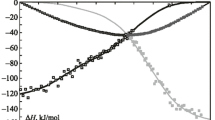
The mixing enthalpies of liquid binary Eu–In alloys (0 < x In < 0.66, 0.78 < x In < 1) are determined by isoperibol calorimetry at 1170–1300 K. The thermodynamic properties of the liquid Eu–In alloys are described in the entire composition range using the model of ideal associated solution. The thermodynamic activities of components in the Eu–In melts demonstrate negative deviations from the ideal behavior, and the mixing enthalpies are characterized by significant exothermic effects. The minimum value of the mixing enthalpy is −35.1 ± 0.5 kJ/mol at x In = 0.52 (T = 1300 K) and −41.2 ± ± 0.5 kJ/mol at x In = 0.50 (T = 1170 K).
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Indium and indium-containing alloys are applied in aircraft and automotive industries as corrosion-resistant coatings, bearing lubricants, and mirrors with high reflectivity, as well as in semiconductor technology, electronics, electrical engineering, nuclear power, tool industry, chemical engineering (as alloys resistant to alkaline corrosion), glass industry, etc. [1, 2]. Recent improvements in the methods to produce and purify indium undertaken to satisfy demands of modern semiconductor technology promote the application of this metal, including that as a component of various alloys. Although literature data on the physicochemical parameters of interaction between indium and most rare metals (for example, lanthanides) are still scarce, we assume that they would be increasingly needed for modern science. In particular, the respective phase diagrams indicate that lanthanides and indium form many intermetallics that are often more refractory than the associated pure components. Along with data on the formation enthalpies for these intermetallics (commonly rich in indium), this is indicative of intensive interaction in In–Ln alloys, other systems of lanthanides with p-metals being no exception. These data are not sufficient from a fundamental or applied point of view. They should be obtained over wide composition and temperature ranges, and dependences of interactions in the In–Ln systems on the nature of Ln should be determined. Hence, europium and ytterbium, compared to other lanthanides, show abnormalities in interaction with many elements. Unfortunately, there is also a lack of experimental data for these elements, which is also due to their volatility at high temperatures.
The Eu–In alloys are studied by calorimetry at 1300 K in [3]. It is established that \( \varDelta {\overline{H}}_{\mathrm{In}}^{\infty } \) = −100 kJ/mol (though \( \varDelta {\overline{H}}_{\mathrm{In}} \) = −111 kJ/mol at x In = 0.11), \( \varDelta {\overline{H}}_{\mathrm{Eu}}^{\infty } \) = −160 kJ/mol, and ΔH min = −41.5 kJ/mol at x In = 0.53. Hence, there is a noticeable asymmetry of the mixing enthalpies toward indium. Based on measurement of the thermodynamic properties of heterogeneous EuIn2 (sol.)–Eu–In (liq.) alloys at 743–1023 K, it is determined in [4] that \( \varDelta {\overline{H}}_{\mathrm{Eu}} \) = −102.3 kJ/mol at x Eu ≈ 0.1, being close to \( \varDelta {\overline{H}}_{\mathrm{Eu}}^{\infty }. \) According to [4], the solubility of Eu in liquid In has temperature dependence lg(x Eu) = 0.88–3790/T and constitutes 0.046% at 900 K, 0.123% at 1000 K, and 0.368% (the value reported in [4]) or 0.272% (calculated from temperature dependence) at 1100 K. This solubility is very low for such high temperatures and does not agree with the phase diagram. However, the handbook [5] refers to [4] to state that \( \varDelta {\overline{H}}_{\mathrm{Eu}} \) = −152.6 kJ/mol (instead of 102.3 kJ/mol) at x Eu ≈ 0.1, and the solubility of Eu in liquid In at 800, 900, and 1000 K equals 7.4, 10.4, and 14.4% according to lg(x Eu) = 0.319–1160/T. There was probably a mistake in [4] which was then corrected, and [5] presented the corrected values. The results reported in [3, 4] are not in adequate agreement, especially if we take into account the typical pattern of decrease in exothermic effects during high-temperature formation of the melts.
A complete thermodynamic assessment of the Eu–In alloys, employing the phase diagram, is still to be conducted. Hence, study of the Eu–In alloys is significant. We examined the alloys by melt calorimetry and then tried to model a series of thermodynamic properties, both obtained experimentally and based on literature data.
Our objective is to study the mixing enthalpies of Eu–In melts over a wide composition range by calorimetry and model a series of thermodynamic properties of liquid and solid Eu–In alloys.
Experimental Procedure
The experiment was performed using an isoperibol calorimeter. The experimental procedure is described in [6]. Indium (99.99%) and europium (99.9%) were used in the experiment. Indium samples weighing 0.012–0.03 g and europium samples weighing 0.014–0.033 g in solid state at T = 298 K were introduced into the melt in a crucible made of molybdenum, inert to both components.
Europium is rather a volatile metal, which is very undesirable: first, its vapors interact with some parts of the calorimeter, so the latter can break down; second, the amount of metal in the crucible decreases with time and the exact amount of this decrease at a certain moment cannot be determined. In this regard, we tried to perform all the experiments at the lowest possible temperature when the alloy was still liquid. In particular, the alloys in the range 0 < x In < 0.34 were examined at 1170 K, those with 0.34 < x In < 0.55 at 1240 K, and those with 0.55 < x In < < 0.66 and 0.78 < x In < 1 at 1300 K. The calorimeter was calibrated against six or seven samples of the pure metal that was put into the crucible and against molybdenum samples 0.017–0.036 g in weight. To calculate the thermal effects that accompanied the dissolution of samples, the Tian equation was applied:
where ΔH T298 is the enthalpy of heating 1 mol of addition from 298 to 1300 K [7]; K is the calorimeter constant; n i is the amount of addition (mol); τ ∞ is time for temperature relaxation in recording the heat-exchange figure; T – T 0 = = ΔT is the difference in temperatures of the crucible with melt and the calorimeter’s isothermal shell; and t is time.
The partial mixing enthalpies of one component were used to calculate the same parameters for the other component by integrating the Gibbs–Duhem equation and then finding the integral values. We combined the found data to obtain the partial and integral mixing enthalpies for Eu–In melts over the entire composition range (Fig. 1).
We processed the experimental and literature data with the software package that we developed employing the model of ideal associated solution (IAS). We used this technique previously to process the data of calorimetric analysis of melts and literature data on phase equilibria and thermodynamic properties of different binary systems, particularly those containing europium [8]. We introduced all available experimental data, as well as a list of compounds in solid alloys (according to the phase diagram) and associates in melts, into the software package.
Arbitrary initial values were assigned to the enthalpies and entropies for forming these compounds from pure components in solid and liquid alloys, which became variable parameters during optimization of the software. If the list of associates is set correctly and the literature data are consistent, then these parameters satisfactorily agree with all experimental data.
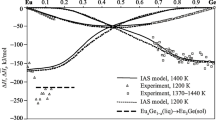
For calculation, we selected four associates corresponding in composition to Eu–In intermetallics (though this correspondence is not required in the general case). It turned out that the amount and composition of the associates were necessary and sufficient to describe thermodynamic data within the experimental error. The calculations were performed for 1300 K, at which the Eu–In alloys are liquid over the entire composition range. The IAS model allows other thermodynamic properties of these alloys to be calculated, such as activities of components and molar contents of associates (Fig. 2), temperature dependences of mixing enthalpies (Fig. 3), liquidus curve (Fig. 4), and mixing entropies and Gibbs energies at any temperatures. The optimized parameters of the IAS model are provided in Table 1.
Liquidus and solidus lines (lines) in the Eu–In phase diagram based on our calculation with the IAS model versus literature data [10]
The fact that integral mixing enthalpies of the melts measured on the sides of two components in the binary Eu–In system somewhat disagree (even considering the small difference between experimental temperatures) may be attributed to oxide impurities in the samples, which could not be eliminated. Thus, oxygen adsorbed on the indium sample can increase exothermic effects when this sample gets into the crucible with Eu because the formation of Eu oxide is very thermodynamically favored. On the contrary, Eu2O3 that covers the surface of Eu samples decreases the exothermic effect from interaction with indium in the crucible, resulting from heat loss in heating this oxide to the crucible temperature.
To ensure agreement between the mixing enthalpies of melts measured from the sides of Eu and In, the computational procedure can be modified. A large compact Eu sample in a crucible oxidized only slightly prior to the beginning of experiments on the Eu side because of a small surface-to-volume ratio. It can be expected that the mixing enthalpies on the Eu side are more reliable. At the same time, small Eu samples added to the crucible with In oxidized noticeably. The Eu2O3 weight content of Eu samples can be assessed using the enthalpy of heating Eu2O3 from 298 K to experimental temperature (1300 K) [9]. The data [9] were fitted to a polynomial to obtain C p(Eu2O3) = 133.31 + 0.01856T – 1,258,530/T 2, J/(mol · K); ΔH T298 (Eu2O3) = − 44791 + 133.306T + 0.00928T 2 + + 1,258,530/T, J/mol. Thus, ΔH 1300298 (Eu2O3) = 145.16 kJ/mol. The thermal effect in dropping the sample into the crucible consists in heating Eu and Eu2O3 to experimental temperature and Eu mixing with the melt (containing mostly In). Oxide Eu2O3 can be considered an inert impurity. Hence,
where m is the weight of the sample in which the contents of Eu and Eu2O3 constitute 1 – x and x, respectively; M Eu and \( {M}_{{\mathrm{Eu}}_2{\mathrm{O}}_3} \) are the molar masses of Eu and Eu2O3. Then we can choose the value of x at which \( \varDelta {\overline{H}}_{\mathrm{Eu}} \) and ΔH agree best with the values obtained by extrapolation of the ΔH curve on the Eu side. It turned out that this was reached at x = 0.065.
The experimental error is due to several factors, mainly includingFootnote 1: 1) inaccurate proportionality of the area under peaks of the heat pattern \( \left({\displaystyle \underset{0}{\overset{\tau_{\infty }}{\int }}\left(T-{T}_0\right)dt}\right) \) and true thermal effects in dissolution of the samples, since the proportionality factor somewhat depends on the place where the sample drops into the crucible and on fluctuation in concentrations of components in the crucible; 2) insufficient chemical purity of the samples, especially oxygen and oxide impurities; 3) error in determining the area under peaks of the heat pattern; 4) error in weighing the samples; 5) inaccurate measurement of temperature in the crucible; 6) registration of various noises by calorimeter instrumentation, causing the distortion of peaks in the heat pattern.
Favorably, factors 1, 3, 4, and 6 do not cause systematic errors, so their overall impact can be assessed from the scatter of experimental data points (first of all, partial mixing enthalpies of components) relative to the smooth fitting curve. In particular, the standard deviation of data points from the curve for the IAS model is \( \sigma \left(\varDelta {\overline{H}}_{\mathrm{In}}\right) \) = 3.8 and \( \sigma \left(\varDelta {\overline{H}}_{\mathrm{Eu}}\right) \) = 12.0 kJ/mol, which was used for the calculation taking into account the Student’s test for random contributors to the error: \( {\updelta}_{\mathrm{rand}}\left(\varDelta {\overline{H}}_{\mathrm{In}}\right) \) = 0.9 kJ/mol and \( {\delta}_{\mathrm{rand}}\left(\varDelta {\overline{H}}_{\mathrm{Eu}}\right) \) = 4.1 kJ/mol. The following standard deviations correspond to two sides of integral mixing enthalpies of the Eu–In alloys: σ(ΔH)1 = 0.27 kJ/mol (0 < x In < 0.66) and σ(ΔH)2 = 0.42 kJ/mol (0.78 < x In < 1). The impact of random errors greatly decreases because the accumulated effects are averaged in calculation of the integral enthalpies from partial ones.
The magnitude of deviation of ΔH and \( \varDelta {\overline{H}}_i \) points for each side can be regarded as the lowest estimate since these integral and partial functions are mathematically interrelated but not to the extent to which the data points on a given side deviate from the fitting curve because of systematic errors. The average values of these deviations are (kJ/mol) 0.2 for \( \varDelta {\overline{H}}_{\mathrm{In}} \), 3.0 for \( \varDelta {\overline{H}}_{\mathrm{Eu}} \), and 0.06 and 0 for ΔH in the ranges 0 < x In < 0.66 and 0.78 < x In < 1, respectively. The total error can be assessed as the root of the sum of random and systematic squared errors:
The resulting partial and integral mixing enthalpies and entropies at 1300 and 1170 K were fitted to polynomial dependences (kJ/mol), giving less accurate approximation of thermodynamic functions than the IAS model but accelerating the calculation in case of multicomponent systems based on the binary Eu–In system:
Therefore, we obtained \( \varDelta {\overline{H}}_{\mathrm{In}}^{\infty } \) = −107.6 ± 1.0, \( \varDelta {\overline{H}}_{\mathrm{Eu}}^{\infty } \) = −122.1 ± 5.2, and ΔH min = −35.1 ± 0.5 kJ/mol at x In = 0.52 for 1300 K. The values for 1170 K are as follows: \( \varDelta {\overline{H}}_{\mathrm{In}}^{\infty } \) = −119.5 ± 1.0, \( \varDelta {\overline{H}}_{\mathrm{Eu}}^{\infty } \) = −124.2 ± 5.2, and ΔH min = −41.2 ± 0.5 kJ/mol at x In = 0.50. The errors may be higher since the values indicated do not allow for many additional effects, such as the error in fitting thermodynamic data to the IAS model and polynomials.
Discussion of Results
The mixing enthalpies of Eu–In melts that we determined by calorimetry agree well with the data [3] obtained by the same method. The only exceptions are the range 0 < x In < 0.1, in which an inverse composition dependence of \( \varDelta {\overline{H}}_{\mathrm{In}} \) was observed [3], and the range 0.7 < x In < 1, while we received much lower exothermic values of \( \varDelta {\overline{H}}_{\mathrm{Eu}} \), closer to the results provided in [4]. Our integral mixing enthalpies of the melts are also somewhat less exothermic on the indium side and more symmetric than the data in [3]. These distinctions can be attributed to different effects exerted by oxidation of the samples and evaporation of components in our experiments and in the study [3]. Temperature may also influence the data obtained, which is substantial in the range 500–2000 K according to Fig. 3.
In creating the thermodynamic model of Eu–In alloys, we used our own experimental and literature data. In particular, we tried to reach the best agreement with the published data [10] for the liquidus line of the Eu–In phase diagram (Fig. 4).
Figure 4 shows that our liquidus agrees well with the data reported in [10] at 0 < x In < 0.5, while the agreement is much lower in the range of EuIn2 equilibrium with the melt. It should be noted that the attempts to change input parameters of the IAS model have not improved this. For example, removal of EuIn4 associate from the model, whose concentration turned out to be the lowest, has almost no effect on the description of equilibrium between the solid and liquid alloys but substantially worsens the fitting of our experimental mixing enthalpies of the melts since the abrupt decrease in the magnitude of \( \varDelta {\overline{H}}_{\mathrm{Eu}} \) when Eu is added to In (0.78 < x In < 1) is no longer described in this case. The excess steepness [10] of the liquidus in the eutectic region between EuIn and EuIn2 can hardly be explained by any agreed thermodynamic model. In our opinion, this region of the phase diagram is to be studied further. At the same time, new data for the In–Ln systems allow understanding the dependence of their properties (in particular, mixing enthalpies of melts, formation of intermetallics, and temperatures of invariant reactions on phase diagrams) on the Ln atomic number. A critical analysis would probably resolve this situation.
It was of interest to compare the thermochemical properties of Eu–In melts and data for the related Eu–Me systems; so far, we have examined the Al–Eu system [11]. It turned out that interaction of Eu with In is much stronger (ΔH min = −36.5 kJ/mol) than with Al (ΔH min = −23.0 kJ/mol). This is probably due to a smaller effect of the size factor for the Eu–In system (preventing effective interaction of components in the Al–Eu system) and a much greater difference in electronegativities in the Eu–In system, compared to the Al–Eu system (Table 2).
There are currently many data on the formation enthalpies of intermetallics in the In–Ln systems, most of them being related to In3Ln compounds (Fig. 5). Note that there is no complete agreement between these data [14–24]. The properties reported in [14] demonstrate weaker dependence on lanthanide atomic number and are more exothermic for heavy lanthanides, compared to the data in [15]. The Eu–In system contains no In3Ln, and experimental data for other compounds are missing. In this regard, this system can be compared with other In–Ln systems only indirectly, by calculating the formation enthalpy of solid EuIn3 alloy, representing a mixture of EuIn2 and EuIn4 compounds, as (3Δ f H(EuIn2) + 5Δ f H(EuIn4))/8 = (−3 ⋅ 46.0 − 5 ⋅ 28.8)/8 = − 35.3 kJ/mol at., where data for EuIn2 and EuIn4 result from IAS modeling (Table 1). This value is much less exothermic than experimental literature data for similar systems. This can be attributed to different atomic sizes of the components, preventing their efficient interaction (Fig. 6). Hence, it is assumed that ytterbium will also show this abnormality in the formation enthalpy of In3Yb. However, these data should be verified since the abnormality was not observed experimentally [15].
Size factor Δr/Σr = = |r Ln − r In|/(r Ln + r In) for the In–Ln systems versus the Ln atomic number (z Ln) [12]
Conclusions
Isoperibol calorimetry is used at 1170–1300 K to determine the mixing enthalpies of binary liquid Eu–In alloys (0 < x In < 0.66; 0.78 < x In < 1). They show significant negative values: ΔH min = −35.1 ± 0.5 kJ/mol at x In = = 0.52 (1300 K) and ΔH min = −41.2 ± 0.5 kJ/mol at x In = 0.50 (1170 K). The results qualitatively agree with the data reported in [3]. The thermodynamic properties of liquid and solid Eu–In alloys and the liquidus line of the phase diagram have been modeled in the entire composition range. The liquidus agrees well with the data provided in [10]. The data obtained are explained by comparison with similar Eu–Me and In–Me systems.
Notes
The factors are indicated in descending order of significance.
References
S. P. Yatsenko, Indium. Properties and Applications [in Russian], Nauka, Moscow (1987), p. 256.
P. I. Fedorov and R. Kh. Achkurin, Indium [in Russian], Nauka, Moscow (2000), p. 276.
V. D. Bushmanov, E. G. Fedorova, and S. P. Yatsenko, “Enthalpy of forming liquid alloys of europium with indium,” Zh. Fiz. Khim., 61, No. 7, 1797–1799 (1987).
V. A. Dubinin, V. I. Kober, V. I. Kochkin, and I. F. Nichkov, “Thermodynamic properties of liquid-metal europium–indium alloys,” Zh. Fiz. Khim., 59, No. 5, 1258–1260 (1985).
V. A. Lebedev, V. I. Kober, and L. F. Yamshchikov, Thermochemistry of Rare Earth and Actinide Element Alloys: Handbook [in Russian], Metallurgiya, Chelyabinsk (1989), p. 336.
M. Ivanov, V. Berezutski, and N. Usenko, “Mixing enthalpies in liquid alloys of manganese with the lanthanides,” J. Mater. Res., 102, 277–281 (2011).
A. T. Dinsdale, “SGTE data for pure elements,” Calphad, 15, No. 4, 319–427 (1991).
M. I. Ivanov, V. V. Berezutskii, M. O. Shevchenko, et al., “Interaction in alloys of systems containing europium,” Dop. Nats. Akad. Nauk Ukrainy, No. 8, 90–99 (2013).
I. Barin and G. Platzki (ed.), Thermochemical Data of Pure Substances, 3rd ed., VCH Verlagsgesellschaft mbH, Weinheim, Germany (1995), p. 2003.
T. B. Masalsky, Binary Alloy Phase Diagrams, 2nd ed., ASM International, Metals Park, Ohio (1990), p. 1741.
M. I. Ivanov, V. V. Berezutskii, M. O. Shevchenko, et al., “Thermodynamic properties of Al–Eu liquid alloys,” Powder Metall. Met. Ceram., 50, No. 7–8, 538–543 (2011).
V. A. Rabinovich and Z. Ya. Khavin, Concise Chemical Handbook [in Russian], Khimiya, Leningrad (1977), p. 376.
David R. Lide, CRC Handbook of Chemistry and Physics. Electronegativity, 90th ed., CRC Press Taylor and Francis, Boca Raton, USA (2010).
S. V. Meschel and O. J. Kleppa, “Standard enthalpies of formation of some lanthanide indium compounds by high temperature direct synthesis calorimetry,” J. Alloys Compd., 337, 115–119 (2002).
A. Palenzona and S. Cirafici, “Dynamic differential calorimetry of intermetallic compounds. III. Heats of formation, heats and entropies of fusion of REIn3 and RETl3 compounds,” Thermochim. Acta, 9, 419–425 (1974).
A. Borsese, A. Calabreta, S. Delfino, and R. Ferro, “Measurements of heats of formation in the lanthanum-indium system,” J. Less-Common Met., 51, 45–49 (1977).
V. A. Novozhenov, T. M. Shkol’nikova, and V. V. Serebrennikov, “Formation heats for alloys of lanthanum with indium,” Zh. Fiz. Khim., 49, No. 11, 3012 (1975).
V. V. Serebrennikov, V. A. Novozhenov, and T. M. Shkol’nikova, “Formation heats for alloys of praseodymium with indium,” Zh. Fiz. Khim., 50, No. 9, 2401–2402 (1976).
V. A. Novozhenov, T. M. Shkol’nikova, and V. V. Serebrennikov, “Formation heats for alloys of neodymium with indium,” Zh. Fiz. Khim., 53, No. 8, 2117 (1979).
V. A. Degtyar’, L. A. Vnuchkova, A. P. Bayanov, and V. V. Serebrennkikov, “Thermodynamic study of liquid cerium–indium alloys,” Zh. Fiz. Khim., 45, No. 6, 1594 (1971).
V. A. Degtyar’, A. P. Bayanov, L. A. Vnuchkova, and V. V. Serebrennikov, “Thermodynamics of liquid praseodymium–indium alloys,” Zh. Fiz. Khim., 45, No. 7, 1816–1818 (1971).
V. A. Degtyar’, A. P. Bayanov, and V. V. Serebrennikov, “Thermodynamics of interaction between neodymium and indium,” in: Collected Papers of Tomsk University [in Russian], Vol. 204, Tomsk (1971), pp. 401–402.
A. P. Bayanov, E. N. Ganchenko, and Yu. A. Afanasiev, “Study of thermodynamic properties of alloys of terbium with indium and lead by e.m.f. method,” Zh. Fiz. Khim., 51, 2381–2382 (1977).
V. P. Vasiliev and Wu Ding Khui, “Thermodynamic properties of phases in the Gd–In system in the range to 50 at.%,” Izv. Akad. Nauk SSSR. Neorg. Mater., 21, No. 7, 1144–1148 (1985).
Author information
Authors and Affiliations
Corresponding author
Additional information
Translated from Poroshkovaya Metallurgiya, Vol. 53, Nos. 11–12 (500), pp. 93–103, 2014.
Rights and permissions
About this article
Cite this article
Berezutskii, V.V., Ivanov, M.I., Shevchenko, M.O. et al. Thermodynamic Properties of Eu–In Alloys. Powder Metall Met Ceram 53, 693–700 (2015). https://doi.org/10.1007/s11106-015-9665-z
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11106-015-9665-z