Abstract
The ZmRXO1 gene is a nucleotide-binding site leucine-rich repeat (NBS–LRR) type of R gene in maize (Zea mays). To understand the regulatory mechanism of ZmRXO1 gene expression, we isolated and characterized the ZmRXO1 promoter (PZmRXO1)—the 5′ flanking region of ZmRXO1. A series of PZmRXO1 deletion derivatives, R1–R4, from the translation start code (−1,576, −934, −829, and −582) were fused to the GUS reporter gene, and each deletion construct was analyzed by Agrobacterium-mediated transformation into tobacco. Sequence analysis showed that several cis-acting elements (MBS, Box-I, TGA-element and CCAAT-box) were located within the promoter. Deletion analysis of the promoter suggested that the 1,576-bp fragment upstream of ZmRXO1 gene showed a high level of GUS expression in tobacco. The promoter sequence (−582 to −1) was sufficient to improve transcription of GUS gene under hormones (MeJA, GA, ABA), drought and low temperature. Moreover, there might be repressor elements in the region (−1,576 to −934 bp) to repress ZmRXO1 gene expression under treatment with salicylic acid.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Adverse environmental stresses such as drought, salinity, low temperature and pathogen attacks have drastic effects on plant growth and development, which significantly limit agricultural productivity. To increase crop yield under stress conditions, molecular breeding technologies relying on genetic engineering represent an important addition to conventional breeding technology. The cloning of key genes related to adverse stress is the core to solving the problem. Importantly, cloning and understanding stress-related promoters are the keys to realizing molecular breeding goals. In current applications, constitutive promoter expression, such as that from the cauliflower mosaic virus 35S (CaMV35S) and ubiquitin promoters, is crucial.
Constitutively expressed promoters, such as CaMV35S, have been used to assess the effects of transgene expression in various plant species. But the high expression of transgenes in all tissues during all developmental periods increases the metabolic burden to a great extent, leading to a loss of material and energy in the transgenic plants (Vaucheret et al. 1998; Malnoy et al. 2006). Therefore, an inducible promoter to drive gene expression in transgenic plants could minimize the negative effects on plants.
Nowadays, many inducible promoters have been identified in the search to understand the molecular mechanisms of signaling pathways. Several inducible promoters, such as the pathogen-inducible promoter Pgst1 (Malnoy et al. 2006), light-regulated promoter AtPolλ (Sujit et al. 2012), wounding and tensile stress-inducible gene promoter from Brassica napus (Katherine AE and Anil HS. 1998), and oxidative stress-inducible peroxidase SWPA2 promoter from sweet potato (Kee-Yeun et al. 2003), have been studied in plants. The cloning and use of these inducible promoters have contributed to the generation of high-quality transgenic plants (Rushton et al. 2002).
The ZmRXO1 gene, which involves in signaling pathways to induce basic defense reactions, could specifically activate large numbers of genes (Bingyu et al. 2005; Yong-Li et al. 2010). But there is little information about the response of its core element to some abiotic stresses, such as methyl jasmonate (MeJA), salicylic acid (SA), gibberellin (GA) and abscissic acid (ABA), as well as to drought and low temperature. To better understand how the ZmRXO1 promoter is regulated, we characterized the ZmRXO1 promoter region using β-glucuronidase (GUS) gene as a reporter gene. Our results showed that the 5′-flanking sequence of the ZmRXO1 promoter was induced by hormones (MeJA, GA, ABA), drought and low temperature. These results provide valuable insights into the role of the ZmRXO1 promoter in regulating gene expression patterns under biotic and abiotic stresses.
Materials and Methods
Plant Materials and Growth Conditions
Seeds of Zea mays (B73) were obtained and used as the source material for this study. Maize plants were raised in pots under greenhouse conditions (16/8 h photoperiod; 25 °C). Tobacco Nicotiana tabacum (NC89) plants used for tissue culture, were raised on Murashige-Skoog (MS) medium supplemented with 30 g/L sucrose, 7 g/L agar, 3 mg/L 6-BA, 0.2 mg/L NAA and pH 5.8. The plants were maintained in a growth chamber with a 16/8 h light/dark at 25 °C. The fully developed tobacco leaves were then used for genetic transformation experiments.
Promoter Cloning and Sequence Analysis
To determine the structure of the ZmRXO1 promoter, polymerase chain reaction (PCR) was performed using the primer pairs (RXO1-F/RXO1-R, Table 1) with maize genomic DNA as template. The PCR conditions were as follows: 94 °C for 5 min and 30 cycles of 94 °C for 45 s, 56 °C for 40s, 72 °C for 2 min and 72 °C for 10 min. The PCR products were cloned into the vector pMD18-T vector and the recombinant clones obtained were sequenced. Sequences analysis of the ZmRXO1 promoter from maize was carried out using the PlantCARE database (Lescot et al. 2002).
Construction of Expression Vectors and Genetic Transformation
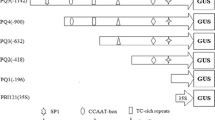
In order to study the functional regions of the ZmRXO1 promoter, 5′-end deletion analysis was carried out. A series of deletions of the ZmRXO1 promoter was generated by PCR amplifications. Four deletion fragments were named as R1 (−1,576 bp), R2(−934 bp), R3 (−829 bp), R4 (−582 bp) with the common reverse primer (RXO1-R) and the forward primers R2-F, R3-F, R4-F, respectively (Table 1). The PCR products were cloned into the pCMBIA1301 plasmid to replace the CaMV35S promoter (Fig. 1). The recombinant plasmids were introduced into the Agrobacterium tumefaciens strain EHA105 for Agrobacterium-mediated transformation of tobacco leaves. To evaluate promoter activity, expression of the gene reporter beta-glucuronidase (GUS) was measured.
Schematic representation of the PZmRXO1: GUS construct. The insertion position of the ZmRXO1 promoter in the vector is indicated with restriction enzyme sites (EcoRI and NcoI). LB Left border, RB right border, 35s-ter Cauliflower Mosaic virus 35S terminator, 35s Pro Cauliflower Mosaic virus 35S promoter, GUS β-glucuronidase gene, HPTII hygromycin phosphotransferase (II) coding region, NOS-ter nopaline synthase terminator, ZmRXO1 Pro ZmRXO1 promoter
Plant Treatment
To understand the effects of different stresses on GUS expression mediated by the ZmRXO1 promoter, plants were treated for different durations with dehydration, low temperature (4 °C), abscisic acid (ABA), methyl jasmonate (MeJA), salicylic acid (SA), gibberellin (GA) before sampling. For drought stress treatment, the roots of transgenic tobacco plants were soaked in 20 % polyethylene glycol 6000 (PEG6000). For low temperature treatment, the transgenic plants were put into a 4 °C growth chamber. For different hormone stimuli treatments, the transgenic tobacco plants were sprayed with 100 μM MeJA, 1 mM SA, 100 μM ABA and 100 μM GA, respectively. Transgenic tobacco plants of different deletion promoters and the CaMV35S (pCAMBIA1301 vector) transformants treated with water were used as negative and positive controls, respectively. The tobacco samples were treated for 1 h, 3 h, 5 h, 10 h and 24 h. After treatment, tobacco leaves were harvested, quickly frozen in liquid nitrogen, and stored at −80 °C for total RNA extraction and fluorometric GUS assay.
Total RNA Extraction and Real-Time Quantitative RT-PCR Analysis
To understand the effects of different stresses on GUS expression mediated by the ZmRXO1 promoter, the fourth–sixth leaves of the stress-treated transgenic tobacco were used as the plant materials. Total RNA from tobacco leaves was extracted using the RNAiso Reagent (Takara, Changchun, China) and single-stranded cDNA was synthesized from total RNA with reverse transcriptase M-MLV and the oligo (T) 18 primer (Takara). Real-time quantitative RT-PCR (qRT-PCR) analysis was performed using an Applied Biosystems 7500 apparatus (Applied Biosystems, Foster City, CA). The ACTIN gene (GenBank accession No. U60491) was used as an endogenous control gene (ACTIN-F/ACTIN-R, Table 1). GUS-F/GUS-R primers (Table 1) were used for GUS gene. The PCR conditions were: 95 °C for 30s, 95 °C for 5 s, 56 °C for 40s, 40 cycles. Data from the individual runs were collated using the 2−ΔΔCT method (Livak and Schmittgen 2001). Mean expression levels and standard deviations (SD) were calculated from the results of three repeat experiments.
Histochemical Staining and Fluorometric GUS Assay
Histochemical staining and fluorometric GUS assay analysis for GUS activity was carried out as described by Jefferson et al. (1987). The leaves of stress-treated transgenic tobaccos were incubated in GUS reaction buffer (3 mg/mL X-gluc, 40 mM sodium phosphate pH 7, 10 mM EDTA, 0.1 % Triton X-100, 0.5 mM ferricyanatum kalium, 0.5 mM ferrocyanatum kalium and 20 % methanol). After overnight incubation at 37 °C, the stained samples were bleached with 70 % (v/v) ethanol to remove chlorophyll. Photos of those stained samples were obtained by a Nikon SMZ1000 microscope under white light. For fluorometric GUS assay, leaves of stress-treated transgenic tobaccos were used to determine GUS enzyme activity by measuring the fluorescence of 4-methylumbelliferone produced by GUS cleavage of 4-methylumbelliferyl-β-d-glucuronide (4-MUG) (Jefferson 1988). GUS activity is expressed as nanomoles of methylumbelliferone per minute per milligram of protein. Protein amount was determined using a Protein Assay kit (Bio-Rad, Hercules, CA) using bovine serum albumin as a standard.
Results
Promoter Sequence Analysis
For a more comprehensive identification of cis-acting regulatory elements, the 1,576 bp upstream sequence before the translation initiation codon (indicated with +1, Fig. 1) was analyzed using the PLACE and the PlantCARE databases. Bioinformatics analysis of ZmRXO1 promoter detected some putative cis-acting regulatory elements known to modulate gene expression in different plant species (Fig. 2). Several core fragments were identified in the ZmRXO1 promoter sequence (Table 2). They consisted of four CAAT-box elements, five TATA-box elements, one CATT motif (GCATTC), one AE-box (AGAAACAA), one ATCT-motif (AATCTGATCG), one GAG-motif (AGAGAGT), one MBS (TAACTG), two gibberellin-responsive elements (P-box and GARE-motif) and many other cis-acting regulatory elements, such as CAT-box, SP1, Box-W1.
GUS Expression Under the Control of the ZmRXO1 Promoter in Response to Different Stimuli
To determine the regulatory mechanism controlling expression of the ZmRXO1 gene, the 1,576 bp full-length ZmRXO1 promoter was fused to the GUS reporter gene in a plant expression vector and transferred into tobacco plants. The GUS gene expression levels in the transgenic tobacco by histochemical GUS staining clearly revealed the inducible activity of the ZmRXO1 promoter. The results indicated that expression of the GUS gene was decreased after SA treatment (Fig. 3a, c, e), but increased after the other treatments (Fig. 3a, c, d, f–i). From Fig. 3a–c, we can see that the untreated transgenic tobacco showed a certain degree of GUS expression, which was apparently lower than that of the CaMV35S transformant tobacco plants. Moreover, GUS gene expression occurred mainly in the aerial parts of the plants and there was hardly expression in roots.
Histochemical staining of GUS activity in six-week-old transgenic tobacco plants. a, b Beta-glucuronidase (GUS) expression in wild type (a) and CaMV35S-transformed (b) tobacco plants. c Untreated transgenic tobacco plants. d–l Transformed tobacco plants were treated with 100 μM methyl jasmonate (MeJA), 1 mM salicylic acid (SA), 100 μM abscisic acid (ABA), 20 % polyethylene glycol (PEG), 100 μM gibberellin (GA) and low temperature treatment (4 °C), respectively
To further verify the above findings, GUS expression from the ZmRXO1 promoter was analyzed by qRT-PCR and fluorometric GUS assay (Figs. 4, 5). The GUS gene transcript level could be induced substantially by ABA and PEG with a maximal level at 10 h. GUS gene transcription in plants treated with MeJA, GA or low temperature also increased significantly at 1 h, 3 h or 5 h, respectively. However, the GUS expression level of SA-treated plants was lower than that of untreated plants (0 h). From Fig. 5, we found the same phenomenon as that shown in Fig. 4. ZmRXO1 promoter-mediated GUS activity increased under dehydration, cold stresses, MeJA, GA, ABA treatment, but declined under SA treatment.
Time course of the ZmRXO1 transcript levels in the leaves of the transgenic tobacco under methyl jasmonate (MeJA), salicylic acid (SA), abscisic acid (ABA), polyethylene glycol (PEG), gibberellin (GA) and low temperature (4 °C) treatment. CK (wild-type) and 0 h-treated tobacco plants were left untreated as controls. At least three independent determinations were performed for each sample
GUS activity of the ZmRXO1 promoter in response to methyl jasmonate (MeJA), salicylic acid (SA), abscisic acid (ABA), polyethylene glycol (PEG), gibberellin (GA) and low temperature (4 °C) treatments. GUS activity from the CaMV35S (pCAMBIA1301 vector) transformants, wild type and untreated transformants served as controls
Activity Analysis of ZmRXO1 Deletion Promoters
To further study the stress-inducible expression of the gene promoter, a series of 5′ end deletion promoter:: GUS constructs was also produced, and then transferred to tobacco plants for analysis of transient expression. The deletion promoters were named as R1 (−1,576 to −1), R2 (−934 to −1), R3 (−829 to −1) and R4 (−582 to −1), respectively. Leaves of stress-treated transgenic tobaccos were subjected to the fluorometric GUS activity assay. The results indicated that the GUS activity of R1-promoter plants was higher than that of R2-, R3- and R4-promoter plants (Fig. 6). The deletion promoters were subjected to abiotic stress and phytohormones (MeJA, GA, ABA); GUS activity of the R1, and R4 promoters increased by 1.88-fold and 2.67-fold (compared with the untreated control), respectively, after GA treatment. The two gibberellin-responsive elements (GARE-motif and P-box) might play an important role in improving the GUS activity of R1 and R4. However, R1-mediated GUS activity was reduced significantly (about 0.48-fold) by SA treatment, and while that mediated by R2, R3, and R4 showed no differences from that of untreated plants.
Analysis of ZmRXO1 promoter deletion mutants. GUS activity in methyl jasmonate (MeJA), salicylic acid (SA), abscisic acid (ABA), polyethylene glycol (PEG), gibberellin (GA) and low temperature (4 °C) treatments of plants carrying the ZmRXO1 promoter deletion series is shown. GUS activity from the CaMV35S (pCAMBIA1301 vector) transformants, wild type and untreated transformants served as controls
Discussion
PZmRXO1 is a Photosynthesis Tissue-Specific Promoter
Expression of many plant genes, including structural, metabolic and many regulatory genes (Eckes et al. 1986; Simpson et al. 1986; Kaldenhoff et al. 1993; Sheen 1993), is induced by light . These genes are expressed preferentially under light conditions, but exhibit low expression or even no expression in dark conditions. Therefore, they are defined as light inducible genes and are generally expressed in leaves. In this study, the fusion construct (PZmRXO1: GUS) was transformed into the tobacco genome to study the activity of this promoter. Transgenic tobacco plants exhibited blue staining in the leaves and stems, but not in the roots (Fig. 3). This result indicated that the ZmRXO1 promoter might be regulated by light. Several types of light-responsive elements, including Sp1, GAG-motif, AE-box, CATT-motif and Box-I, were found in the ZmRXO1 promoter sequence (Fig. 2). Box-I has been identified in nuclear extracts from tobacco and tomato leaves and cotyledons (Borello et al. 1993). LeMYBI is reported to bind specifically to Box-I and to activate transcription in plant leaves (Rose et al. 1999).
Deletion Analysis of the ZmRXO1 Promoter
To determine the function of the cis-acting elements, deletion analysis of the ZmRXO1 promoter was performed. The deletion promoter constructions were transformed into tobaccos using Agrobacterium-mediated leaf-disc transformation.
As shown in Fig. 6, GUS activity decreased as the ZmRXO1 promoter length decreased from R1 to R5. The full-length ZmRXO1 promoter (R1) had the highest activity among all the deletion promoters. This suggests that the sequence between −1,576 and −934 bp of the ZmRXO1 promoter may contain cis-elements involved in up-regulation of GUS expression.
The ZmRXO1 promoter exhibited inducible expression in response to different stress stimuli (ABA, drought and low temperature; Fig. 6). Sequence analysis showed that there were two cis-acting elements—MBS and the CCAAT-box (MYBHv1 binding site)—in the ZmRXO1 promoter. We hypothesized that these two elements played an important role in increasing GUS activity in these cases. MBS is the binding site for the transcription factor MYB, which has been identified as a target of other regulators (Dubos et al. 2010). Transient expression analyses indicated that AtMYB2 could transactivate gene expression via MBS-1 by binding to the MYB recognition site MBS-1 in the AtADH1 promoter (Hoeren et al. 1998). The CCAAT box is one of the most ubiquitous of promoter elements, being present in 30 % eukaryotic promoters (Bucher 1990). Many housekeeping and inducible promoters have a CCAAT box (Roy and Lee 1995), and many DNA-binding proteins binds the CCAAT element, including CTF/NF1 (transcription factor/nuclear factor 1), C/EBP (CCAAT/enhancer binding protein), CDP (CCAAT displacement protein) (Edwards et al. 1998). In this study, we also found that GUS activity changes in transgenic tobacco under drought stress, low temperature and ABA exhibited the same pattern (Fig. 6). This may be related to ABA signal transduction. ABA is one of the most important signaling molecules in plants, and is also a cross-talking signal molecule. Under drought and low temperature conditions, various biochemical and physiological responses are triggered. The expression of many stress-inducible genes was improved and ABA accumulated in plants (Yamaguchi-Shinozaki and Shinozaki 1993). Promoters sequence analyses have revealed that ABA-responsive gene expression needs ABA-responsive elements (ABREs or CE3). This suggested that new ABA-responsive elements might exist in the ZmRXO1 promoter.
The ZmRXO1 gene is reported as a single dominant gene in maize that controls elicitation of the hypersensitive response to X. oryzae pv. oryzicola (Zhao et al. 2004). Plant defense responses are regulated by SA, ET-MeJA and other signaling molecules (Dong 1998; McDowell and Dangl 2000). The SA-dependent response is deployed against a biotrophic pathogen that obtains nutrients from living cells, but the ET–JA response is activated by necrotrophic pathogens that kill plant tissue. In this study, R1, R2, R3, R4-mediated GUS activity increased significantly under MeJA treatment (compared to untreated controls). However, R1-mediated GUS activity declined significantly under SA treatment (Fig. 6). This result indicated that expression of the ZmRXO1 gene might be a MeJA-dependent response within the overall plant defense response.
The TGA-element (AACGAC) is an auxin response element. Xing reported 31 encoding ARF genes, whose products interact with the TGA-element (AACGAC) in the maize genome (Xing et al. 2011). Among these genes, 14 had auxin response elements in their promoter regions, 7 of which were not affected by exogenous auxin. Sequence analysis of the ZmRXO1 promoter showed that one TGA-element was present in the R1 promoter. R1-mediated GUS activity declined significantly under SA treatment (compared with untreated controls). However, no significant difference in GUS activity of R2-, R3-, and R4-mediated expression was observed; the TGA-element of these deletion promoters was deleted (Fig. 6). We suspected that the TGA-element might have a negative effect on the ZmRXO1 promoter under SA treatment. Or that there is other negative cis-element that is not found in the 642 bp fragment (−1,576 to −934).
In conclusion, GUS expression assays indicated that a 1,576-bp fragment upstream of the ZmRXO1 gene coding sequence confers a high level of GUS expression in tobacco. The results demonstrated that elements needed for high expression might be located in a 642-bp fragment (−1,576 to −934). Also, R4 (−582) was sufficient to induce transcription of the GUS gene. Moreover, there might be repressor elements of ZmRXO1 gene expression under SA in the region from −1,576 to −934 bp.
References
Bingyu Z, Xinghua L, Jesse P, Harold T, Jan L, Scot H (2005) A maize resistance gene functions against bacterial streak disease in rice. Proc Natl Acad Sci USA 102(43):15383–15388
Borello U, Ceccarelli E, Giuliano G (1993) Constitutive, light-responsive and circadian clock-responsive factors compete for the different I box elements in plant light-regulated promoters. Plant J 4:611–619
Bucher P (1990) Weight matrix descriptions of four eukaryotic RNA polymerase II promoter elements derived from 502 unrelated promoter sequences. J Mol Biol 212(4):563–578
Dong X (1998) SA, JA, ethylene, and disease resistance in plants. Curr Opin Plant Biol 1(4):316–323
Dubos C, Stracke R, Grotewold E, Weisshaar B, Martin C, Lepiniec L (2010) MYB transcription factors in Arabidopsis. Trends Plant Sci 15(10):573–581
Eckes P, Rosahl S, Schell J, Willmitzer L (1986) Isolation and characterization of a light-inducible, organ-specific gene from potato and analysis of its expression after tagging and transfer into tobacco and potato shoots. Mol Gen Genet 205(1):14–22
Edwards D, Murray JA, Smith AG (1998) Multiple genes encoding the conserved CCAAT-box transcription factor complex are expressed in arabidopsis. Plant Physiol 117(3):1015–1022
Hoeren FU, Dolferus R, Wu Y, Peacock WJ, Dennis ES (1998) Evidence for a role for AtMYB2 in the induction of the arabidopsis alcohol dehydrogenase gene (ADH1) by low oxygen. Genetics 149(2):479–490
Jefferson RA (1988) Plant reporter genes: the GUS gene fusion system. Genet Eng 10:247–263
Jefferson RA, Kavanagh TA, Bevan MW (1987) GUS fusions:betaglucuronidase as a sensitive and versatile gene fusion marker in higher plants. EMBO J 6(13):3901–3907
Kaldenhoff R, Kölling A, Richter G (1993) A novel blue light-and abscisic acid-inducible gene of Arabidopsis thaliana encoding an intrinsic membrane protein. Plant Mol Biol 23(6):1187–1198
Kee-Yeun K, Suk-Yoon K, Haeng-Soon L, Yunkang H, Jae-WB S-SK (2003) A novel oxidative stress-inducible peroxidase promoter from sweetpotato: molecular cloning and characterization in transgenic tobacco plants and cultured cells. Plant Mol Biol 51:831–838
Lescot M, Déhais P, Thijs G, Marchal K, Moreau Y, Van de Peer Y, Rouzé P, Rombauts S (2002) PlantCARE, a database of plant cis-acting regulatory elements and a portal to tools for in silico analysis of promoter sequences. Nucleic Acids Res 30:325–327
Livak KJ, Schmittgen TD (2001) Analysis of relative gene expression data using real-time quantitative PCR and the2− ∆∆ct method. Methods 25(4):402–408
Malnoy M, Reynoird JP, Borejsza-Wysocka EE, Aldwinckle HS (2006) Activation of the pathogen-inducible Gst1 promoter of potato after elicitation by Venturia inaequalis and Erwinia amylovora in transgenic apple (Malus × domestica). Transgenic Res 15:83–93. doi:10.1007/s11248-005-2943-7
McDowell JM, Dangl JL (2000) Signal transduction in the plant immune response. Trends Biochem Sci 25(2):79–82
Rose A, Meier I, Wienand U (1999)The tomato I-box binding factor LeMYBI is a member of a novel class of Myb-like proteins. Plant J 20:641–652
Roy B, Lee AS (1995) Transduction of calcium stress through interaction of the human transcription factor CBF with the proximal CCAAT regulatory element of the GRP78/BIP promoter. Mol Cell Biol 15(4):2263–2274
Rushton PJ, Reinstädler A, Lipka V, Lippok B, Somssich IE (2002) Synthetic plant promoters containing defined regulatory elements provide novel insights into pathogen- and wound-induced signaling. Plant Cell 14:749–762
Sheen J (1993) Protein phosphatase activity is required for light-inducible gene expression in maize. EMBO J 12(9):3497
Simpson J, Schell J, Van Montagu M, Herrera-Estrella L (1986) Light-inducible and tissue-specific pea lhcp gene expression involves an upstream element combining enhancer-and silencer-like properties. Nature 323:551–554
Sujit R, Swarup RC, Sanjay KS, Kali PD (2012) Functional analysis of light-regulated promoter region of AtPolλ gene. Planta 235:411–432
Vaucheret H, Beclin C, Elmayan T, Feuerbach F, Godon C, Morel JB, Mourrain P, Palauqui JC, Vernhettes S (1998) Transgene-induced gene silencing in plants. Plant J 16:651–659
Xing H, Pudake R, Guo G, Xing G, Hu Z, Zhang Y, Sun Q, Ni Z (2011) Genome-wide identification and expression profiling of auxin response factor (ARF) gene family in maize. BMC Genomics 12(1):178
Yamaguchi-Shinozaki K, Shinozaki K (1993) The plant hormone abscisic acid mediates the drought-induced expression but not the seed-specific expression of RD22, a gene responsive to dehydration stress in arabidopsis thaliana. Mol Gen Genet MGG 238(1–2):17–25
Yong-Li Z, Mei-Rong X, Ming-Fu Z, Xue-Wen X, Ling-Hua Z, Bin-Ying F, Zhi-Kang L (2010) Genome-wide gene responses in a transgenic rice line carrying the maize resistance gene Rxo1 to the rice bacterial streak pathogen, Xanthomonas oryzae pv. BMC Genomics 11(78):1471–2164
Zhao B, Ardales EY, Raymundo A, Bai J, Trick HN, Leach JE, Hulbert SH (2004) The avrRxo1 gene from the rice pathogen xanthomonas oryzae pv. Oryzicola confers a nonhost defense reaction on maize with resistance gene Rxo1. Mol Plant-Microbe Interact 17(7):771–779
Acknowledgments
This research was funded by the Twelfth Five Year Plan Project of Science and Technology Support, P.R. China (2012BAD19B04, 2014BAD14B02), the Ministry of Agriculture Key Project of GM Cultivation of New Varieties, P. R. China (2013ZX08004004), the Project of International Collaboration Plan in Jilin Province (20100723) and the Research and Development of Industrial Technology Special at Jilin Provincial Development and Reform Commission (2013C001).
Author information
Authors and Affiliations
Corresponding author
Additional information
Ye Tao and Fengting Wang contributed equally to this work and are co-first authors.
Rights and permissions
About this article
Cite this article
Tao, Y., Wang, F., Jia, D. et al. Cloning and Functional Analysis of the Promoter of a Stress-inducible Gene (ZmRXO1) in Maize. Plant Mol Biol Rep 33, 200–208 (2015). https://doi.org/10.1007/s11105-014-0741-1
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11105-014-0741-1