Abstract
Geochemical approximation of Earth’s atmospheric O2 level over geologic time prompts hypotheses linking hyper- and hypoxic atmospheres to transformative events in the evolutionary history of the biosphere. Such correlations, however, remain problematic due to the relative imprecision of the timing and scope of oxygen change and the looseness of its overlay on the chronology of key biotic events such as radiations, evolutionary innovation, and extinctions. There are nevertheless general attributions of atmospheric oxygen concentration to key evolutionary changes among groups having a primary dependence upon oxygen diffusion for respiration. These include the occurrence of Devonian hypoxia and the accentuation of air-breathing dependence leading to the origin of vertebrate terrestriality, the occurrence of Carboniferous-Permian hyperoxia and the major radiation of early tetrapods and the origins of insect flight and gigantism, and the Mid-Late Permian oxygen decline accompanying the Permian extinction. However, because of variability between and error within different atmospheric models, there is little basis for postulating correlations outside the Late Paleozoic. Other problems arising in the correlation of paleo-oxygen with significant biological events include tendencies to ignore the role of blood pigment affinity modulation in maintaining homeostasis, the slow rates of O2 change that would have allowed for adaptation, and significant respiratory and circulatory modifications that can and do occur without changes in atmospheric oxygen. The purpose of this paper is thus to refocus thinking about basic questions central to the biological and physiological implications of O2 change over geological time.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
1 Introduction
Texts on historical geology chronicle how the structure of the Earth’s surface has been modified over time by forces of climate and tectonics. They also date the progression in the diversity, abundance, and complexity of life on Earth as well as its ebb and flow as a result of climatic and tectonic influences and events such as asteroid impacts. The record of biosphere evolution was first revealed by the fossil record which documented the continuity between extinct and extant organisms throughout the Phanerozoic Eon, the time span “of visible life” extending from the Cambrian (550 million years ago, MYA) to the present. Nearly the entire history of the appearance, proliferation, and diversification of plants and animals and their integration into complex ecological communities defining Earth’s biosphere plays out over Phanerozoic time.
Although a somewhat later addition to the documentation of Earth’s history , studies of the paleoatmosphere , including the origin and growth of atmospheric O2, verify this molecule’s gradual accumulation and significant changes over time as well as its critical role in biosphere evolution [3, 13]. The primitive atmosphere’s O2 content (about 0.001 present atmospheric level, PAL = 20.95 %) came from the photochemical dissociation of water vapor and was largely consumed in the oxidation of lithosphere sediments. Further O2 increases awaited the evolution of photosynthesis about 2800 MYA. When atmospheric O2 reached 0.01 PAL, it was sufficient to block ultraviolet light penetration into water, which expanded opportunities for life in the ocean. Additional radiation of life awaited the rise to 0.1 PAL, which opened land habitats [2, 3, 6].
The biological significance of atmospheric O2 for organism and biosphere evolution is thus tied to step functions associated with its gradual accumulation in sufficient amounts to form the ultraviolet-ozone shield, enable the appearance of the diffusion-limited Ediacaran fauna, support the Cambrian explosion, and possibly even drive the radiation and metabolic physiology of various taxa and contribute to the occurrence of global extinctions [26]. Indeed, biologists have long attributed the occurrence of some phenomena documented in the fossil record as being the result of O2 changes, and some have further considered atmospheric O2 to be an ecological resource that affected biodiversity [15, 23, 30]. In the case of the giant dragonflies of the Carboniferous Period (354–290 MYA), Schidlowski [49] observed that their tracheal respiratory system would not have been adequate for sustaining flight metabolism at PAL and thus predicted a hyperoxic Carboniferous atmosphere.
Whereas early models of atmospheric evolution indicated a gradual increase in O2, through the first half of the Phanerozoic, more advanced, geochemically based studies show a more complex pattern. The model published in 1989 by Berner and Canfield [5] was one of the first to present data in a manner that drew additional interest from biologists in examining the role of O2 in evolution and the enrichment of biodiversity [30]. This model (Figs. 27.1 and 27.2) showed a rise of O2 to above 30 % in the Carboniferous and Permian followed by a drop to below 15 % in the Late Permian and Triassic. In the same way that a new fossil stimulates a paleontologist to compare its size, structure, and ecological setting with other fossils and extant species, the Berner and Canfield O2 curve provided a window into deep time for comparative biologists and physiologists. This paper refocuses thinking about the basic questions that are central to the biological and physiological implications of O2 change over geological time. These questions concern the extent to which the resolution of these models can actually be used in support of physiological inference about O2 effects, given both the millions of years involved in the completion of these O2 transitions and the rate and extent to which physiological systems could, through mechanisms of phenotypic plasticity, homeostasis, and natural selection, become adjusted to them.
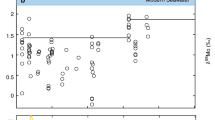
Estimated paleoatmospheric O2 levels from four different models. Estimates are shown in relation to the present atmospheric level (PAL; dashed line = 20.95 %). Geologic period abbreviations: C Carboniferous, Cam Cambrian, Cr Cretaceous, D Devonian, E Ediacaran, J Jurassic, O Ordovician, P Permian, S Silurian, T Tertiary, Tr Triassic. Geologic era abbreviations: Cen Cenozoic, Neo Neoproterozoic
(a) Comparison of the estimated paleoatmospheric O2 levels reported by Berner and Canfield [5] and Berner [4]. Dashed line is the present atmospheric level (PAL) of O2 (20.95 %). (b) Percentage differences in the 2009 and 1989 paleoatmospheric curves. Geologic period and era abbreviations are given in Fig. 27.1
2 Models of Paleoatmospheric Oxygen
Most of the geochemical models of paleoatmospheric O2 and CO2 have their basis in estimates of fixed carbon and exchange processes with the inorganic, carbon-silicate cycle. They attempt to quantify sedimentary records reflecting the formation rate of oxidized and reduced forms of iron, carbon, sulfur, and other elements. Their basis is in estimates of the degree of O2 shuttling between Earth’s large carbon and sulfur reservoirs. When these reservoirs are oxidized, atmospheric O2 is lowered; when these reservoirs are buried, O2 increases. Figure 27.1 compares the paleoatmospheric O2 models of Cloud [13], Berkner and Marshall [3], Bergman et al. [2], and Berner and Canfield [5]. Cloud’s model is not based on geochemical relationships and assumes a gradual but steady rise in O2 with time. It shows an increase from 0.25 to nearly 1 PAL over the 300 MY (million years) of the Paleozoic Era. By contrast, the geochemical models of Berkner and Marshall [3] and Bergman et al. [2] show a lower O2 in the Early Paleozoic but then a rise to levels exceeding those indicated by Cloud [13]. This differs greatly from the Berner and Canfield [5] construction which shows Early Paleozoic levels close to PAL. The three geochemical models do agree in terms of a marked O2 rise in the Carboniferous followed by an abrupt decline in the Permian (Fig. 27.1). However, there is little agreement among them for O2 over the 250 MY span from the beginning of the Triassic to the present.
The differences in the O2 curves generated by these different studies reflect the parameters of the models as well as operating assumptions. Berner and Canfield [5], for example, developed separate models for O2 and CO2, whereas Bergman et al. [2] combined these in the same model. Also, a fire-feedback effect on O2 added to the Bergman et al. [2] model reduced the Devonian-Carboniferous-Permian excursion of their O2 curve. Thus, while the three geochemical models shown in Fig. 27.1 vary greatly in their resultant O2 curves, there is some agreement between them insofar as the rise and then fall in O2 during the Carboniferous and Permian. This said, the timing of these events shown in the different models still differs by as much as 20 MY or more and there are also considerable differences in the magnitude of the shifts.
Berner and colleagues have consistently reported sampling error estimates with all of their O2 curves [4–6]. However, model refinements and successive iterations are a normal course of events in this type of research and subsequent models usually differ from earlier ones. The summary of 20 years of model refinement by Berner and colleagues is captured in Fig. 27.2 which compares the curve of Berner and Canfield in 1989 [5] with that of Berner in 2009 [4] and quantifies the percentage differences between them (Fig. 27.2b). Relative to the 1989 curve, the 2009 version indicates a generally higher O2 for the early part of the Phanerozoic and for the Triassic, but shows much lower O2 levels than previously estimated in the Late Paleozoic and from the Jurassic to the present. Although the general structure of the 1989 and 2009 curves remains similar, the newer model identifies a new O2 spike in the early Devonian (also supported by ocean O2 models, Ref. [14]) and indicates less extreme changes in the upward and downward O2 oscillations occurring across the Carboniferous, Permian, and Triassic.
3 The General Premise for Phanerozoic O2 Effects
The initial hypothetical exploration of the biological significance of the Berner and Canfield [5] paleoatmospheric O2 model sought to identify signature respiratory and metabolic transformations having the potential to impact the physiology and life history of key groups in a way that influenced biosphere evolution [30]. The premise was that while hyperoxia held the potential for enhanced respiratory and metabolic function and increased biosphere complexity, atmospheric hypoxia had the potential not only to refine respiratory function but also to limit it. The latter could have impacted fitness leading to the winnowing of certain groups, thus setting the stage for extinction and the subsequent reconstitution of biosphere complexity. The focus of Graham et al. [29, 30] was on the biological processes that unfolded over the Devonian, Carboniferous, and Permian Periods in the Late Paleozoic . This segment of time was chosen because of the general agreement among the geochemical models for the rise and fall of atmospheric O2 and because there were numerous “biological firsts” and other significant events documented by the fossil record. Also, central to the initial theory was the effect of paleoatmosphere O2 shifts on organisms (insects, amphibious tetrapod s, and others) having respiratory systems entirely or somewhat dependent upon the diffusive flux of O2 for respiration.
4 The Problem: Fitting Paleoatmospheric O2 Models to Fossils and Physiology and Vice Versa
In view of the variation in the modeled curves for paleoatmospheric O2, the question of fitting or correlating these with evolutionary events that were possibly linked to a change in O2 is problematic, especially for times outside of the Late Paleozoic. However, even within this narrow sector of geologic time, there are still differences among the models in both the magnitude of the O2 changes and their timing.
Another timing problem is that the O2 changes of interest in the Late Paleozoic span millions of years. Physiologists interested in studying the effects of variable O2 on metabolism or other processes do so most often by evaluating responses over time spans ranging from fractions of a second to a few months [24, 27, 32, 44, 46, 47]. For Drosophila, Caenorhabditis, and other small model organisms, experiments with O2 exposure effects can extend over generations [1, 33, 42, 54, 55]. However, to put this into perspective, the 2009 Berner curve (Fig. 27.2a) shows about a 12 % O2 rise over the approximately 45 MY between the Late Carboniferous to Middle Permian, a rate of O2 increase of 2 Torr MY−1. Referring again to the 2009 Berner curve [4], the decline in O2 between the Late Permian and the Early Triassic is about 14 % over 35 MY, 3 Torr MY−1. Given that the estimated time of existence for most biological species is less than 2 MY, these slow changes in atmospheric O2 need to be considered not for their effect on a species but rather on the fates of an assemblage of clades derived from species living at the time O2 began to change and prevailing through its duration.
In terms of vertebrate adaptive mechanisms for the gradual Late Permian-Triassic O2 decline, this may have occurred slowly enough to be compensated by functional plasticity in Hb-O2 affinity through, for example, intracellular shifts in allosteric modulators. Two natural occurrences of gradual O2 decline are the uplifting events in the Andes and Himalaya mountains, both of which appear to have had effect on the respiratory physiology of resident vertebrates.
Each autumn bar-headed geese (Anser indicus), living on the plains of Siberia, migrate over the Himalaya to winter feeding grounds in India, Pakistan, and Myanmar. Over the span of time that this migration has occurred (40–50 MY), the Himalaya has slowly ascended at a rate that lowers migratory route PO2 by about 2 Torr MY−1 or about 100 Torr total [47]. A higher Hb-O2 affinity (lower P50), which enhances O2 uptake in the lungs, is a general adaptive feature of endotherms living at high altitude, and the bar-headed goose has a higher Hb-O2 affinity than other Anser species that do not fly as high. This affinity difference is due to one amino acid substitution in the α Hb chain [39]. Assuming a general rate of amino acid substitution of 1 per 5 MY (determined for β globin by Ref. [22]), this affinity increase is well within the time frame for selection to have acted on the ancestral clade of the bar-headed goose and to thereby fix a random mutation into a high-altitude adaptation [47], even though exposure to hypoxia on this high altitude migration is abrupt and temporary (bar-headed geese cross the Himalaya in 1 day).
A similar pattern is seen for two altitude dwelling Andean camelids, the vicuña (Vicugna vicugna) and the guanaco (Lama guanicoe), both of which have a left-shifted Hb-O2 dissociation curve relative to other mammals [47]. Ghosh et al. [25] estimated the rate of ascent of the Bolivian Altiplano 7–11 MYA to be about 1 mm per year, which, over a span of about 5 MY, is 5000 m. This altitude shift reduced atmospheric PO2 from 160 to 87 Torr or about 15 Torr MY−1, which is much greater than the estimated rate for the Permian-Triassic O2 reduction. Assuming that the ancestral groups of the vicuňa and guanaco rode along with this geologic transit to higher elevation, there would have been a slowly paced natural selection for life at high altitude which, in addition to a lowered PO2, would have also involved colder temperature, less precipitation, increased ultraviolet light, less productivity leading to fewer ecological resources, and a reduced habitat complexity [35, 52].
Comparative studies of human and other high-altitude dwellers provide insight into the roles of phenotypic plasticity, physiological acclimatization, and genetic change associated with high-altitude colonization [52]. Recent work documents how intense selection for survival at high altitude among human populations in Tibet and in the Andes has induced the increased expression of genes enhancing respiration in low O2 and blunting erythropoietic and pulmonary vasoconstriction responses to hypoxia demonstrated for low land populations placed at altitude. However, the extrapolation of the migration of extant species into hypoxic zones is not the same as the gradual atmospheric shifts experienced by Late Paleozoic organisms [28, 30, 33, 35]. In this regard, one of the key omissions in much of the thinking about the effect of O2 fluctuations on vertebrates has been a lack of distinction between the diffusive and convective aspects of O2 transport. Changes in ambient PO2 affect diffusion and thus ventilation; however, as long as lung PO2 remains above P50, a greater or lower environmental PO2 will not impact convective transport [30, 33, 47]. Thus, attribution of a hyperoxic atmosphere to changes in body size or specialized physiology in vertebrates with fully functional circulatory systems [18] largely ignores the important regulation of O2 delivery by hemoglobin and other factors [29, 30].
5 Examining the Premise: The Case for and Against Atmospheric O2 in Landmark Respiratory Transformations
This section surveys the occurrence of landmark transformations possibly related to respiration and metabolism as suggested by the fossil record and recent investigations. It sets the stage for evaluating the merits of attributing such changes to shifts in atmospheric O2 as opposed to other factors. These considerations are informed by Fig. 27.3, which shows the 1989 Berner and Canfield [5] and 2009 Berner [4] O2 curves in relation to the timing of several significant biological events (numbered), including transformations in the respiratory function of specific groups.
Comparison of the estimated paleoatmospheric O2 levels reported by Berner and Canfield [5] and Berner [4] in relation to the present atmospheric level (PAL). Vertical grey fields indicate the timing of certain events that may have been influenced by changes in atmospheric O2 and specific groups for which “transformative” modifications in respiration, physiology, functionality, or ecological radiation could potentially relate to paleo-oxygen level: (1) Ediacara radiation; (2) Radiation of large predatory fishes; (3) Vertebrate land invasion; (4) Early tetrapod radiation; (5) First synapsids; (6) Insect gigantism and the origin of flight; (7) Mammal-like reptiles; (8) Permian Extinction; (9) Probable origin of synapsid endothermy; (10) Early crocodilians; (11) Dinosaurs (Ornithischia and Saurischia); (12) Early mammals; (13) First birds; (14) Majungasaurus; (15) First lungless salamanders; (16) Icefish. Geologic period and era abbreviations are given in Fig. 27.1
5.1 O2, Ediacara, and the Age of Fishes (Events 1, 2)
Dahl et al. [14] reported analyses of the isotopic composition and the concentration of molybdenum in sedimentary rocks that identify two periods of ocean oxygenation. The first of these corresponds to the Pre-Cambrian (550–560 MYA) appearance of the Ediacaran fauna, which were large, thin, motile, bilaterally symmetrical, and diffusion-limited animals. The second oxygenation event occurred in the Devonian and correlates with both the radiation of vascular plants (which by fixing carbon and emitting O2 contributed to atmosphere oxygenation) and the radiation and increase in body size of large predatory fishes (the Devonian Period is referred to as the “Age of Fishes”). While the Pre-Cambrian ocean oxygenation is not reflected in paleoatmosphere models, the Devonian episode described by Dahl et al. [14] appears to line up with the O2 rise shown in Berner’s [4] latest curve (Figs. 27.2 and 27.3).
Assumptions regarding the benefit of increased O2 to diffusion-limited animals like the Ediacaran fauna are consistent with the basic hypothesis linking O2 to transformative evolutionary events. On the other hand, the extension of the idea of O2 enhancement contributing to an increase in Devonian fish size misses a fundamental point: For a Devonian fish (or any vertebrate) having a functional circulation and effective Hb-O2 binding and transport mechanisms complete with the capacity for HIF-1α modulation of respiratory adaptation, gradual changes in O2 over millions of years would arguably contribute less to increases in body size and respiration than would the myriad of ecological changes afforded by increased productivity (more O2), such as opening new niches in which there would be selection for increased swimming speed and body size.
5.2 Vertebrate Land Invasion and the Early Tetrapods (Events 3, 4)
Vertebrates evolved in water, and the first air-breathing vertebrates were fishes living in lowland environments subject to drought and hypoxia [27]. A critical lowering of aquatic oxygenation in shallow water habitats is believed to have been the main driving force for the independent origin of air breathing in many Devonian fishes [12]. The occupation of land by vertebrates was initiated by a lineage of the lobe-finned (Sarcopterygii) osteichthyan fishes, the tetrapod omorphs, which came ashore during the Middle Devonian (370 MYA) and became the ancestral group of all tetrapods [10, 28, 50]. With respect to the question of how atmospheric O2 may have related to the land invasion, both atmospheric hypoxia (a Middle Devonian nadir) and an O2 rise throughout the Middle to Late Devonian (Figs. 27.1, 27.2, and 27.3) have been invoked to explain it [10, 12, 30].
Paralleling these events, the 250 MY history of fish evolution since the Paleozoic has seen the persistence of other air-breathing fish taxa as well as the independent origin of air breathing among many fish groups, novel air-breathing organs distinct from the lung, and amphibious behavior [27, 28]. The extant air-breathing fishes present proxy models for the Paleozoic evolution of vertebrate aerial respiration and terrestriality [31, 40, 41, 45]. Particularly relevant are the mudskippers (family Gobiidae, subfamily Oxudercinae), a modern teleost group that appeared in the early to mid Tertiary (about 40–50 MYA), in which the characters of air breathing and terrestriality are well developed. Mudskippers live on mudflats and can respire aerially and aquatically, although species in the genus Periophthalmodon are obligate air breathers and will drown if denied air access [38, 53]. They engage in complex air management behaviors in order to brood their eggs in an air phase in hypoxic mud burrows [36, 37]. Recent work has shown that mudskipper aerial respiration and exercise capacity are limited by hypoxia and enhanced in hyperoxia [40]. Their capacity to repay an exercise-induced O2 debt is also enhanced in a hyperoxic atmosphere [40].
Based on these findings, global atmospheric hyperoxia likely aided the vertebrate invasion of land in several ways. Breathing hyperoxic air reduced the ratio of evaporative water loss to O2 uptake thus lowering desiccation. Hyperoxia also increased lung efficiency. (Early tetrapod s had both gills and lungs, but gills are not effective for aerial gas exchange, particularly CO2 discharge, which, because of its implications for acid–base balance, is a more acute constraint for prolonged aerial respiration.) Early tetrapods were heavily dependent upon the lungs because their large size and thick body armor limited their capacity for aerial cutaneous respiration. The rise in atmospheric O2 coupled with a reduction in CO2 would have elevated primitive lung effectiveness. Subsequent changes in these gas ratios could have influenced the transition from ventilation by buccal force pumping to aspiration and also led to a greater role of CO2 in the control of breathing. A reduced ratio of water loss to O2 uptake possible in hyperoxic air may have also been an important factor in the origin of the cleidoic egg. Finally, hyperoxia would have also enhanced tetrapod metabolic capacity and thus contributed to the sustained power production required to overcome gravity. As a result, increased metabolic performance expanded ecological options within the rapidly expanding terrestrial biosphere.
5.3 Gigantism and the Origin of Insect Flight (Event 6)
The Carboniferous and Permian saw the rise of gigantism in protodont dragonflies, such as Meganeura (wing span up to 71 cm, thorax diameter 2.8 cm), and other insect groups including Ephemeroptera (mayflies), Palaeodictyoptera, Diplura (bristletails), and Thysanura (silverfish) [11, 33]. Gigantism also occurred in other Carboniferous arthropods (millipedes and scorpions), the extant relatives of which are diffusion-dependent for respiration [30]. Tests to model Carboniferous and Permian O2 atmosphere effects on insect body size have been done by rearing Drosophila in hypoxia and normoxia over several generations [42]. Such studies (reviewed by Ref. [33]) demonstrate that Drosophila and most other insects are smaller when reared in hypoxia, while in hyperoxia some develop and evolve larger sizes. In addition, insects reared in higher PO2 developmentally and evolutionarily reduce their proportional investment in the tracheal system, suggesting that there are significant costs associated with its structure and function. Thus, the hyperoxic atmosphere of the Late Paleozoic was likely beneficial in both overcoming diffusion limitation and relaxing investment in the energetically expensive and elaborate tracheal system.
Flight is an essential mechanism for insect dispersal and diversification. The earliest proto-wings had a possible respiratory function and were used for locomotion and predator escape. Some insects evolved flight in the Devonian, but it was likely a hyperoxic and a more dense Carboniferous atmosphere that aided the origin of flight in many groups by increasing the lift generated by small winglets [15, 16, 30]. The need to power flight required sustained high levels of oxidative metabolism which would have been facilitated by a hyperoxic atmosphere. Recent work [11] has shown that fossil insect wing length parallels estimates of atmospheric O2 , concentration throughout the Carboniferous and Permian when O2 levels were at their highest and then through the Triassic which saw a decrease in both atmospheric O2 and insect wing length (large flying insects did not survive beyond the Permian when 27–30 % of the known insect orders went extinct). However, following the Jurassic, the correlation of insect wing length and atmospheric O2 decoupled as biotic factors (e.g., the rise of insect-eating birds and the advent of vertebrate flight) are hypothesized to have applied selective pressures that reduced flying insect size, even in the wake of rising O2 concentrations during the Cretaceous [11].
5.4 The Permian Extinction (Event 8)
About 80 % of marine and terrestrial species were lost in the Permian Extinction. Included in this loss were 75 % of the tetrapods (amphibians and amniotes). Causal factors suggested for the Permian Extinction include the formation of the Pangea supercontinent and the corresponding changes in habitat surface area, primary production and glaciation, as well as extreme volcanism associated with formation of the Siberian Steps [17]. Also occurring was a sharp drop in global atmospheric O2, which is registered in each of the geochemical models except that of Bergman et al. [2] (Figs. 27.1, 27.2, and 27.3). Model-indicated values for hypoxia at this time range between 0.2 and 0.9 PAL. This fall in global O2 would have been restrictive and may have modified or eliminated some taxa that had radiated in the preceding period of hyperoxia ; however, given the slow rate of change in O2, these levels of hypoxia cannot be regarded as the primary cause of the Permian Extinction [30].
5.5 Synapsids , Mammal-Like Reptiles , Endothermy , and Early Mammals (Events 5, 7, 9, 12)
Tetrapod diversification began in the Devonian and accelerated in the Carboniferous when at least 11 of 16 known basal lineages, including the three primary groups of amniotes, the synapsids (ancestral group of mammals), diapsids (ancestral group of reptiles and birds), and anapsids, all appeared. While tetrapod diversity decreased in the late Permian, the synapsids underwent a pronounced diversification from the pelycosaurs to the therapsids, a diverse assemblage of herbivores and carnivores known as the mammal-like reptiles [8]. Synapsid success has been partially attributed to the effects of hyperoxia and to a denser atmosphere, which enhanced specializations such as metabolic heat retention, an important precursor for endothermy that was thought to be present in certain groups [29, 30]. Although the mammal-like reptiles flourished in the Late Permian and Early Triassic, they were later outcompeted by the diapsids.
5.6 Diapsids : Crocodiles, Dinosaurs, and Birds (Events 10, 11, 13, 14)
The Middle Triassic saw the diapsids become the dominant terrestrial vertebrates. Appearing at this time were the archosaurian reptiles, a group that includes crocodiles, dinosaurs, and birds. This group holds the interest of comparative biologists for the possible influence of paleoatmospheric O2 in the development of respiratory and metabolic physiology [44, 57]. It is now known that the highly specialized respiratory system of birds, which features fixed-volume lungs that have a unidirectional airflow driven by bellow-like air sacs, is a shared feature with dinosaurs such as the late Cretaceous Majungasaurus (= Majungatholus) [43], and that unidirectional pulmonary airflow is also found in crocodilians [19]. Another structural similarity for dinosaurs and birds is that the air sacs extend into the skeleton where they form air pockets that are presumed to lessen body mass [58].
The bird pulmonary system is essential for the maintenance of stable flight and is thought to be closely tied to this group’s high metabolism and endothermy. Birds did not appear until the Jurassic; however, the presence of a structurally similar lung in dinosaurs and unidirectional pulmonary airflow in crocodilians [19] suggest that the metabolic physiology of birds may be synapomorphic within the archosaur lineage. Although the crocodiles differ from birds by lacking air sacs and ventilating using a hepatic piston [19], these differences may reflect the secondary requirements for an aquatic existence such as apnea and buoyancy regulation. Fossils suggest that many of the early archosaurs including ancestral crocodilians were highly active and some were bipedal [7, 8]. Moreover, the presence of a four chamber heart in crocodiles and birds implies that this too was a synapomorphy for the group, and, by extension, higher metabolic rates, and possibly endothermy, were within the repertoire of the early archosaurs. Finally, the unidirectional cross flow of blood in the bird lung is highly effective for respiration in hypoxia, and this suggests that a similar lung in the early archosaurs could have enabled their survival, radiation, and competitive dominance over other groups in the relatively low O2 regime of the Mesozoic (Fig. 27.3). However, the recent discoveries of unidirectional pulmonary airflow in both monitor lizards and iguanas (lepidosaurian reptiles) calls into question such hypotheses and suggests this ventilatory mechanism may have evolved in early diapsids before the archosaur and lepidosaur lineages split [9, 48].
5.7 Lungless Amphibians (Event 15)
For amphibians, but no other tetrapods, the lungs appear to be an expendable commodity that can be traded for other life-history advantages. Loss of this organ has occurred independently at least three times in amphibians. The majority of salamanders are lungless, including all of the nearly 380 species (9 genera) in the family Plethodontidae. Lunglessness also occurs in two other salamanders (both in the genus Onychodactylus, family Hynobiidae), a South American caecilian (Atretochoana, Typhlonectidae), and in an Indonesian frog (Barbourula, Bombinatoridae). Although Barbourula is small and has a favorable surface area to volume ratio for cutaneous gas exchange, Atretochoana is one of the largest caecilians and is the largest lungless amphibian.
In some groups, lunglessness is accompanied by the complete loss of the pulmonary circulation and closure of the nasal choanae. Lunglessness is possible for amphibians because of their low metabolic rates and their well-developed capacity for cutaneous respiration. Because respiratory capacity defines metabolic scope and the potential for life-history specialization, lunglessness implies the presence of a complex of selective trade-offs between buoyancy and metabolic performance and feeding mechanisms [20, 21]. The lungless frog and caecilian, as well as many plethodontids, occur in flowing highland streams where cool oxygenated water optimizes cutaneous O2 uptake, while the absence of a lung lessens buoyancy, which optimizes locomotion and reduces the risk of being swept downstream by strong flow. Plethodontids, however, are highly diverse and some are terrestrial. This means that even if initial selection for the loss of lungs was rooted in buoyancy compensation and cutaneous respiration, this limitation for aerobic metabolism has not precluded this group’s radiation into ecological niches where life without a lung remains manageable. In all lungless amphibians, elimination of the buccal chamber’s involvement in breathing has led to altered feeding mechanisms and largely precluded vocalization. The origin of the Plethodontidae in the Early Tertiary (some estimates put this in the Late Cretaceous, Ref. [56]) and this group’s radiation in the Early to Middle Tertiary probably encompasses the timing of lung loss in other lungless amphibians, implying that this feature evolved in O2 levels at or near PAL (Fig. 27.3). This argues that biological forcing functions, and not a relaxation of respiratory drive attributable to elevated O2, were the agents for amphibian lung loss.
5.8 Antarctic Icefish (Event 16)
Icefish (Channichthyidae) live in extremely cold (+1.5 to −1.9 °C) waters of the Antarctic continental shelf and are the only adult vertebrates without Hb or red cells [51]. Channichthyids are one of eight families in the perciform suborder Notothenioidei, which is the dominant fish group in Antarctic waters. The loss of Hb in icefish occurred at the time (2–5.5 MYA) of the group’s separation from other Antarctic notothenioids, which have hematocrits and mean corpuscular Hb concentrations that are comparable to those of other fish species inhabiting cold water [51]. All of the 11 genera in the Channichthyidae lack the β globin gene and only have remnants of the gene for α globin. Of the 16 species in this family, none express myoglobin (Mb) in their striated muscle, and only ten have Mb in their cardiac myocytes [51].
Icefish demonstrate that the loss of seemingly vital components of the respiratory system can occur in the absence of pronounced changes in atmospheric O2 (Fig. 27.3). The absence of these proteins in icefish is not, as initially hypothesized, an adaptation to cold, oxygenated water. Rather, the loss of Hb and Mb are mutations that would not support physiological function in warmer temperatures or in a habitat having much greater levels of predatory pressure and interspecific competition. Sidell and O’Brien [51] describe Hb loss in this group as a “naturally occurring genetic knockout,” noting that the subsequent loss of cardiac Mb within some species is the result of four independent mutational events.
Blood lacking Hb transports less than 10 % of the O2 carried in blood having red cells with Hb. It was initially thought that the higher solubility of O2 in extremely cold plasma compensated for the loss of red cells and provided physiological advantage by reducing blood viscosity. However, the work of Hemmingsen [34] and colleagues and others demonstrates that the heart function of icefish has undergone adaptations that compensate for the lack of Hb. Relative to other notothenioids, channichthyid hearts are larger, have a larger mass-specific cardiac output, and the cost of their cardiac work for circulation is much greater (22 % of total VO2). Icefish also have a fourfold greater blood volume and larger capillary diameters which allows a larger, faster, and lower-pressure blood flow through tissues. The absence of Hb and Mb in striated and cardiac muscle also limits the nitric oxide (NO)-oxygenase activities which may have accelerated the acquisition of morphological changes such as increased tissue vascularization, capillary diameters, and the mitochondrial densities needed to compensate for hypoxia.
6 Conclusions
In the time since the publication of the Berner and Canfield [5] paleoatmospheric model and the first synthesis of its implications for life in the Phanerozoic [30], over 50 contributions have provided new ideas and suggested specific ways that changes in atmospheric O2 could have exercised signature effects on respiration, metabolism, and biodiversity. The main focus of the Graham et al. [30] hypothesis was the correlation between changes in Late Paleozoic (Devonian, Carboniferous, Permian) O2 levels and transformative changes in the pattern of life that took place at that time, particularly in organisms having respiratory systems dependent upon the diffusive flux of O2 for respiration. However, many more recent formulations of the “O2 correlation” have extended into regions of the Phanerozoic where the paleoatmosphere O2 is less well known by modelers, and to a range of biological processes, from the formation of shells, segments, and body shapes and sizes in various phyla, to behavioral and ecological changes. While all of these can be loosely correlated to O2, implying a cause-effect relationship is simplistic. Critically important in many of these newer contributions is an apparent lack of understanding of how the presence of a circulatory system, replete with an effective O2 binding respiratory pigment, would obviate slight changes in atmospheric O2 over geologic time. Simply stated, the O2 affinity of a respiratory pigment is set at a level that maximizes O2 loading at partial pressures far less than atmospheric, and modulation of this affinity in response to changes in atmospheric O2 over geologic timescales is a reasonable expectation. This paper verifies the “O2 correlation” by stressing the importance and clear relevance of O2 access to the radiation of certain groups such as the diffusion-dependent Ediacaran fauna and the giant Carboniferous arthropods, and the development of innovative functional changes such as insect flight, vertebrate terrestrial locomotion, and the cleidoic egg. However, it also describes cases in which there have been large-scale changes in vertebrate respiratory and circulatory systems (lungless amphibians and Hb-lacking icefish) in the absence of marked changes in atmospheric O2, thus demonstrating that the composite effects of habitat and life history can both select for and sustain major changes in organismal physiology. Thus, when attempting to understand the causation of transformative evolutionary events, it is essential to consider the interplay of potentially numerous abiotic and biotic factors.
References
Azad P, Haddad GG. Survival in acute and severe low O2 environment: use of a genetic model system. Ann N Y Acad Sci. 2009;1177:39–47.
Bergman NM, Lenton TM, Watson AJ. COPSE: a new model of biogeochemical cycling over Phanerozoic time. Am J Sci. 2004;304:397–437.
Berkner LV, Marshall LC. On the origin and rise of oxygen concentration in the earth’s atmosphere. J Atmos Sci. 1965;22:225–61.
Berner RA. Phanerozoic atmospheric oxygen: new results using the GEOCARBSULF model. Am J Sci. 2009;309:603–6.
Berner RA, Canfield DE. A new model of atmospheric oxygen over Phanerozoic time. Am J Sci. 1989;289:333–61.
Berner RA, VandenBrooks JM, Ward PD. Oxygen and evolution. Science. 2007;316:557–8.
Carrier DR, Farmer CG. The evolution of pelvic aspiration in archosaurs. Paleobiology. 2000;26:271–93.
Carroll RL. Vertebrate paleontology and evolution. New York, NY: WH Freeman and Company; 1988. p. 698.
Cieri RL, Craven BA, Schachner ER, Farmer CG. New insight into the evolution of the vertebrate respiratory system and the discovery of unidirectional airflow in iguana lungs. Proc Natl Acad Sci U S A. 2014;111:17218–23.
Clack JA. Gaining ground: the origin and evolution of tetrapods. Bloomington, IN: Indiana University Press; 2002. p. 369.
Clapham ME, Karr JA. Environmental and biotic controls on the evolutionary history of insect body size. Proc Natl Acad Sci U S A. 2012;109:10927–30.
Clement AM, Long JA. Air-breathing adaptation in a marine Devonian lungfish. Biol Lett. 2010;6:509–12.
Cloud P. The biosphere. Sci Am. 1983;249:176–89.
Dahl TW, Hammarlund EU, Anbar AD, Bond DPG, Gill BC, Gordon GW, Knoll AH, Nielsen AT, Schovsbo NH, Canfield DE. Devonian rise in atmospheric oxygen correlated to the radiations of terrestrial plants and large predatory fish. Proc Natl Acad Sci U S A. 2010;107:17911–5.
Dudley R. Atmospheric oxygen, giant Paleozoic insects and the evolution of aerial locomotor performance. J Exp Biol. 1998;201:1043–50.
Dudley R. The evolutionary physiology of animal flight: paleobiological and present perspectives. Annu Rev Physiol. 2000;62:135–55.
Erwin DH. The great Paleozoic crisis: life and death in the Permian. New York, NY: Columbia University Press; 1993. p. 327.
Falkowski PG, Katz ME, Milligan AJ, Fennel K, Cramer BS, Aubry MP, Berner RA, Novacek MJ, Zapol WM. The rise of oxygen over the past 205 million years and the evolution of large placental mammals. Science. 2005;309:2202–4.
Farmer CG, Sanders K. Unidirectional airflow in the lungs of alligators. Science. 2010;327:338–40.
Feder ME. Integrating the ecology and physiology of plethodontid salamanders. Herpetologica. 1983;39:291–310.
Feder ME, Olsen LE. Behavioral and physiological correlates of recovery from exhaustion in the lungless salamander Batrachoseps attenuatus (Amphibia: Plethodontidae). J Comp Physiol. 1978;128:101–7.
Fitch WM, Langley CH. Protein evolution and molecular clock. Fed Proc. 1976;35:2092–7.
Flück M, Webster KA, Graham J, Giomi F, Gerlach F, Schmitz A. Coping with cyclic oxygen availability: evolutionary aspects. Integr Comp Biol. 2007;47:324–31.
Frazier MR, Woods HA, Harrison JF. Interactive effects of rearing temperature and oxygen on the development of Drosophila melanogaster. Physiol Biochem Zool. 2001;74:641–50.
Ghosh P, Garzione CN, Eiler JM. Rapid uplift of the Altiplano revealed through 13C-18O bonds in paleosol carbonates. Science. 2006;311:511–5.
Graham JB. Ecological and evolutionary aspects of integumentary respiration: body size, diffusion, and the invertebrate. Am Zool. 1988;28:1031–45.
Graham JB. Air-breathing fishes: evolution, diversity, and adaptation. San Diego, CA: Academic; 1997. p. 299.
Graham JB, Wegner NC. Breathing air in water and in air: the air-breathing fishes. In: Nilsson GE, editor. Respiratory physiology of vertebrates: life with and without oxygen. Cambridge: Cambridge University Press; 2010. p. 174–221.
Graham JB, Aguilar N, Dudley R, Gans C. The late Paleozoic atmosphere and the ecological and evolutionary physiology of tetrapods. In: Sumida SS, Martin KLM, editors. Amniote origins. San Diego, CA: Academic; 1997. p. 141–68.
Graham JB, Dudley R, Aguilar NM, Gans C. Implications of the late Palaeozoic oxygen pulse for physiology and evolution. Nature. 1995;375:117–20.
Graham JB, Wegner NC, Miller LA, Jew CJ, Lai NC, Berquist RM, Frank LR, Long JA. Spiracular air breathing in polypterid fishes and its implications for aerial respiration in stem tetrapods. Nat Commun. 2014;5:3022. doi:10.1038/ncomms4022.
Harrison JF, Lighton JRB. Oxygen-sensitive flight metabolism in the dragonfly Erythemis simplicicollis. J Exp Biol. 1998;201:1739–44.
Harrison JF, Kaiser A, VandenBrooks JM. Atmospheric oxygen level and the evolution of insect body size. Proc R Soc Lond B Biol Sci. 2010;277:1937–46.
Hemmingsen EA. Respiratory and cardiovascular adaptation in hemoglobin-free fish: resolved and unresolved problems. In: di Prisco G, Maresca B, Tota B, editors. Biology of Antarctic fish. New York, NY: Springer; 1991. p. 191–203.
Huey RB, Ward PD. Hypoxia, global warming, and terrestrial late Permian extinctions. Science. 2005;308:398–401.
Ishimatsu A, Graham JB. Roles of environmental cues for embryonic incubation and hatching in mudskippers. Integr Comp Biol. 2011;51:38–48.
Ishimatsu A, Hishida Y, Takita YT, Kanda T, Oikawa S, Takeda T, Huat KK. Mudskippers store air in their burrows. Nature. 1998;391:237–8.
Ishimatsu A, Aguilar NM, Ogawa K, Hishida Y, Takeda T, Oikawa S, Kanda T, Huat KK. Arterial blood gas levels and cardiovascular function during varying environmental conditions in a mudskipper, Periophthalmodon schlosseri. J Exp Biol. 1999;202:1753–62.
Jessen TH, Weber RE, Fermi G, Tame J, Braunitzer G. Adaptation of bird hemoglobins to high altitudes: demonstration of molecular mechanisms by protein engineering. Proc Natl Acad Sci. 1991;88:6519–22.
Jew CJ, Wegner NC, Yanagitsuru Y, Tresguerres M, Graham JB. Atmospheric oxygen levels affect mudskipper terrestrial performance: implications for early tetrapods. Integr Comp Biol. 2013;53:248–57.
Kawano SM, Blob RW. Propulsive forces of mudskipper fins and salamander limbs during terrestrial locomotion: implications for the invasion of land. Integr Comp Biol. 2013;53:283–94.
Klok CJ, Harrison JF. Atmospheric hypoxia limits selection for large body size in insects. PLoS One. 2009;4:e3876.
O’Connor PM, Claessens LPAM. Basic pulmonary design and flow-through ventilation in non-avian theropod dinosaurs. Nature. 2005;436:253–6.
Owerkowicz T, Elsey RM, Hicks JW. Atmospheric oxygen level affects growth trajectory, cardiopulmonary allometry and metabolic rate in the American alligator (Alligator mississippiensis). J Exp Biol. 2009;212:1237–47.
Pierce SE, Hutchinson JR, Clack JA. Historical perspectives on the evolution of tetrapodomorph movement. Integr Comp Biol. 2013;53:209–23.
Porteus C, Hedrick MS, Hicks JW, Wang T, Milsom WK. Time domains of the hypoxic ventilatory response in ectothermic vertebrates. J Comp Physiol B. 2011;181:311–33.
Powell FL. Studying biological responses to global change in atmospheric oxygen. Respir Physiol Neurobiol. 2010;173S:S6–12.
Schachner ER, Cieri RL, Butler JP, Farmer CG. Unidirectional pulmonary airflow patterns in the savannah monitor lizard. Nature. 2014;506:367–70.
Schidlowski M. Probleme der atmosphärischen Evolution im Präkambrium. Geol Rundsch. 1971;60:1351–84.
Shubin N. Your inner fish: a journey into the 35-billion-year history of the human body. New York, NY: Random House; 2008. p. 229.
Sidell BD, O’Brien KM. When bad things happen to good fish: the loss of hemoglobin and myoglobin expression in Antarctic icefishes. J Exp Biol. 2006;209:1791–802.
Storz JF, Runck AM, Moriyama H, Weber RE, Fago A. Genetic differences in hemoglobin function between highland and lowland deer mice. J Exp Biol. 2010;213:2565–74.
Takeda T, Ishimatsu A, Oikawa S, Kanda T, Hishida Y, Khoo KH. Mudskipper Periophthalmodon schlosseri can repay oxygen debts in air but not in water. J Exp Zool. 1999;284:265–70.
Van Voorhies WA. Metabolic function in Drosophila melanogaster in response to hypoxia and 100% oxygen. J Exp Biol. 2009;212:3132–41.
Van Voorhies WA, Ward S. The response of the nematode Caenorhabditis elegans to varied oxygen tensions. J Exp Biol. 2000;203:2467–78.
Vieites DR, Min M-S, Wake DB. Rapid diversification and dispersal during periods of global warming by plethodontid salamanders. Proc Natl Acad Sci U S A. 2007;104:19903–7.
Ward PD. Out of thin air: dinosaurs, birds, and Earth’s early atmosphere. Washington, DC: John Henry Press; 2006. p. 282.
Wedel MJ. Origin of postcranial skeletal pneumaticity in dinosaurs. Integr Zool. 2006;2:80–5.
Acknowledgments
The authors thank W. Milsom, J. Harrison, and R. Roach for their comments on this manuscript and their help with its presentation at the 2011 International Hypoxia Symposium. N.C. Wegner and C.J. Jew were supported by NSF grants IOS-0922569 and IOS-0817774 during the writing of this paper.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2016 Springer Science+Business Media New York
About this chapter
Cite this chapter
Graham, J.B., Jew, C.J., Wegner, N.C. (2016). Modeling Variable Phanerozoic Oxygen Effects on Physiology and Evolution. In: Roach, R., Hackett, P., Wagner, P. (eds) Hypoxia. Advances in Experimental Medicine and Biology, vol 903. Springer, Boston, MA. https://doi.org/10.1007/978-1-4899-7678-9_27
Download citation
DOI: https://doi.org/10.1007/978-1-4899-7678-9_27
Published:
Publisher Name: Springer, Boston, MA
Print ISBN: 978-1-4899-7676-5
Online ISBN: 978-1-4899-7678-9
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)