rate near 1 cm3 O2/cm3 tissue/h (Fig. 3). Additional size increase requires respiratory organs to increase the effective surface area for gas exchange, explaining the prevalence of lungs, gills, or tracheae in large animals.
It is exceedingly difficult in practice to calculate a maximum size for organisms with complex respiratory organs because surface area for gas exchange is difficult to determine and allometric scaling of the respiratory system can free organisms from the simple area:volume scaling relationships that exist when shape is conserved. In mammals, for example, lung capacity scales approximately linearly with body mass (Stahl 1967); consequently, there is no decrease in the ability of the lung to supply oxygen at larger size.
Oxygenic photoautotrophs present additional difficulties for deriving a theoretical maximum tissue thickness. First, oxygen is produced within the organism during photosynthesis and so internal oxygen concentrations commonly exceed environmental values. C4 plant cells can contain more than twice atmospheric pO2 (Raven 1991). Second, O2 competes with CO2 for RUBISCO (ribulose-1,5-bisphosphate carboxylase oxygenase), the enzyme responsible for CO2 binding during photosynthesis. Consequently, high environmental O2:CO2 tends to inhibit growth (Quebedeaux and Hardy 1975; Raven 1991).
Mass-specific metabolic rate scales allometrically with body mass at a power less than one (e.g., Peters 1983; Schmidt-Nielsen 1984), suggesting that respiratory and circulatory systems do not entirely compensate for increased metabolic demand with size and that any satisfactory prediction of maximum possible size must incorporate allometric scaling of metabolic rate. The precise mechanism by which this allometric scaling occurs is controversial, in part because the value of the scaling exponent is subject of ongoing debate. Simple isometric scaling would yield an exponent of 0.66 following the relationship between surface area and volume. Exponents greater than 0.66 have commonly been observed in comparisons across diverse groups of animals (Brown 1995; Kleiber 1932; Mori et al. 2010; Peters 1983; Savage et al. 2004; Schmidt-Nielsen 1984), leading to alternative proposals and vigorous debate regarding the variables controlling allometric scaling (Brown et al. 2005; Darveau et al. 2002; Dodds et al. 2001; Kozlowski and Konarzewski 2005; Makarieva et al. 2008; Mori et al. 2010; West et al. 1997). Moreover, the allometric scaling exponent appears to decrease with size and structural grade (DeLong et al. 2010; Johnson et al. 2009; Mori et al. 2010), although arguments for increase with size have also been advanced (Makarieva et al. 2003).
Allometric scaling of metabolic rate clearly follows an exponent <1 for macroscopic organisms and so, in principle, maximum size could be limited by a minimum viable mass-specific metabolic rate. Once this rate is reached, size increase should cease or the scaling of metabolic rate with mass should become linear. Makarieva et al. (2003) suggest that large mammals approach or exceed the mass at which this critical rate is achieved, but do not demonstrate statistically that a piecewise linear regression is a better fit to their data set than a single regression line. Given the small number of very large taxa in their data set, it appears unlikely that a null hypothesis of allometric scaling at an exponent less than 1.00 across the entire data set can be rejected. Consequently, it remains unclear if the maximum size of animals is limited by oxygen acquisition and transport, or if other factors are more important. No method exists for calculating an oxygen-imposed upper limit on size because such calculations pertain to thickness or the volume:area ratio rather than mass per se.
Anaerobic organisms are more constrained than their aerobic counterparts due to the lesser energy yield of anaerobic metabolism, which may explain the absence of any living or fossil macroscopic anaerobes. Gilbert (1960, 1996) and several subsequent authors (Catling et al. 2005; Koch and Britton 2008; Thannickal 2009) have pointed out that oxygen is the optimal electron acceptor for respiration. Oxygen yields more energy per electron transfer than any other element available in appreciable concentrations, is sufficiently stable to build up to appreciable concentrations in Earth’s atmosphere (unlike the more reactive halogens such as fluorine, chlorine, and bromine), and is abundant in a biologically available form—liquid water. Catling et al. (2005) argue that alternative metabolic pathways are likely too inefficient to permit the growth of large organisms. They focus on differences in growth efficiency; for animals, values are near 50%, whereas anaerobic organisms never achieve more than 20% (Catling et al. 2005). The inefficiency of energy transfer up the food chain would limit the abundance of larger organisms, which tend to be at higher trophic levels. Considering Eq. 1, however, we suggest that the transport of electron acceptors into the body can also present a severe limitation. As with aerobic organisms, the maximum possible thickness for anaerobic organisms is limited by availability of the electron acceptor, metabolic rate, and geometry. The lower energy yield of anaerobic reactions means that when compared with a similar-sized aerobic organism, an anaerobic organism would require a higher concentration of the electron acceptor, exhibit a much lower metabolic rate, or have a lower volume:area ratio. If the Precambrian oceans lacked any suitably abundant electron acceptor, and if all life is limited by some minimum viable metabolic rate, then the failure of large organisms to evolve prior to the rise in oxygen may reflect this fundamental energetic limitation.
The theoretical results presented above demonstrate that oxygen can limit body size; they do not demonstrate that it does (or did) limit body size. The structural complexity of large organisms and the fact that oxygen availability limits thickness rather than mass make it difficult to predict an oxygen-limited maximum size for fungi, plants, or animals. Moreover, selection on body size in any given species reflects numerous trade-offs between advantages and disadvantages of size increase or decrease (Brown 1995; Brown et al. 1993, 2004; Calder 1984; Peters 1983; Schmidt-Nielsen 1984; Sebens 2002). Below we address the empirical question of whether oxygen does in fact impose a limit on size within or among living species and whether historical variation in atmospheric oxygen concentrations has significantly influenced macroevolutionary trends in body size.
Experiments on oxygen and size
Hypoxia and hyperoxia each affect growth and development of algae, plants, and animals in laboratory settings. The vast majority of studies have focused on animals. The few whole-organism experiments on plants have used agriculturally important taxa such as wheat, corn, soybeans, and lettuce.
In animals, size decrease is nearly universal under hypoxia, occurring in fruit flies, snakes, fish, rats, and even human populations (Table 1) whereas hyperoxia is associated with increased final size and growth rate in a minority of studies (Table 1). The most pronounced acceleration of growth under experimental hyperoxia occurred in the domestic chicken (Metcalfe et al. 1981; Stock et al. 1983), a species which has been bred specifically for rapid growth. In studies finding size effects associated with both hypoxia and hyperoxia, the magnitude of change in body size under hyperoxia has been much less than that observed under hypoxia (Frazier et al. 2001; Owerkowicz et al. 2009). Several other studies have found size decrease under hypoxia but no increase under hyperoxia (Andrews 2002; Greenberg and Ar 1996; Herman and Ingermann 1996; Klok et al. 2009).
Multi-generation studies point toward differences in the mechanisms of size change in response to hypoxia versus hyperoxia. Klok et al. (2009) reared individual flies under a variety of oxygen levels, finding size reduction under hypoxia but no change in size under hyperoxia. However, exposing multiple generations of a population to hyperoxia produced size increase that was maintained even after return to normal oxygen, suggesting an evolutionary response favoring larger size under hyperoxia. In contrast, lineages exposed to hypoxia returned to pre-exposure sizes after one or two generations reared under normal atmospheric levels. These findings suggest size reduction under hypoxia resulted entirely from developmental plasticity whereas size increase under hyperoxia was evolutionary (Klok et al. 2009). Experimental selection for large size yielded similar results. Lineages raised under normoxia and hyperoxia exhibited heritable size increase, whereas lineages raised under hypoxia did not exhibit larger size until subsequent generations were reared under normoxia (Klok and Harrison 2009). Thus, not only are the effects of hypoxia versus hyperoxia asymmetric, but also the mechanisms underlying size responses differ as well.
Performance studies have also been used to assess the extent to which oxygen supply limits size evolution. One potential consequence of size limitation by oxygen is a higher critical oxygen level (the value below which the organism cannot function indefinitely) at large body size or a smaller safety margin between resting and maximum metabolic rate. There is little experimental evidence for such an effect. Critical oxygen level does not scale with size across species of grasshoppers (Greenlee et al. 2007) or during development in grasshoppers and caterpillars (Greenlee and Harrison 2004, 2005). In fact, the metabolic safety margin increases with size in the grasshopper Schistocerca americana (Greenlee and Harrison 2004). Decrease in endurance during aerobic exercise with size in grasshoppers appears to reflect an increase in mass-specific metabolic rate with size rather than a decrease in the absolute capacity of the organism to supply its muscles with oxygen (Kirkton et al. 2005). Performance reduction in pycnogonids (sea spiders) under hypoxia is not correlated with size (Woods et al. 2009). Critical oxygen levels were unaffected by multi-generational rearing of Drosophila melanogaster under hypoxia and hyperoxia (Klok et al. 2010). These findings have been interpreted by some (e.g., Woods et al. 2009) to indicate that maximum size in the studied clades is not oxygen-limited. However, it is also possible that the findings simply reflect the fact that the need for metabolic scope is unrelated to size in these organisms. Species cannot evolve to sizes where resting metabolism is oxygen-limited because of the behavioral constraints such a size would impose, and small species may have no need for greater scope than larger species. Therefore, these findings should be interpreted cautiously with respect to constraints on size evolution.
The limited response of organism size to experimental hyperoxia could indicate that little evolutionary response would occur during intervals of elevated pO2, but these observations are perhaps better interpreted to reflect asymmetry in nature. Many animals encounter hypoxia during growth and development, in an egg, in utero, or within microenvironments such as decaying organic matter and burrows. In contrast, there are few natural settings under which animals encounter hyperoxia, as noted by Harrison (2010) with respect to D. melanogaster. Consequently, there is likely little selective advantage associated with developmental plasticity that can take advantage of elevated pO2. The rarity of natural hyperoxic environments can explain why hemoglobin in the American alligator becomes saturated with oxygen under present-day oxygen levels (Busk et al. 2000), limiting the benefits of hyperoxia, and the observation that flight performance in several insects appears to be generally unaffected by experimental hyperoxia (Harrison 2010).
Growth response to experimental hypoxia is more complex in plants and algae than in animals. Vegetative growth generally increases under hypoxia as severe as 25% PAL in plants without carbon-concentrating mechanisms (i.e., C3 plants) such as soybeans (Glycine max), liverworts (Marchantia polymorpha), and the scarlet monkeyflower (Mimulus cardinalis) (Björkman et al. 1969, 1968; Quebedeaux and Hardy 1973, 1975), although lettuce (Lactuca sativa) exhibited reduced growth at 25% PAL (He et al. 2007). In contrast to the increase in vegetative growth, seed growth decreased under hypoxia in soybeans, reaching zero in soybeans at 24% PAL (Quebedeaux and Hardy 1973, 1975). Plants with carbon concentrating mechanisms (i.e., C4 and CAM plants), such as sorghum wheat (Sorghum bicolor) and corn (Zea mays), did not exhibit increased vegetative growth under experimental hypoxia covering the above-ground portion of the plant (Björkman et al. 1969; Quebedeaux and Hardy 1973, 1975). Musgrave and Strain (1988) were the first to conduct whole-plant growth experiments under hypoxia, using two strains of Triticum aestivum (wheat). They observed increased vegetative growth under hypoxia and under high CO2 (1000 ppm). Quebedeaux and Hardy observed a decline in wheat seed production under hypoxia (1973), whereas Guo et al. (2008) observed slightly increased yield at 50 and 25% PAL, with yield declining to near zero at 12% PAL. Thus, although experimental results are somewhat mixed, hypoxia generally tends to increase vegetative growth for C3 plants but not for C4 plants, consistent with differences in mechanisms for CO2 acquisition and O2 release (Raven 1991). As hypoxia negatively impacts seed production, it is difficult to extrapolate these findings to expected differences in fitness and resulting trends in size over multiple-generation timescales.
Hyperoxia inhibited growth in all studies of plants, algae, and cyanobacteria. Torzillo et al. (1998) observed decreased biomass production under hyperoxia in the cyanobacterium Spirulina plantensis. Pruder and Bolton (1980) observed a reduction in total carbon content (though not cell number) at high pO2 in the estuarine diatom Thalassiosira pseudonana clone 3H. McMinn et al. (2005) observed decreased growth in the diatoms Fragilariopsis cylindrus, F. curta, Pseudonitzschia sp., Porosira glacialis, Endomoneis kjellmannii, and Nitzschia frigida under hyperoxia. Quebedeuax and Hardy (1975) observed decreases in both vegetative and reproductive growth in soybeans and wheat under hyperoxia (190% PAL). No experiments have reported cell size directly, although Pruder and Bolton’s (1980) results appear to require a decrease in mean cell size.
The effects of oxygen on size in oxygenic photoautotrophs suggest photorespiration exerts a greater negative effect on growth than any beneficial effects from greater oxygen availability. Vegetative growth appears not to be inhibited by hypoxia at oxygen levels above 25% PAL, although reproduction may be optimized by higher pO2 or higher pCO2. The extent to which decreased seed production under hypoxia could be modified by selection during long-term hypoxia is currently unknown. Experimental coverage is limited primarily to clades that radiated during the Mesozoic (e.g., angiosperms and diatoms), but these findings suggest that Phanerozoic oxygen levels have been consistently above the minimum level required for vegetative growth. In contrast to findings for animals, experimental results suggest high pO2 and low (i.e., near-modern) pCO2 during the Carboniferous and Permian periods may have negatively impacted growth rates in plants.
Comparative biological perspectives on oxygen and body size
Additional insights into the effect of oxygen on maximum size in higher taxa arise from comparative studies across living species. Biological variation across environmental gradients in the modern world can serve as a useful analog for the temporal variation in the same parameters. As is the case for experimental studies, most comparative studies have focused on animals and a minority on marine protists.
The influence of oxygen availability on the structure of marine communities has been investigated most thoroughly in studies of the oceans’ oxygen minimum zones (OMZs) (Levin 2003; Rhoads and Morse 1971), regions at depths of a few hundred meters where respiration of sinking organic matter exceeds oxygen supply through physical mixing. These zones contain oxygen concentrations below atmospheric equilibrium and are locally completely anoxic. These studies have often been conducted with an eye toward reconstructing oxygen gradients using fossil data (e.g., Rhoads and Morse 1971). Levin (2003) argued that change in size structure is the most pervasive response of marine benthic invertebrate communities to the reduced oxygen availability in the OMZ. Megafauna such as echinoids, large gastropods, asteroids, holothurians, and decapods are reported from OMZs down to concentrations less than 0.25 ml/l (McClain and Barry 2010), but are typically absent from the most oxygen-starved settings (<0.1 ml/l), which tend to be dominated by protists and invertebrate animals of ca. 0.1–1.0 mm.
Extremely low oxygen levels exclude macrofauna in the oceans, but patterns of size variation with oxygen within species and higher taxa is more complex. For example, Gooday et al. (2000) observed smaller mean (but not maximum) size when comparing foraminiferan communities from the OMZ and to those from deep water in the Arabian Sea off of Oman. Perez-Cruz and Machain-Castillo (1990) observed reduction in average size from the shelf to the OMZ in the common species Hanzawaia nitidula and Bolivina seminuda in the Gulf of Tehuantepec, Mexico. However, Phleger and Soutar (1973) speculated that small size may reflect an adaptive strategy to high food availability rather than low oxygen, a prediction consistent with life-history modeling by Hallock (1985).
Interestingly, there are also cases of increased size with lower oxygen (Levin et al. 1994), perhaps due to the greater food availability in OMZs (Levin 2003). A similar inverse correlation between the availability of food and oxygen occurs in many deep sea environments (McClain et al. 2005, 2006; Rex et al. 2006). There have been few studies explicitly assessing the relationship between oxygen availability and size that have controlled for the effects of food availability, the presence of competitor species and other potential confounders. McClain and Rex (2001) did find a significant relationship between oxygen concentrations and intra- and inter-specific size even after controlling for depth (as a proxy for food) in non-OMZ deep sea systems. Differentiating the effects of food and oxygen on size is challenging because oxygen and food availability are rarely decoupled in the modern ocean, as oxygen minima exist when and where the supply of food exceeds the supply of oxygen required to respire the organic carbon aerobically (Levin 2003).
There are few hard data concerning the relationship between size and oxygen availability for large, photosynthetic marine protists, such as brown algae. A recent study of kelp suggests that they depend primarily upon high nutrient levels within the photic zone. Consequently, they occur where nutrient-enriched (and oxygen-depleted) waters from below the mixed layer impinge upon hard substrates within the photic zone (Graham et al. 2007). Based upon experimental findings for plants and algae, giant kelp may if anything benefit from lower oxygen levels in nutrient-rich deeper waters. These findings are consistent with evidence that size evolution of diatoms, planktonic foraminifers, and dinoflagellates through Cenozoic time has been controlled primarily by nutrient availability in surface waters (Finkel et al. 2005, 2007; Schmidt et al. 2004).
Size clines also occur over other oxygen gradients. Chapelle and Peck (1999, 2004; Peck and Chapelle 2003) have compiled size distributions for amphipod crustaceans across water bodies varying in salinity, temperature, and dissolved oxygen concentration. The upper 95th percentile of size among species is highly correlated with water oxygen content across a wide range of marine basins and large lakes (Chapelle and Peck 1999; Peck and Chapelle 2003). As the rate of oxygen uptake depends on partial pressure (which is constant at sea level) and solubility (which varies with temperature and salinity), the larger maximum size of freshwater amphipods relative to marine environments of similar temperature results from the greater solubility of oxygen at lower salinity (Peck and Chapelle 2003). The consistency of the relationship between maximum size and oxygen concentrations across both marine and fresh water environments and across elevation strongly supports the hypothesis that oxygen limits maximum size in amphipods. Chapelle and Peck (2004) further found that oxygen availability is not only associated with maximum size; in fact, it is positively associated with every size quantile, with the slope of the relationship becoming steeper for the higher size quantiles. The linear relationship between oxygen concentration and body length suggests that respiration in these amphipods is aided by circulation and/or allometric scaling of gill size, rather than occurring simply via diffusion. Jacobsen et al. (2003) examined the effects of oxygen availability on the macroinvertebrate fauna of freshwater streams along an elevation gradient in Ecuador, spanning more than 3 km in elevation. They found a slightly higher proportion of large-bodied families in the low-elevation streams, but the difference in mean size between high- and low-elevation was not statistically significant. As discussed with respect to the OMZ, however, there remains the potential that size variation with elevation is controlled, at least in part, by other correlates of elevation.
Cross-species comparisons also hold promise for understanding structural and physiological constraints on the evolution of body size. The best example of this approach is a recent study by Kaiser et al. (2007), in which they characterized the relationship between tracheal volume and body size in beetles and used this scaling to estimate the maximum size physiologically possible. They used synchrotron radiation to image tracheae in situ, finding an allometric scaling exponent of 1.29. Their results suggest a maximum length for beetles of 32 cm, twice the observed maximum. However, they note that oxygen enters the beetle body at the thorax and must pass through local constrictions to reach the head and limbs of the organism. Their scaling relationship suggests tracheae would occupy 90% of the leg joint orifice at a length of 16 cm, preventing any further growth by limiting the space available for connective tissue and hemolymph. This predicted value is similar to the size of the largest living beetle (Titanus giganteus), suggesting it is not the overall scaling of tracheal volume to body volume that limits beetle size, but rather the scaling of particular anatomical features (Kaiser et al. 2007). As insects exhibit developmental plasticity such that tracheal volume is reduced when individuals are reared under high oxygen conditions (cf. Henry and Harrison 2004), increased atmospheric oxygen levels could permit larger beetles than currently exist. The study by Kaiser et al. (2007) provides good evidence for a particular morphological bottleneck that currently serves to limit maximum size in a diverse group of animals (Lighton 2007). Such bottlenecks may be widespread, but they have yet to be identified in other clades.
Historical correlation between oxygen and size
The simple fact that both atmospheric oxygen and life’s maximum size have increased through geological time does not prove a causal relationship. For example, secular increase in life’s maximum size could simply reflect expansion away from a small initial size (Gould 1988, 1996; McShea 1994; Stanley 1973); indeed, one would expect this to be the case in the absence of any ecological or environmental selective pressures. Improved documentation of size trends and of Earth’s atmospheric oxygen history has recently enabled more detailed examination of the covariation between body size evolution and changing atmospheric composition. Many observations in the fossil record appear to reflect a strong influence of oxygen availability on size evolution, but considerable complexity remains.
Bonner (1965) was the first to report the sizes of the largest organisms through the entire geological record, updating this record in subsequent publications (Bonner 1988, 2006). He illustrated a smooth trend in maximum size from the Archean to the Recent. Interpretation of the rate of change implied by his graph is complicated by his use of a logarithmic time axis, but the smooth trend line implies that if maximum size was tightly controlled by oxygen availability, then oxygenation of the atmosphere must have been gradual. Over time, the pattern of size evolution implied by Bonner’s illustration came to stand in contrast to geochemical evidence for stepwise oxygenation of Earth’s surface environments derived from a wide range of proxies (Fig. 1).
Payne et al. (2009) recently revisited the evolution of size over time, finding a stepwise pattern of increase in life’s maximum size coinciding approximately with the inferred steps in atmospheric pO2. The first size step occurred early in the Proterozoic and the second in the late Neoproterozoic and early Paleozoic (Fig. 1b). The magnitude of the initial size jump may have been smaller than reported by Payne et al. (2009). Javaux et al. (2010) discovered Paleoarchaean (~3.2 Gya) acritarchs (organic-walled microfossils of uncertain taxonomic affinity) with diameters up to 300 μm (Fig. 1b). It remains uncertain even whether these microfossils derive from prokaryotic or eukaryotic organisms. Similarly large microfossils are currently unknown from younger Archaean rocks, but this finding highlights the extent of current uncertainty in the size distribution and evolution of early life. Interestingly, the first major size jump appears to post-date the initial rise in oxygen (~2.35 Gya) by 350–700 Mya. However, permanent oxygenation of the atmosphere may not have occurred until less than 2.0 Gya (Frei et al. 2009).
The duration of the lag between oxygenation and size increase is uncertain not only because the timing of permanent oxygenation remains uncertain, but also because taxonomic interpretation of the early fossil record of eukaryotes remains challenging. Sterane molecules in 2.7 Gya rocks from Western Australia have been interpreted as the earliest fossil signature of eukaryotic cells (Brocks et al. 1999), but more recent work suggests these molecular fossils may not be indigenous to the sediments that contain them. Instead, they appear to result from post-burial contamination by much younger overlying strata (Rasmussen et al. 2008). The oldest putative eukaryotic macrofossils occur in the Negaunee Iron Formation of Michigan (1.9 Gya; Schneider et al. 2002), but these and other specimens of similar age have been alternatively interpreted as composite microbial filaments (Samuelsson and Butterfield 2001). Recently, macrofossils possibly derived from multicellular eukaryotes were reported from the 2.1 Gya Francevillian B Formation of Gabon (Albani et al. 2010). The oldest uncontroversial eukaryotic macrofossils occur in the 1.6 Gya Vindhyan Supergroup of India (Kumar 1995), post-dating the earliest evidence for an increase in atmospheric oxygen by more than 700 Mya—an interval longer than the entire animal fossil record. In sum, these observations suggest oxygen availability played a role in triggering the initial evolution of macroscopic organisms. This scenario must be viewed cautiously, however, given the large uncertainties in the timing of size increase and the taxonomic affinities of these ancient fossils.
The second step in maximum size, during the Ediacaran, Cambrian, and Ordovician periods (635–445 Mya), began essentially coincident with the second major oxygenation event (Fig. 1). Size increase began with the appearance of the taxonomically problematic Ediacaran organisms and continued during the Cambrian and Ordovician radiation of animals. The largest fossils from this interval all appear to be stem- or crown-group animals, but macroscopic algae also exhibit a trend toward larger size through Neoproterozoic time (Xiao and Dong 2006). During Cambrian and Ordovician time, numerous animal phyla independently achieved size orders of magnitude larger than any pre-Ediacaran fossils (Payne et al. 2009). Several recent studies have reported geochemical evidence for increased oxygenation of seawater during the latest Neoproterozoic (Canfield et al. 2007; Fike et al. 2006; Scott et al. 2008), providing stronger support for an increase in oxygen availability at this time. Runnegar (1982) used the approaches of Raff and Raff (1970) and Alexander (1971) to calculate the minimum ambient oxygen concentrations required by Dickinsonia, a flat, ovoid, segmented fossil of Ediacaran age (635–543 Mya). Due to its simple morphology and lack of any obvious gills, Dickinsonia provides one of the more attractive opportunities to use organismal size as a constraint on oxygen availability. Assuming the organism was filled with muscle tissue, Runnegar calculated that larger individuals (~5 mm thick) would have had difficulty meeting their metabolic needs if acquiring oxygen by simple diffusion, even at modern oxygen levels. If, alternatively, Dickinsonia contained a circulatory system, then it would have required only about 10% PAL (Runnegar 1982). This value may provide a minimum estimate of Ediacaran oxygen levels because Dickinsonia does not appear to have had any respiratory organ that would have increased its effective surface area. However, it is possible that Dickinsonia and other Ediacaran organisms contained metabolically inert material surrounded by a thin layer of metabolically active cells, similar to living cnidarians (Norris 1989). If so, its effective thickness may have been much less than 1 mm and its overall thickness may provide little constraint on ambient oxygen concentrations. Larger Cambrian animals used respiratory and circulatory systems, making quantification of the relationship between pO2 and maximum size more challenging.
The temporal relationship between episodes of oxygenation and size increases (Fig. 1) points toward oxygen as a contributing factor, but the taxonomic distribution of the pattern indicates that rising oxygen alone was not sufficient. The first increase in maximum size coincides with the appearance of fossils that were likely eukaryotes. Moreover, no prokaryote before or since has reached the size of early putative eukaryotes such as Grypania and Chuaria (Fig. 1b). The second increase in maximum size occurred only among multicellular eukaryotes; no single-celled eukaryote has achieved the sizes of the largest Ediacaran and Cambrian organisms (Fig. 1b). Thus, even if oxygen concentrations limited organismal sizes for long stretches of geological time, increases in structural complexity were also required for each stepwise increase in maximum size. Of course, these structural changes may also have required increased oxygen availability for other reasons (e.g., Acquisti et al. 2007)—potentially making oxygen both a proximate and ultimate control on the evolution of body size. Multicellular forms have evolved numerous times independently within the eukaryotes. The earliest multicellular form in the fossil record—a 1.2 Gya bangiophyte red alga (Butterfield 2000)—predates the increase in maximum size by 600 Mya. Consequently, the evolution of decimeter- to meter-scale organisms in multiple animal clades during Ediacaran and Cambrian time suggests the removal of an environmental barrier, although ecological pressures favoring large size (and hard parts) in predators and prey and the evolution of more genetic regulatory systems controlling tissue-grade organisms may also have been important factors (Knoll and Carroll 1999; Marshall 2006). Similarly, the later appearance of large vascular plants, during Devonian time (Fig. 1), suggests that the proximal barrier to large size was not oxygen availability but, rather, the biochemical and anatomical modifications associated with the production of wood.
The post-Cambrian fossil record points toward a link between variation in atmospheric pO2 and the evolution of body size in taxa as disparate as insects, mammals, and protists. For example, Carboniferous gigantism in several animal clades has been attributed to high oxygen concentrations. In fact, prior to the geochemical modeling of Phanerozoic oxygen levels by Berner and colleagues (Berner 2004, 2006; Berner and Canfield 1989), Rutten (1966) argued that insect gigantism was the best evidence for high pO2 during Carboniferous time. Later authors inverted the argument, using the geochemical model predictions of high Carboniferous oxygen levels to argue for oxygen as a contributing cause of gigantism (Berner et al. 2007; Dudley 1998; Graham et al. 1995). Flying insects are the most widely cited Late Paleozoic giants, particularly dragonflies (Protodonata) with wingspans reaching 70 cm (Carpenter 1960; Shear and Kukalová-Peck 1990) and mayflies reaching 45 cm (Kukalová-Peck 1985), but other lineages appear to have exhibited gigantism as well, such as meter-long arthropleurid arthropods (Rolfe and Ingham 1967; Shear and Kukalová-Peck 1990) and marginal marine eurypterids, which have left tracks up to a meter in width (Whyte 2005). Gigantism in marine animals may have been unusually widespread at this time as well; marine eurypterids also exhibit very large sizes (Braddy et al. 2008). In addition, Newell’s (1949) examples of phyletic size increase in his classic paper on Cope’s Rule draw largely on Late Paleozoic examples: foraminifera, bryozoans, echinoids, brachiopods, and rugose corals. Moreover, the sizes of the largest arthropods, mollusks, and chordates decline from the Carboniferous to the Permian, dramatically so in the arthropods, the group likely to have been most sensitive to oxygen concentrations for anatomical reasons (Payne et al. 2009).
Despite widespread awareness of Late Paleozoic gigantism, there have been few attempts to determine whether organisms the size of Carboniferous giants would be prohibited at present-day oxygen levels or whether the magnitude of temporal variation in maximum size within the relevant taxa has been of the magnitude predicted by modeled changes in pO2. Okajima (2008) was the first to examine the link between insect size and oxygen concentration quantitatively through the Phanerozoic, using newly compiled data on the sizes of fossil dragonflies. She found that the variation in maximum size of dragonflies through time has been much greater than predicted by variation in atmospheric oxygen concentrations, assuming respiration via diffusion through tracheae, and assuming that the sizes of Carboniferous dragonflies represent an oxygen-limited maximum size. If oxygen limited maximum body size in the Carboniferous, it has not consistently done so during other periods. Alternatively, if oxygen is limiting in the modern, then anatomical or physiological differences must exist between the Protodonata and Odonata to explain the inability of the Odonata to achieve similarly large sizes. The latter interpretation is suggested by the fact that all of the largest Paleozoic specimens belong to the Protodonata; Paleozoic members of the Odonata exhibit sizes comparable to the largest in the Mesozoic and Cenozoic. Alternatively, the simplifying assumption of oxygen diffusion through tracheae may be inaccurate; there is emerging evidence for active tracheal breathing in insects (Socha et al. 2008; Westneat et al. 2003). Okajima (2008) proposed still another alternative: although variation in oxygen may have contributed to size evolution, maximum size of Mesozoic and Cenozoic dragonflies was limited by ecological competition with flying vertebrates. A further possibility, not examined by Okajima (2008), is that the trend in maximum size of fossils is poorly correlated with the true evolutionary pattern. Temporal variation in the quality of the insect fossil record (Labandiera 2005; Smith and Cook 2001) makes it difficult to determine the extent to which variation in maximum size in the fossil record reflects biological reality versus variation in the quality of available material. For example, the Carboniferous contains an unusually extensive record of the coastal marsh environments that may be most likely to house large insects.
Carboniferous gigantism is the most widely cited link between oxygen and the evolution of animal size, but variation in oxygen levels may also have significantly influenced gigantism among marine invertebrates during the Late Ordovican, size reduction during the Permian–Triassic transition, size increase during the Cenozoic radiation of mammals, and Cenozoic size variation in deep-sea benthic foraminifera.
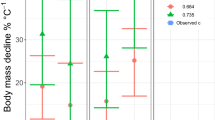
Late Ordovician faunas in tropical carbonate environments are widely known for exceptionally large invertebrates, including the largest ever trilobite (Rudkin et al. 2003), orthocone cephalopod (Teichert and Kummel 1960), Paleozoic gastropod (Rohr and Blodgett 1992), and conspicuously large brachiopods and other marine invertebrates (Jin 2001; Nelson 1959). [It should be noted that our prior documentation of the size of this cephalopod specimen (Payne et al. 2009) contained an error, which is corrected in Fig. 1 with biovolume of 8.4 log mm3.] The causes of widespread gigantism at this time are unclear. Oxygen levels may have been increasing at this time, but it has not be reconstructed as an interval of unusually high pO2 (Berner 2006).
Increased oxygen availability may have facilitated the radiation of mammals by enabling higher metabolic rates as well as larger sizes. As indicated in Eqs. 1 and 2 and Fig. 3, increased oxygen availability enables higher metabolic rate at any given size. The mass-specific metabolic rates of birds and mammals are three to six times those of reptiles (Else and Hulbert 1981). Thus, the Mesozoic evolution of birds and mammals and the subsequent Eocene diversification of large placental mammals may have been facilitated by a doubling of atmospheric oxygen levels from the Jurassic to the Recent (Falkowski et al. 2005).
Although oxygen concentrations on land and in the mixed layer of the surface ocean are largely determined by bulk atmospheric concentrations, oceanographic factors can produce substantial marine oxygen gradients in space and time that are decoupled from atmospheric variations. Kaiho (1998) examined temporal variation in the maximum size of trochospiral, deep-sea benthic foraminifera over the past 120 Mya, using material from several drill-cores. He found that minima and maxima in the history of foraminiferan size corresponded to minima and maxima in the oxygen isotope composition of seawater—a proxy for paleotemperature. He postulated that this link reflected climatically driven variation in dissolved oxygen concentration in the deep sea.
Body size reduction within lineages and across higher taxa is associated with episodes of mass extinction and ocean anoxia. In the case of the end-Permian mass extinction—the most severe biotic crisis of the Phanerozoic (Raup and Sepkoski 1982)—extinction and size reduction have been attributed, at least in part, to marine anoxia and rapid reduction in atmospheric oxygen concentrations (Huey and Ward 2005; Knoll et al. 2007; Retallack et al. 2003; Wignall and Hallam 1992; Wignall and Twitchett 1996). In particular, reduction in maximum size among marine gastropods as a whole and within some bivalve, gastropod, and brachiopod lineages has been attributed at least in part to oxygen stress (Fraiser and Bottjer 2004; Payne 2005; Twitchett 2007). Reduction in burrow diameters in sedimentary rocks following the end-Triassic mass extinction has similarly been ascribed to marine anoxia (Barras and Twitchett 2007; Twitchett and Barras 2004). Payne (2005) found that although the end-Permian extinction event did not show obvious evidence of size bias across the full spectrum of gastropod size, the reduction in maximum size was too large to be explained by the reduction in diversity alone. Other studies have not addressed this issue statistically. Moreover, it remains unclear whether oxygen stress was more important than other coeval environmental and ecological factors such as reduced predation pressure (Payne 2005) or decreased primary productivity (He et al. 2010).
Discussion
Although oxygen is not strictly necessary for the evolution of macroscopic life, the high growth efficiency allowed by aerobic respiration and the coincidence of step-wise increases in life’s maximum size with increases in atmospheric oxygen concentrations (Fig. 1) strongly suggest that oxygen availability has in fact limited maximum size for long intervals of Earth history.
Has variation in Earth’s atmospheric oxygen concentration further influenced size evolution, beyond constraining tissue thickness and the geometry of distributary networks? Despite the wide range of scales at which the problem has been examined, the answer remains unclear. Oxygen deprivation should limit size in anatomically simple organisms and does so in experimental settings even for anatomically complex bilaterian animals (Table 1). On the other hand, oxygen availability is inversely correlated with vegetative growth rate in algae and plants. Thus, above a threshold value well below modern values, increased oxygen availability appears to lead to size increase primarily in aerobic heterotrophs. However, only a few comparative biological studies provide quantitative evidence for oxygen as a control on maximum size within diverse animal clades (Chapelle and Peck 1999, 2004; Kaiser et al. 2007; McClain and Rex 2001). Evidence from the fossil record remains largely qualitative, with the few exceptions enumerated above. Moreover, recent study of fossil dragonflies suggests that one of the most famous examples of animal gigantism cannot be explained solely by variation in oxygen (Okajima 2008).
Differences in taxonomic and temporal scale make it exceedingly difficult to make quantitative links across theory, experimental biology, comparative biology, and paleobiology. Theoretical predictions are difficult to apply to living animals because of the wide range of morphological and physiological mechanisms that animals use to compensate for variation in oxygen concentration. Consequently, we lack a predictive model for the maximum size of morphologically complex animals as a function of oxygen concentration. Laboratory findings that hypoxia has a much greater influence on size than hyperoxia could be read to suggest a non-linear relationship between oxygen and body size with an optimum value for large size near modern oxygen levels, but are perhaps more likely to reflect an asymmetric need for developmental plasticity due to the prevalence of hypoxia but not hyperoxia in nature. Correlation between oxygen levels and size in field studies is often complicated by covariation of oxygen with variables such as temperature or food availability, not to mention co-occurring species. Modern oxygen gradients cannot fully mimic the variation in selective pressures associated with temporal variation in atmospheric pO2 and provide no analog for historical hyperoxia. Moreover, there may be hysteresis in the evolutionary response to variation in oxygen concentration. For example, if size increase is made possible by an evolutionary novelty that is difficult or impossible to reverse (e.g., metazoan multicellularity), then the response to subsequent decrease in oxygen availability is unlikely to be symmetrical with the initial response to oxygen increase. Threshold transitions in the allometric scaling of metabolic rate with size (DeLong et al. 2010; Mori et al. 2010) may be indicative of such hysteresis or ratcheting.
In contrast to animals and benthic protists, there is little evidence that the details of size evolution in vascular plants and algae can be explained by variation in atmospheric oxygen levels beyond the constraints lifted when oxygen first accumulated in the atmosphere and when it first exceeded a threshold value near 10% PAL. First, vascular plants achieve large size primarily through the production of wood, which is not metabolically active. Secondly, metabolically active cells are maintained near the outer surface of the plant (e.g., leaves), where they can be supplied with oxygen and carbon dioxide via diffusion through stomata. Third, the competition between CO2 and O2 for RUBISCO results in an inhibition of growth by high O2:CO2, suggesting that higher oxygen levels would, if anything, increase the cost of growth to large size (Raven 1991). Fourth, maximum height in vascular plants appears to be limited by hydraulic and possibly mechanical factors, rather than by metabolic and nutrient demands (Niklas 2007; Ryan and Yoder 1997). These anatomical and physiological constraints are consistent with biogeographic data showing that kelp are environmentally constrained by nutrient availability rather than oxygen concentration (Graham et al. 2007) and fossil data indicating that the size evolution of dinoflagellates and diatoms has responded most strongly to rates of nutrient upwelling into surface waters rather than to oxygen availability (Finkel et al. 2005, 2007; Schmidt et al. 2004).
The challenges enumerated above are in many ways inherent to any interdisciplinary problem in the Earth and life sciences because experimental and field observations must be extrapolated across vast spatial and temporal scales, whereas the fossil record often contains little or no information constraining potentially important variables. That said, the findings emerging at all scales of investigation into the role of oxygen in size evolution suggest that more integrated efforts could yield important new insights using techniques and data already available. Below we outline what we believe may be the most fruitful lines of inquiry. This is intended to be a representative list, not an exhaustive one.
-
1.
Despite decades of speculation, there remains little systematic analysis of size evolution in the fossil record with respect to oxygen history. The examples of Carboniferous gigantism are undoubtedly real—counterexamples would certainly have come forth by now. However, Okajima’s (2008) recent study represents the only systematic examination of size data with respect to oxygen for a clade with a late Paleozoic maximum in size. Similar analyses for other taxa are critical. It remains unknown not only whether size variation in other clades is over- or under-predicted by variation in atmospheric oxygen but also whether other clades exhibit gigantism during intervals not characterized by high pO2. Until such studies are conducted, it will remain unclear whether the widely cited examples of Carboniferous and Permian gigantism represent selection bias or whether gigantism was truly more common during the time of Earth’s highest oxygen levels. The fossil record is replete with diverse and well-fossilized clades; such analyses are well within the scope of the size data included implicitly or explicitly in the taxonomic literature.
-
2.
Although prediction of maximum (or optimum) size from first principles alone is likely impossible in light of the anatomical and physiological complexity of animals, several lines of empirical research could improve our understanding of the role of oxygen in the evolution of body size. Work on amphipods by Chapelle and Peck (1999, 2004) highlights several potential avenues for future research. First, it remains unknown whether other clades exhibit a similarly strong relationship between size quantiles and oxygen availability. Are such relationships common in other arthropod clades? Among animals more generally? Constraints on the prevalence of such relationships, or lack thereof, would greatly aid our understanding of the extent to which oxygen availability governs size distributions in animals. If such relationships are common in some higher taxa but not others, comparative analyses may shed light on the anatomical, physiological, or ecological factors that determine the importance of oxygen in the evolution of size. Such data could even be applied to analysis of fossil data. Given an empirically determined relationship between oxygen availability and maximum size, one could then assess the extent to which temporal variation in size matches predictions based on spatial variation among living species. Over- or under-prediction of size change relative to past oxygen concentrations could even shed light on additional factors governing size evolution. Unfortunately, amphipods likely have too poor a fossil record for such an exercise, but other diverse higher taxa with good fossil records may present opportunities. Bivalves, gastropods, and ostracods are likely among the best candidates.
-
3.
The observed scaling of the entire size distribution with oxygen availability in amphipods further highlights a shortcoming of previous theoretical work on the size-oxygen relationship: all theoretical work has focused on oxygen as a factor limiting maximum size. Size distributions among species within higher taxa are widely thought to be determined by energetic considerations and mortality schedules (Brown et al. 1993; Hallock 1985; Sebens 2002). As oxygen availability can affect both efficiency and rate of growth (Owerkowicz et al. 2009), variation in oxygen availability should affect selection across the size spectrum, not simply at the maximum. For example, time-dependent risk of death due to predation or disease tends to select against large size because of the time required to grow larger prior to reproduction. An increase in growth efficiency due to increased oxygen availability could allow an organism to achieve larger size without the trade-off of waiting longer prior to reproduction. Figure 4 illustrates a schematic example of how an effect of oxygen on the efficiency of energy intake as a function of body mass could affect fitness across the size spectrum. Higher oxygen concentrations also come with a cost of increased oxidative damage. Any complete assessment of the precise effects of variation in oxygen levels on the evolution of body size would take these into account as well. However, the ability of many animals to grow normally under experimental conditions of extremely high pO2 (e.g., Herman and Ingermann 1996; Metcalfe et al. 1981; Stock et al. 1983) suggests that the stress of oxidative damage is comparatively minor, at least over experimental timescales. Of course, any change in oxygen availability will occur for all organisms within an ecosystem. Consequently, factors allowing for size increase at higher oxygen levels may be offset by ecological interactions with other similarly affected organisms, either through increased competition for resources or through increased predation pressure on prey species. On the other hand, the strong circumstantial evidence for oxygen as an important control on size evolution in the fossil record suggests its effects are expressed despite the many additional ecological factors influencing size evolution.
Fig. 4 Schematic illustration of how variation in atmospheric oxygen concentrations could affect energetically determined minimum, optimum, and maximum sizes. In this case, cost is assumed to be a linear function of mass, whereas intake is assumed to scale allometrically with mass with a scaling exponent less than one. Optimum size is assumed to occur where energy surplus is maximized. The range of viable sizes is assumed to span those sizes where an energy surplus occurs. Assuming a proportional increase in intake under high oxygen causes an increase in both optimum and maximum size. Such a pattern is consistent with the observations of Chapelle and Peck (2004) for amphipods. Varying cost as a function of oxygen concentration rather than intake yields qualitatively similar results. a Energy intake and energy cost as a function of body mass. b Energy surplus as a function of mass
-
4.
Systematic experimentation across taxa differing in anatomy and physiology can help to clarify which anatomical and physiological characteristics are most likely to lead to a relationship between oxygen and body size. Extrapolation of experimental findings to evolutionary timescales is difficult because experimental systems cannot capture the effects of ecological selection pressures on body size or phenotypic variability introduced by new mutations. However, they can provide additional insight through comparative analysis among taxa. Experiments on taxa for which relevant field observations also exist, such as the amphipods, could even be used to test the extent to which size responses in the laboratory can be extrapolated. Such comparisons could potentially help to calibrate an empirical relationship between ecophenotypic and evolutionary response to variation in oxygen availability.
-
5.
Experimental work suggests that the relationship between oxygen availability and size evolution is much more complex for oxygenic photoautotrophs than for aerobic heterotrophs and that high O2:CO2 negatively impacts growth at modern pO2. However, there are few fossil data sets and no experimental data for the clades of vascular plants that were most diverse and abundant through the majority of Phanerozoic time. Basic observations are still required to better understand the interactions between O2, CO2, and plant anatomy in governing the evolution of maximum size.
-
6.
Experiments suggest that size reduction may be more easily achieved during oxygen decrease than size increase during oxygen increase. As there is likely stronger selective pressure on taxa for ecophenotypic responses to oxygen deprivation than to oxygen surplus, simply because oxygen deprivation occurs more commonly in nature, it is possible that most taxa can respond more quickly to reduction in oxygen availability over evolutionary time. Of course, the response times for size increase and decrease may be shorter than the temporal resolution of the fossil record or the rate of change in pO2, but such a potential asymmetry in evolutionary pattern may warrant a careful search for appropriate study organisms. Moreover, hysteresis in the evolutionary response may extend beyond asymmetry in phenotypic plasticity to size thresholds requiring the gain or loss of a key innovation such as a respiratory organ or circulatory system. Parasites may provide interesting test cases because many lineages are much larger than their free-living relatives (A. Curis, pers. comm. 2010) and parasites occur in unique microenvironments where O2 availability may differ substantially from the surrounding environment.
Conclusions
Eons and eras are demarcated on the basis of evolutionary events. Among these, the Archean-Proterozoic (2.5 Gya), Proterozoic-Phanerozoic (543 Mya), and Paleozoic–Mesozoic (252 Mya) transitions have long been recognized as among the most significant, in part on account of the major changes in maximum body size occurring during or after these events. The evidence we have now points to change in atmospheric oxygen as a major cause of these size changes. This possibility has been appreciated for at least half a century, but quantitative support has grown rapidly in recent years and now derives from a wide spectrum of disciplines, including physical chemistry, geochemistry, cell and developmental biology, physiology, ecology, and paleontology. The case is still somewhat uncertain, of course; and other factors were doubtless involved.
Future work in experimental and comparative biology holds the potential to provide better quantitative models of the potential influence of oxygen on size evolution. When applied to the fossil record, these models may shed light on the extent to which size evolution in different clades and at different times can be ascribed to the influence of variation in atmospheric oxygen levels versus other environmental and ecological factors.
Abbreviations
- PAL:
-
Present atmospheric level (of oxygen)
- Mya:
-
Millions of years ago
- Gya:
-
Billions of years ago
References
Acquisti C, Kleffe J, Collins S (2007) Oxygen content of transmembrane proteins over macroevolutionary time scales. Nature 445:47–52
Albani AE, Bengtson S, Canfield DE, Bekker A, Macchiarelli R, Mazurier A, Hammarlund EU, Boulvais P, Dupuy J-J, Fontaine C, Fürsich FT, Gauthier-Lafaye F, Janvier P, Javaux E, Ossa FO, Pierson-Wickmann A-C, Riboulleau A, Sardini P, Vachard D, Whitehouse M, Meunier A (2010) Large colonial organisms with coordinated growth in oxygenated environments 2.1 Gyr ago. Nature 466:100–104
Alexander RM (1971) Size and shape. Edward Arnold, London
Allwood AC, Walter MR, Kamber BS, Marshall CP, Burch IW (2006) Stromatolite reef from the Early Archaean era of Australia. Nature 441:714–718
Andrews RM (2002) Low oxygen: a constraint on the evolution of viviparity in reptiles. Physiol Biochem Zool 75:145–154
Barghoorn ES, Tyler SA (1963) Fossil organisms from Precambrian sediments. Ann N Y Acad Sci 108:451–452
Barghoorn ES, Tyler SA (1965) Microorganisms from Gunflint Chert—these structurally preserved Precambrian fossils from Ontario are most ancient organisms known. Science 147:563–575
Barras CG, Twitchett RJ (2007) Response of the marine infauna to Triassic-Jurassic environmental change: ichnological data from southern England. Palaeogeogr Palaeoclimatol Palaeoecol 244:223–241
Belcher CM, McElwain JC (2008) Limits for combustion in low O2 redefine paleoatmospheric predictions for the Mesozoic. Science 321:1197–1200
Bergman NM, Lenton TM, Watson AJ (2004) COPSE: a new model of biogeochemical cycling over Phanerozoic time. Am J Sci 304:397–437
Berkner LV, Marshall LC (1965) History of major atmospheric components. Proc Natl Acad Sci USA 53:1215–1226
Berner RA (2004) The Phanerozoic carbon cycle: CO2 and O2. Oxford University Press, New York
Berner RA (2006) GEOCARBSULF: a combined model for Phanerozoic atmospheric O2 and CO2. Geochim Cosmochim Acta 70:5653–5664
Berner RA, Canfield DE (1989) A new model for atmospheric oxygen over Phanerozoic time. Am J Sci 289:333–361
Berner RA, Beerling DJ, Dudley R, Robinson JM, Wildman RA (2003) Phanerozoic atmospheric oxygen. Annu Rev Earth Planet Sci 31:105–134
Berner RA, VandenBrooks JM, Ward PD (2007) Oxygen and evolution. Science 316:557–558
Björkman O, Hiesey WM, Nobs MA, Nicholson F, Hart RW (1968) Effect of oxygen concentration in higher plants. Carnegie Inst Wash Year B 66:228–232
Björkman O, Gauhl E, Hiesey WM, Nicholson F, Nobs MA (1969) Growth of Mimulus, Marchantia, and Zea under different oxygen and carbon dioxide levels. Carnegie Inst Wash Year B 67:477–478
Blankenship RE, Sadekar S, Raymond J (2007) The evolutionary transition from anoxygenic to oxygenic photosynthesis. In: Falkowski PG, Knoll AH (eds) Evolution of primary producers in the sea. Academic Press, Amsterdam, pp 21–35
Bonner JT (1965) Size and cycle. Princeton University Press, Princeton, NJ
Bonner JT (1988) The evolution of complexity by means of natural selection. Princeton University Press, Princeton, NJ
Bonner JT (2006) Why size matters: from bacteria to blue whales. Princeton University Press, Princeton, NJ
Braddy SJ, Poschmann M, Tetlie OE (2008) Giant claw reveals the largest ever arthropod. Biol Lett 4:106–109
Brocks JJ, Logan GA, Buick R, Summons RE (1999) Archean molecular fossils and the early rise of eukaryotes. Science 285:1033–1036
Brown JH (1995) Macroecology. University of Chicago Press, Chicago
Brown JH, Marquet PA, Taper ML (1993) Evolution of body-size—consequences of an energetic definition of fitness. Am Nat 142:573–584
Brown JH, Gillooly JF, Allen AP, Savage VM, West GB (2004) Toward a metabolic theory of ecology. Ecology 85:1771–1789
Brown JH, West GB, Enquist BJ (2005) Yes, West, Brown and Enquist’s model of allometric scaling is both mathematically correct and biologically relevant. Funct Ecol 19:735–738
Brusca RC, Brusca GJ (2003) Invertebrates. Sinauer Associates, Sunderland, MA
Busk M, Overgaard J, Hicks JW, Bennett AF, Wang T (2000) Effects of feeding on arterial blood gases in the American alligator Alligator mississippiensis. J Exp Biol 203:3117–3124
Butterfield NJ (2000) Bangiomorpha pubescens n. gen., n.sp.: implications for the evolution of sex, multicellularity, and the Mesoproterozoic/Neoproterozoic radiation of eukaryotes. Paleobiology 26:386–404
Calder WA (1984) Size, function, and life history. Harvard University Press, Cambridge, MA
Canfield DE (2005) The early history of atmospheric oxygen: homage to Robert M. Garrels. Annu Rev Earth Planet Sci 33:1–36
Canfield DE, Poulton SW, Narbonne GM (2007) Late-Neoproterozoic deep-ocean oxygenation and the rise of animal life. Science 315:92–95
Carpenter FM (1960) Studies on Carboniferous insects. 1. The Protodonata. Psyche 67:98–110
Catling DC, Glein CR, Zahnle KJ, McKay CP (2005) Why O2 is required by complex life on habitable planets and the concept of planetary “oxygenation time”. Astrobiology 5:415–438
Chan T, Burggren W (2005) Hypoxic incubation creates differential morphological effects during specific developmental critical windows in the embryo of the chicken (Gallus gallus). Respir Physiol Neurobiol 145:251–263
Chapelle G, Peck LS (1999) Polar gigantism dictated by oxygen availability. Nature 399:114–115
Chapelle G, Peck LS (2004) Amphipod crustacean size spectra: new insights in the relationship between size and oxygen. Oikos 106:167–175
Cloud PE (1965) Significance of the Gunflint (Precambrian) microflora. Science 148:27–35
Cloud PE (1968) Pre-metazoan evolution and the origins of the Metazoa. In: Drake ET (ed) Evolution and environment. Yale University Press, New Haven, pp 1–72
Cloud PE (1972) A working model of the primitive Earth. Am J Sci 272:537–548
Crossley DA II, Altimiras J (2005) Cardiovascular development in embryos of the American alligator Alligator mississippiensis: effects of chronic and acute hypoxia. J Exp Biol 208:31–39
Cunningham EL, Brody JS, Jain BP (1974) Lung growth induced by hypoxia. J Appl Physiol 37:362–366
Dabrowski K, Lee K-J, Guz L, Verlhac V, Gabaudan J (2004) Effects of dietary ascorbic acid on oxygen stress (hypoxia or hyperoxia), growth and tissue vitamin concentrations in juvenile rainbow trout (Oncorhynchus mykiss). Aquaculture 233:383–392
Darveau CA, Suarez RK, Andrews RD, Hochachka PW (2002) Allometric cascade as a unifying principle of body mass effects on metabolism. Nature 417:166–170
DeLong JP, Okie JG, Moses ME, Sibly RM, Brown JH (2010) Shifts in metabolic scaling, production, and efficiency across major evolutionary transitions of life. Proc Natl Acad Sci USA 107. doi: 10.1073/iti2510107
Dodds PS, Rothman DH, Weitz JS (2001) Re-examination of the “3/4-law” of metabolism. J Theor Biol 209:9–27
Dudley R (1998) Atmospheric oxygen, giant Paleozoic insects and the evolution of aerial locomotor performance. J Exp Biol 201:1043–1050
Dutta A, Popel AS (1995) A theoretical analysis of intracellular oxygen diffusion. J Theor Biol 176:433–445
Dzialowski EM, von Plettenberg D, Elmonoufy NA, Burggren WW (2002) Chronic hypoxia alters the physiological and morphological trajectories of developing chicken embryos. Comp Biochem Physiol A 131:713–724
Else PL, Hulbert AJ (1981) Comparison of the “mammal machine” and the “reptile machine”: energy production. Am J Physiol 240:3–9
Falkowski PG, Katz ME, Milligan AJ, Fennel K, Cramer BS, Aubry MP, Berner RA, Novacek MJ, Zapol WM (2005) The rise of oxygen over the past 205 million years and the evolution of large placental mammals. Science 309:2202–2204
Fan C, Iacobas DA, Zhou D, Chen Q, Lai JK, Gavrialov O, Haddad GG (2005) Gene expression and phenotypic characterization of mouse heart after chronic constant or intermittent hypoxia. Physiol Genomics 22:292–307
Fehling J, Stoecker D, Baldauf SL (2007) Photosynthesis and the eukaryote tree of life. In: Falkowski PG, Knoll AH (eds) Evolution of primary producers in the sea. Academic Press, Amsterdam, pp 75–107
Fike DA, Grotzinger JP, Pratt LM, Summons RE (2006) Oxidation of the Ediacaran ocean. Nature 444:744–747
Finkel ZV, Katz ME, Wright JD, Schofield OME, Falkowski PG (2005) Climatically driven macroevolutionary patterns in the size of marine diatoms of the Cenozoic. Proc Natl Acad Sci USA 102:8927–8932
Finkel ZV, Sebbo J, Feist-Burkhardt S, Irwin AJ, Katz ME, Schofield OEM, Young JR, Falkowski PG (2007) A universal driver of macroevolutionary change in the size of marine phytoplankton over the Cenozoic. Proc Natl Acad Sci USA 104:20416–20420
Foss A, Vollen T, Øiestad V (2003) Growth and oxygen consumption in normal and O2 supersaturated water, and interactive effects of O2 saturation and ammonia on growth in spotted wolffish (Anarhichas minor Olafsen). Aquaculture 224:105–116
Fraiser ML, Bottjer DJ (2004) The non-actualistic Early Triassic gastropod fauna: a case study of the Lower Triassic Sinbad Limestone member. Palaios 19:259–275
Fralick P, Davis DW, Kissin SA (2002) The age of the Gunflint Formation, Ontario, Canada: single zircon U–Pb age determinations from reworked volcanic ash. Can J Earth Sci 39:1085–1091
Frappell PB, Mortola JP (1994) Hamsters vs. rats: metabolic and ventilatory response to development in chronic hypoxia. J Appl Physiol 77:2748–2752
Frazier MR, Woods HA, Harrison JF (2001) Interactive effects of rearing temperature and oxygen on the development of Drosophila melanogaster. Physiol Biochem Zool 74:641–650
Frei R, Gaucher C, Poulton SW, Canfield DE (2009) Fluctuations in Precambrian atmospheric oxygenation recorded by chromium isotopes. Nature 461:250–253
Frisancho AR, Baker PT (1970) Altitude and growth: a study of the patterns of physical growth of a high altitude Peruvian Quechua population. Am J Phys Anthropol 32:279–292
Gilbert DL (1960) Speculation on the relationship between organic and atmospheric evolution. Perspect Biol Med 4:58–71
Gilbert DL (1996) Evolutionary aspects of atmospheric oxygen and organisms. In: Fregly MJ, Blatteis CM (eds) Environmental physiology. Oxford University Press, New York, pp 1059–1094
Gillooly JF, Brown JH, West GB, Savage VM, Charnov EL (2001a) Effects of size and temperature on metabolic rate. Science 293:2248–2251
Gillooly JF, Charnov EL, West GB, Savage VM, Brown JH (2001b) Effects of size and temperature on developmental time. Nature 417:70–73
Giussani DA, Salinas CE, Villena M, Blanco CE (2007) The role of oxygen in prenatal growth: studies in the chicken embryo. J Physiol 585:911–917
Gooday AJ, Bernhard JM, Levin LA, Suhr SB (2000) Foraminifera in the Arabian Sea oxygen minimum zone and other oxygen-deficient settings: taxonomic composition, diversity, and relation to metazoan faunas. Deep-Sea Res II 47:25–54
Gould SJ (1988) Trends as changes in variance—a new slant on progress and directionality in evolution. J Paleontol 62:319–329
Gould SJ (1996) Full house: the spread of excellence from Plato to Darwin. Harmony Books, New York
Graham JB, Aguilar NM, Dudley R, Gans C (1995) Implications of the late Paleozoic oxygen pulse for physiology and evolution. Nature 375:117–120
Graham MH, Kinlan BP, Druehl LD, Garske LE, Banks S (2007) Deep-water kelp refugia as potential hotspots of tropical marine diversity and productivity. Proc Natl Acad Sci USA 104:16576–16580
Greenberg S, Ar A (1996) Effects of chronic hypoxia, normoxia and hyperoxia on larval development in the beetle Tenebrio molitor. J Insect Physiol 42:991–996
Greenlee KJ, Harrison JF (2004) Development of respiratory function in the American locust Schistocerca americana I. Across-instar effects. J Exp Biol 207:497–508
Greenlee KJ, Harrison JF (2005) Respiratory changes throughout ontogeny in the tobacco hornworm caterpillar, Manduca sexta. J Exp Biol 208:1385–1392
Greenlee KJ, Nebeker C, Harrison JF (2007) Body size-independent safety margins for gas exchange across grasshopper species. J Exp Biol 210:1288–1296
Guo SS, Tang YK, Gao F, Ai WD, Qin LF (2008) Effects of low pressure and hypoxia on growth and development of wheat. Acta Astronaut 63:1081–1085
Hallam A, Wignall PB (1997) Mass extinctions and their aftermaths. Oxford University Press, New York
Hallock P (1985) Why are larger Foraminifera large? Paleobiology 11:195–208
Harrison JF (2010) Atmospheric oxygen level and the evolution of insect body size. Proc R Soc Lond B 277:1937–1946
Harrison J, Frazier MR, Henry JR, Kaiser A, Klok CJ, Rascón B (2006) Responses of terrestrial insects to hypoxia or hyperoxia. Respir Physiol Neurobiol 154:4–17
He C, Davies FT, Lacey RE (2007) Separating the effects of hypobaria and hypoxia on lettuce: growth and gas exchange. Physiol Plant 131:226–240
He W-H, Twitchett RJ, Zhang Y, Shi GR, Feng Q-L, Yu J-X, Wu S-B, Peng X-F (2010) Controls on body size during the Late Permian mass extinction event. Geobiology. doi: 10.1111/j.1472-4669.2010.00248.x
Henry JR, Harrison JF (2004) Plastic and evolved responses of larval tracheae and mass to varying atmospheric oxygen content in Drosophila melanogaster. J Exp Biol 207:3559–3567
Herman J, Ingermann R (1996) Effects of hypoxia and hyperoxia on oxygen-transfer properties of the blood of a viviparous snake. J Exp Biol 199:2061–2070
Holland HD (2006) The oxygenation of the atmosphere and oceans. Philos Trans R Soc Lond B 361:903–915
Holland HD (2009) Why the atmosphere became oxygenated: a proposal. Geochim Cosmochim Acta 73:5241–5255
Hsia CCW, Carbayo JJP, Yan X, Bellotto DJ (2005) Enhanced alveolar growth and remodeling in Guinea pigs raised at high altitude. Respir Physiol Neurobiol 147:105–115
Huey RB, Ward PD (2005) Hypoxia, global warming, and terrestrial Late Permian extinctions. Science 308:398–401
Jacobsen D, Rostgaard S, Vásconez JJ (2003) Are macroinvertebrates in high altitude streams affected by oxygen deficiency? Freshw Biol 48:2025–2032
Javaux EJ, Marshall CP, Bekker A (2010) Organic-walled microfossils in 3.2-billion-year-old shallow-marine siliciclastic deposits. Nature 463:934–938
Jin J (2001) Evolution and extinction of the North American Hiscobeccus brachiopod Fauna during the Late Ordovician. Can J Earth Sci 38:143–151
Johnson MD, Volker J, Moeller HV, Laws E, Breslauer KJ, Falkowski PG (2009) Universal constant for heat production in protists. Proc Natl Acad Sci USA 106:6696–6699
Julian CG, Vargas E, Armaza JF, Wilson MJ, Niermeyer S, Moore LG (2007) High-altitude ancestry protects against hypoxia-associated reductions in fetal growth. Arch Dis Child 92:F372–F377
Kaiho K (1998) Global climatic forcing of deep-sea benthic foraminiferal test size during the past 120 m.y. Geology 26:491–494
Kaiser A, Klok CJ, Socha JJ, Lee W-K, Quinlan MC, Harrison JF (2007) Increase in tracheal investment with beetle size supports hypothesis of oxygen limitation on insect gigantism. Proc Natl Acad Sci USA 104:13198–13203
Kam Y-C (1993) Physiological effects of hypoxia on metabolism and growth of turtle embryos. Respir Physiol 92:127–138
Kirkton SD, Niska JA, Harrison JE (2005) Ontogenetic effects on aerobic and anaerobic metabolism during jumping in the American locust, Schistocerca americana. J Exp Biol 208:3003–3012
Kleiber M (1932) Body size and metabolism. Hilgardia 6:315–351
Klok CJ, Harrison JF (2009) Atmospheric hypoxia limits selection for large body size in insects. PLoS One 4:e3876
Klok CJ, Hubb AJ, Harrison JF (2009) Single and multigenerational responses of body mass to atmospheric oxygen concentrations in Drosophila melanogaster: evidence for roles of plasticity and evolution. J Evol Biol 22:2496–2504
Klok CJ, Kaiser A, Lighton JRB, Harrison JF (2010) Critical oxygen partial pressures and maximal tracheal conductances for Drosophila melanogaster reared for multiple generations in hypoxia or hyperoxia. J Insect Physiol 56:461–469
Knoll AH (1992) The early evolution of eukaryotes—a geological perspective. Science 256:622–627
Knoll AH (2003) The geological consequences of evolution. Geobiology 1:3–14
Knoll AH, Carroll SB (1999) Early animal evolution: emerging views from comparative biology and geology. Science 284:2129–2137
Knoll AH, Holland HD (1995) Oxygen and Proterozoic evolution: an update. In: Commission on Geosciences EaR (ed) Effects of past global change on life. National Academy Press, Washington, DC, pp 21–33
Knoll AH, Bambach RK, Payne JL, Pruss S, Fischer WW (2007) Paleophysiology and end-Permian mass extinction. Earth Planet Sci Lett 256:295–313
Koch LG, Britton SL (2008) Aerobic metabolism underlies complexity and capacity. J Physiol 586:83–95
Kozlowski J, Konarzewski M (2005) West, Brown and Enquist’s model of allometric scaling again: the same questions remain. Funct Ecol 19:739–743
Kukalová-Peck J (1985) Ephemeroid wing venation based upon new gigantic Carboniferous mayflies and basic morphology, phylogeny, and metamorphosis of pterygote insects (Insecta, Ephemerida). Can J Zool 63:933–955
Kumar S (1995) Megafossils from the Mesoproterozoic Rohtas Formation (the Vindhyan Supergroup), Katni area, central India. Precambr Res 72:171–184
Kump LR (2008) The rise of atmospheric oxygen. Nature 451:277–278
Kump LR, Barley ME (2007) Increased subaerial volcanism and the rise of atmospheric oxygen 2.5 billion years ago. Nature 448:1033–1036
Labandiera CC (2005) The fossil record of insect extinction: new approaches and future directions. Am Entomol 51:14–29
Lane N (2002) Oxygen: the molecule that made the world. Oxford University Press, Oxford
Lenton TM (2003) The coupled evolution of life and atmospheric oxygen. In: Lister A, Rothschild LJ (eds) Evolution on planet earth: impact of the physical environment. Academic Press, San Diego, pp 33–51
Levin LA (2003) Oxygen minimum zone benthos: adaptation and community response to hypoxia. Oceanogr Mar Biol Annu Rev 41:1–45
Levin LA, Plaia GR, Huggett CL (1994) The influence of natural organic enhancement on life histories and community structure of bathyal polychaetes. In: Young CM, Eckelbarger KJ (eds) Reproduction, larval biology, and recruitment of the deep-sea benthos. Columbia University Press, Columbia, SC, pp 261–283
Lighton JRB (2007) Respiratory biology: they would be giants. Curr Biol 17:R969–R971
Loudon C (1988) Development of Tenebrio molitor in low oxygen levels. J Insect Physiol 34:97–103
Lyons TW, Reinhard CT (2009) Early Earth: oxygen for heavy-metal fans. Nature 461:179–181
Makarieva AM, Gorshkov VG, Li B-L (2003) A note on metabolic rate dependence on body size in plants and animals. J Theor Biol 221:301–307
Makarieva AM, Gorshkov VG, Li B-L, Chown SL, Reich PB, Gavrilov VM (2008) Mean mass-specific metabolic rates are strikingly similar across life’s major domains: evidence for life’s metabolic optimum. Proc Natl Acad Sci USA 105:16994–16999
Marshall CR (2006) Explaining the Cambrian “explosion” of animals. Annu Rev Earth Planet Sci 34:355–384
McAlester AL (1970) Animal extinctions, oxygen consumption, and atmospheric history. J Paleontol 44:405–409
McClain CR, Barry J (2010) Habitat heterogeneity, biotic disturbance, and resource availability work in concert to regulate biodiversity in deep submarine canyons. Ecology 91:964–976
McClain CR, Rex M (2001) The relationship between dissolved oxygen concentration and maximum size in deep-sea turrid gastropods: an application of quantile regression. Mar Biol 139:681–685
McClain CR, Rex MA, Jabbour R (2005) Deconstructing bathymetric patterns of body size in deep-sea gastropods. Mar Ecol Prog Ser 297:181–187
McClain CR, Boyer A, Rosenberg G (2006) The island rule and the evolution of body size in the deep sea. J Biogeogr 33:1578–1584
McMinn A, Pankowski A, Delfatti T (2005) Effect of hyperoxia on the growth and photosynthesis of polar sea microalgae. J Phycol 41:732–741
McShea DW (1994) Mechanisms of large-scale evolutionary trends. Evolution 48:1747–1763
Metcalfe J, McCutcheon IE, Francisco DL, Metzenberg AB, Welch JE (1981) Oxygen availability and growth of the chick embryo. Respir Physiol 46:81–88
Mills NE, Barnhart MC (1999) Effects of hypoxia on embryonic development in two Ambystoma and two Rana species. Physiol Biochem Zool 72:179–188
Mojzsis SJ, Arrhenius G, McKeegan KD, Harrison TM, Nutman AP, Friend CRL (1996) Evidence for life on Earth before 3, 800 million years ago. Nature 384:55–59
Mori S, Yamaji K, Ishida A, Prokushkin SG, Masyagina OV, Hagihara A, Rafiqul Hoque ATM, Suwa R, Osawa A, Nishizono T, Ueda T, Kinjo M, Miyagi T, Kajimoto T, Koike T, Matsuura Y, Toma T, Zyryanova OA, Abaimov AP, Awaya Y, Araki MG, Kawasaki T, Chiba Y, Umari M (2010) Mixed-power scaling of whole-plant respiration from seedlings to giant trees. Proc Natl Acad Sci USA 107:1447–1451
Mortola JP, Xu L, Lauzon A-M (1990) Body growth, lung and heart weight, and DNA content in newborn rats exposed to different levels of chronic hypoxia. Can J Physiol Pharmacol 68:1590–1594
Mortola JP, Frappell PB, Aguero L, Armstrong K (2000) Birth weight and altitude: a study in Peruvian communities. J Pediatr 136:324–329
Musgrave ME, Strain BR (1988) Response of two wheat cultivars to CO2 enrichment under subambient oxygen conditions. Plant Physiol 87:346–350
Nelson SJ (1959) Arctic Ordovician fauna: an equatorial assemblage? J Alta Soc Petrol Geol 7:45–47
Newell ND (1949) Phyletic size increase, an important trend illustrated by fossil invertebrates. Evolution 3:103–124
Niklas KJ (2007) Maximum plant height and the factors that limit it. Tree Physiol 27:433–440
Norris RD (1989) Cnidarian taphonomy and affinities of the Ediacara biota. Lethaia 22:381–393
Nursall JR (1959) Oxygen as a prerequisite to the origin of the Metazoa. Nature 183:1170–1172
Okajima R (2008) The controlling factors limiting maximum body size of insects. Lethaia 41:423–430
Owerkowicz T, Elsey RM, Hicks JW (2009) Atmospheric oxygen level affects growth trajectory, cardiopulmonary allometry and metabolic rate in the American alligator (Alligator mississippiensis). J Exp Biol 212:1237–1247
Payne JL (2005) Evolutionary dynamics of gastropod size across the end-Permian extinction and through the Triassic recovery interval. Paleobiology 31:269–290
Payne JL, Boyer AG, Brown JH, Finnegan S, Kowalewski M, Krause RA, Lyons SK, McClain CR, McShea DW, Novack-Gottshall PM, Smith FA, Stempien JA, Wang SC (2009) Two-phase increase in the maximum size of life over 3.5 billion years reflects biological innovation and environmental opportunity. Proc Natl Acad Sci USA 106:24–27
Peck LS, Chapelle G (2003) Reduced oxygen at high altitude limits maximum size. Proc R Soc Lond B 270:S166–S167
Peck LS, Maddrell SHP (2005) Limitation of size by hypoxia in the fruit fly Drosophila melanogaster. J Exp Zool 303A:968–975
Perez-Cruz LL, Machain-Castillo ML (1990) Benthic foraminifera of the oxygen minimum zone, continental shelf of the Gulf of Tehuantepec, Mexico. J Foramin Res 20:312–325
Peters RH (1983) The ecological implications of body size. Cambridge University Press, New York
Phleger FB, Soutar A (1973) Production of benthic foraminifera in three east Pacific oxygen minima. Micropaleontology 19:110–115
Pruder GD, Bolton ET (1980) Differences between cell division and carbon fixation rates associated with light intensity and oxygen concentration: implications in the cultivation of an estuarine diatom. Mar Biol 59:1–6
Quebedeaux B, Hardy RWF (1973) Oxygen as a new factor controlling reproductive growth. Nature 243:477–479
Quebedeaux B, Hardy RWF (1975) Reproductive growth and dry matter production of Glycine max (L.) Merr. in response to oxygen concentration. Plant Physiol 55:102–107
Raff RA, Raff EC (1970) Respiratory mechanisms and the metazoan fossil record. Nature 228:1003–1005
Rasmussen B, Fletcher IR, Brocks JJ, Kilburn MR (2008) Reassessing the first appearance of eukaryotes and cyanobacteria. Nature 455:1101–1104
Raup DM, Sepkoski JJ (1982) Mass extinctions in the marine fossil record. Science 215:1501–1503
Raven JA (1991) Plant responses to high O2 concentrations: relevance to previous high O2 episodes. Palaeogeogr Palaeoclimatol Palaeoecol 97:19–38
Raven JA, Johnston AM, Parsons R, Kübler J (1994) The influence of natural and experimental high O2 concentrations on O2-evolving phototrophs. Biol Rev 69:61–94
Retallack GJ, Smith RMH, Ward PD (2003) Vertebrate extinction across the Permian-Triassic boundary in Karoo Basin, South Africa. Geol Soc Am Bull 115:1133–1152
Rex MA, Etter RJ, Morris JS, Crouse J, McClain CR, Johnson NA, Stuart CT, Thies R, Avery R (2006) Global bathymetric patterns of standing stock and body size in the deep-sea benthos. Mar Ecol Prog Ser 317:1–8
Rhoads DC, Morse JW (1971) Evolutionary and ecologic significance of oxygen-deficient marine basins. Lethaia 4:413–428
Rohr DM, Blodgett RB, Furnish WMF (1992) Maclurina manitobensis (Whiteaves) (Ordovician Gastropoda): the largest known Paleozoic gastropod. J Paleontol 66:880–884
Rolfe WDI, Ingham JK (1967) Limb structure, affinity and diet of the Carboniferous “centipede” Arthropleura. Scot J Geol 3:118–124
Rosing MT (1999) C-13-depleted carbon microparticles in >3700-Ma sea-floor sedimentary rocks from west Greenland. Science 283:674–676
Rudkin DM, Young GA, Elias RJ, Dobrzanski EP (2003) The world’s biggest trilobite—Isotelus rex new species from the Upper Ordovician of northern Manitoba, Canada. J Paleontol 77:99–112
Runnegar B (1982) Oxygen requirements, biology and phylogenetic significance of the late Precambrian worm Dickinsonia, and the evolution of the burrowing habit. Alcheringa 6:223–239
Rutten MG (1966) Geologic data on atmospheric history. Palaeogeogr Palaeoclimatol Palaeoecol 2:47–57
Ryan MG, Yoder BJ (1997) Hydraulic limits to tree height and tree growth. Bioscience 47:235–242
Samuelsson J, Butterfield NJ (2001) Neoproterozoic fossils from the Franklin Mountains, northwestern Canada: stratigraphic and palaeobiological implications. Precambr Res 107:235–251
Savage VM, Gillooly JF, Woodruff WH, West GB, Allen AP, Enquist BJ, Brown JH (2004) The predominance of quarter-power scaling in biology. Funct Ecol 18:257–282
Schidlowski M, Appel PWU, Eichmann R, Junge CE (1979) Carbon isotope geochemistry of the 3.7 × 109-yr-old Isua sediments, West Greenland—implications for the Archaean carbon and oxygen cycles. Geochim Cosmochim Acta 43:189–199
Schlanger SO, Jenkyns HC (1976) Cretaceous oceanic anoxic events: causes and consequences. Geol Mijnb 55:179–184
Schmidt DN, Thierstein HR, Bollmann J, Schiebel R (2004) Abiotic forcing of plankton evolution in the Cenozoic. Science 303:207–210
Schmidt-Nielsen K (1984) Scaling, why is animal size so important. Cambridge University Press, New York
Schneider DA, Bickford ME, Cannon WF, Schulz KJ, Hamilton MA (2002) Age of volcanic rocks and syndepositional iron formations, Marquette Range Supergroup: implications for the tectonic setting of Paleoproterozoic iron formations of the Lake Superior region. Can J Earth Sci 39:999–1012
Schopf JW (1993) Microfossils of the Early Archean Apex Chert—new evidence of the antiquity of life. Science 260:640–646
Schopf JW, Klein C (1992) The Proterozoic biosphere: a multidisciplinary study. Cambridge University Press, New York
Schopf JW, Packer BM (1987) Early Archean (3.3-billion to 3.5-billion-year-old) microfossils from Warrawoona Group, Australia. Science 237:70–73
Scott C, Lyons TW, Bekker A, Shen Y, Poulton SW, Chu X, Anbar AD (2008) Tracing the stepwise oxygenation of the Proterozoic ocean. Nature 452:456–459
Sebens KP (2002) Energetic constraints, size gradients, and size limits in benthic marine invertebrates. Integr Comp Biol 42:853–861
Sessions AL, Doughty DM, Welander PV, Summons RE, Newman DK (2009) The continuing puzzle of the Great Oxidation Event. Curr Biol 19:R567–R574
Shear WA, Kukalová-Peck J (1990) The ecology of Paleozoic terrestrial arthropods: the fossil evidence. Can J Zool 68:1807–1834
Smith DM, Cook A (2001) Beetle bias: how sedimentary environment influences patterns of coleopteran diversity in the fossil record. Geological Society of America Annual Meeting Abstracts with Program 33:267
Socha JJ, Lee W-K, Harrison JF, Waters JS, Fezzaa K, Westneat MW (2008) Correlated patterns of tracheal compression and convective gas exchange in a carabid beetle. J Exp Biol 211:3409–3420
Stahl WR (1967) Scaling of respiratory variables in mammals. J Appl Physiol 22:453–460
Stanley SM (1973) An explanation for Cope’s rule. Evolution 27:1–26
Stephen JW, Dulynn H, Tobias W (1995) Responses to chronic hypoxia in embryonic alligators. J Exp Zool 273:44–50
Stock MK, Francisco DL, Metcalfe J (1983) Organ growth in chick embryos incubated in 40% or 70% oxygen. Respir Physiol 52:1–11
Sundt-Hansen L, Sundström LF, Einum S, Hindar K, Fleming IA, Devlin RH (2007) Genetically enhanced growth causes increased mortality in hypoxic environments. Biol Lett 3:165–168
Tappan H (1974) Molecular oxygen and evolution. In: Hayaishi O (ed) Molecular oxygen in biology. Elsevier, Amsterdam, pp 81–135
Teichert C, Kummel B (1960) Size of endoceroid cephalolopods. Breviora 128:1–7
Thannickal VJ (2009) Oxygen in the evolution of complex life and the price we pay. Am J Respir Cell Mol Biol 40:507–510
Tice MM, Lowe DR (2004) Photosynthetic microbial mats in the 3, 416-Myr-old ocean. Nature 431:549–552
Tintu AN, le Noble FAC, Rouwet EV (2007) Hypoxia disturbs fetal hemodynamics and growth. Endothelium 14:353–360
Torzillo G, Bernardini P, Masojídek J (1998) On-line monitoring of chlorophyll fluorescence to assess the extent of photoinhibition of photosynthesis induced by high oxygen concentration and low temperature and its effect on the productivity of outdoor cultures of Spirulina plantensis (Cyanobacteria). J Phycol 34:504–510
Twitchett RJ (2007) The Lilliput effect in the aftermath of the end-Permian extinction event. Palaeogeogr Palaeoclimatol Palaeoecol 252:132–144
Twitchett RJ, Barras CG (2004) Trace fossils in the aftermath of mass extinction events. Geol Soc Lond 228:397–418
Tyler SA, Barghoorn ES (1954) Occurrence of structurally preserved plants in pre-cambrian rocks of the Canadian Shield. Science 119:606–608
Wangensteen OD, Rahn H, Burton RR, Smith AH (1974) Respiratory gas exchange of high altitude adapted chick embryos. Respir Physiol 21:61–70
West GB, Brown JH, Enquist BJ (1997) A general model for the origin of allometric scaling laws in biology. Science 276:122–126
Westneat MW, Betz O, Blob RW, Fezzaa K, Cooper WJ, Lee W-K (2003) Tracheal respiration in insects visualized with synchrotron X-ray imaging. Science 299:558–560
Whyte MA (2005) Palaeoecology: a gigantic fossil arthropod trackway. Nature 438:576–577
Wignall PB, Hallam A (1992) Anoxia as a cause of the Permian Triassic mass extinction: facies evidence from northern Italy and the western United-States. Palaeogeogr Palaeoclimatol Palaeoecol 93:21–46
Wignall PB, Twitchett RJ (1996) Oceanic anoxia and the end Permian mass extinction. Science 272:1155–1158
Williams JB, Swift K (1988) Oxygen consumption and growth of Northern Bobwhite embryos under normoxic and hyperoxic conditions. The Condor 90:187–192
Woods HA, Moran AL, Arango CP, Mullen L, Shields C (2009) Oxygen hypothesis of polar gigantism not supported by performance of Antarctic pycnogonids in hypoxia. Proc R Soc Lond B 276:1069–1075
Xiao S, Dong L (2006) On the morphological and ecological history of Proterozoic macroalgae. In: Xiao S, Kaufman AJ (eds) Neoproterozoic geobiology and paleobiology. Springer, Dordrecht, pp 57–90
Zamudio S, Postigo L, Illsley NP, Rodriguez C, Heredia G, Brimacombe M, Echalar L, Torricos T, Tellez W, Moldonado I, Balanza E, Alvarez T, Ameller J, Vargas E (2007) Maternal oxygen delivery is not related to altitude- and ancestry-associated differences in human fetal growth. J Physiol 582:883–895
Acknowledgments
This review is a product of the working group on body size evolution (principal investigators JLP, JAS, and MK) funded by the National Evolutionary Synthesis Center (NESCent), National Science Foundation Grant EF-0423641. P. Falkowski, P. Harnik, J. Skotheim, and N. Sleep provided comments that greatly improved the manuscript. We thank G.X. Rothdrake for correcting the body size estimate of the Ordovician cephalopod.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Payne, J.L., McClain, C.R., Boyer, A.G. et al. The evolutionary consequences of oxygenic photosynthesis: a body size perspective. Photosynth Res 107, 37–57 (2011). https://doi.org/10.1007/s11120-010-9593-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11120-010-9593-1