Abstract
Purpose
Understanding the direction of nitrogen flow in the rice-crayfish system (RC) and optimizing the rate of nitrogen fertilizer application (ORN).
Methods
To this end, there were four test treatments—(i) straw with feed (SF), (ii) straw with no feed (SN), (iii) no straw with feed (NF), and (iv) no straw with no feed (NN) that was conducted to delineate the flow of the nitrogen present in the straw (straw-N) and feed (feed-N). Second, we carried out a mesocosm experiment with different rates of 15 N-labeled nitrogen fertilizer application: 0 kg N ha−1 (0 N), 75 kg N ha−1 (75 N), 150 kg N ha−1 (150 N), 225 kg N ha−1 (225 N) to explore the optimal rate under the effects of feed-N and straw-N supply.
Results
The results showed that at 75N, 14% and 1.86% of feed-N and 4.3% and 8.78% of straw-N is utilized by crayfish and rice, respectively. The percentage of feed-N and straw-N lost to the environment was the lowest at 75 N, i.e., 84.48% and 86.92%, respectively. Using straw return to the field and feeding as the basic management measures for RC, we observed that the rice yield was the highest under 75 N. The highest nitrogen use efficiency (NUE) was 25%, and the highest soil storing nitrogen by the fertilizer-derived was 34% under 75 N.
Conclusions
ORN could help in the effective utilization of feed-N and straw-N via a compensation effect, promote the absorption and transport of nitrogen, and ultimately improved NUE in RC.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Nitrogen is essential for crop growth, and it can promote food production (Xu et al. 2012; Yi et al. 2019). Therefore, the use of nitrogen fertilizers is a very important measure in farmland management for intensive agricultural production (Matson et al. 1997). Studies have shown that between 1982 and 2017, grain production in China increased by 74%, while the use of chemical fertilizers tripled (Cui and Shoemaker 2018). In China, the average nitrogen use efficiency (NUE) for crop production was estimated to be 25%, indicating that large amounts of nitrogen are lost to the environment (China National Statistics Bureau 2018).
During crop production, the residual nitrogen fertilizer that is not absorbed by crops damages both the ecosystem and human health (Erisman et al. 2013). High levels of nitrogen fertilizer can directly or indirectly drive soil acidification (Matsuyama et al. 2005; Guo et al. 2010), cause the eutrophication of water bodies (Chen et al. 2013), increasing the greenhouse effect (Davidson 2009). Therefore, the management of nitrogen fertilizers in high-yield rice systems is critical.
The integrated rice cultivation model that was developed in China and subsequently used for thousands of years allows the effective control of nitrogen fertilizer use and can help improve nitrogen use in rice fields (Hu et al. 2016). This model involves agricultural rice production in combination with the breeding of fish, ducks, crayfish, turtles, or loaches (Hu et al. 2016). Studies have shown that the rice–fish system requires, on average, 19% less nitrogen fertilizer than the rice monoculture system (Xie et al. 2011). In the rice–duck system, ducks are active in the paddy field, and the intertillage effect is obvious. This effect can lead to the loosening of paddy soil, promote gas exchange, improve soil permeability, and improve the organic carbon, total nitrogen (TN), and available nitrogen levels in the soil (Li et al. 2008). It has been found that a duck can produce 10 kg of excrement during the rice–duck co-cropping period, equivalent to 47 g of nitrogen, 70 g of phosphorous, and 31 g of potassium, thus fertilizing the rice field (Zhao et al. 2005).
The rice–crayfish (RC) model is an integrated rice cultivation model that has emerged in the middle and lower Yangtze river region in recent years (Yuan et al. 2020a). There are several differences in farmland management measures between traditional local rice fields and RC fields (Si et al. 2017). In RC fields, in the non-rice season, the flooding depth of the field is adjusted to 0.5–1.0 m in order to promote the growth and development of crayfish (Yuan et al. 2020b). In order to grow the crayfish to commercial standards, a large amount of feed is added to the paddy field. After the rice is harvested, rice straw is returned to the field and the field is flooded. This flooding is maintained until June of the following year, before rice crops are planted again. In the rice-growing season, the management and cultivation of rice is performed in a manner similar to that used in local traditional rice fields.
Straw is the carrier of matter, energy and nutrients, and a valuable natural resource and it contains carbon, nitrogen, phosphorus, potassium and other nutrients (Ponnamperuma 1984). Research shows that returning straw to the field can fertilize soil, improve soil quality and increase rice yield (Huang et al. 2013), mainly due to the rapid decomposition and concomitant nutrient release of the incorporated straw, as well as the beneficial effects on soil physical properties (Powlson et al. 2011; Wang et al. 2015). The C/N of rice straw is relatively high, and it is difficult to be decomposed by microorganisms (Thippayarugs et al. 2008). Direct application the straw to the field will stimulate the vigorous activity of microorganisms and will retain part of the available nitrogen, resulting in a shortage with crops for nitrogen, thus affecting the growth of crops (Moritsuka et al. 2004). Therefore, it is necessary to supplement nitrogen fertilizer after returning to the field to make up for the immobilization of nitrogen by soil microorganisms in the process of straw degradation, so as to ensure the supply of nitrogen (Singh and Rengel 2007). Uneaten feed is rich in organic carbon and nitrogen, and organic nitrogen is decomposed into inorganic nitrogen through mineralization, such as ammonia nitrogen and nitrate nitrogen, which can be directly absorbed and utilized by plant (John and Anthonie 2021). The C:N-ratio of the environment plays a key role in organic nitrogen decomposition rate (Xu et al. 2017). Therefor, feed and straw input is closely related to the use efficiency of nitrogen fertilizers in the RC system. Owing to the shift from rice monoculture to the RC model, excess nutrients have accumulated in the soil and there have been negative effects on the environment (Yuan et al., 2022). Research has shown that the amount of nitrogen fertilizer used by RC farmers in paddy fields is 150 kg N ha−1 (Hu et al. ), but it is unclear if this value is optimal. While studies have explored the optimal nitrogen fertilizer application rate in the rice–fish system under feed combinations that allow the best yield without causing environmental pollution (Hu et al. 2013), such studies in the RC system are lacking. Moreover, it is unclear whether rice production can be maintained while preventing environmental pollution in a large-scale commercial RC model.
Therefore, the overall aim of the present study was to identify the optimal nitrogen fertilizer application rate in RC system after straw and feed addition. To this end, we conducted one field experiment with mesocosm experiment. In the field experiment, we compared the impact of the RC system on environmental changes under straw and feed treatment. In the mesocosm experiment, we explored the use efficiency of the nitrogen fertilizer in RC fields under different rates of nitrogen fertilizer application.
Materials and methods
Study site
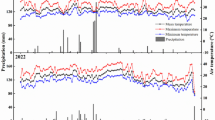
The field experiment was conducted at an experimental station in Houhu Village, Qianjiang City, Hubei Province (30° 39′ N, 112° 71′ E). The experimental site was located in the low-lying lake area of the Jianghan plain. This area has a north subtropical humid monsoon climate with a mean long-term temperature of 16.1 °C and had an annual precipitation of 1100 mm in 2018 (Fig. 1). The studied area had been under continuous RC cultivation for at least 3 years before. The experiment was started in 2018. The soil was fluvo-aquic paddy soil arising from lake sediments and had a pH level of 7.12. The total nitrogen content was 2.41 g kg−1 and the organic matter content was 26.43 g kg−1.
Experimental design
In order to explore the impact of straw return and feed addition under different nitrogen application rates on the environment in RC fields, we set up field and a microcosm experiments. Firstly, straw and feed treatments implemented in field experiments with four test treatments— (i) straw with feed (SF), (ii) straw with no feed (SN), (iii) no straw with feed (NF), and (iv) no straw with no feed (NN) — and each treatment was repeated thrice, yielding a 12-test plot. The area of the rice plantation in each plot was 1000 m2. A ring-shaped ditch (2.0 m wide and 2.0 m deep) was dug around the rice-planting region for crayfish to live in, and a 0.4-m high crayfish escape net was set outside the ring ditch. In the paddy field, there were three replicates for each treatment. One replicate for each treatment was randomly selected for the 15 N isotope tracer microcosms test to explore the optimal rate of nitrogen fertilizer application in the RC model. The following nitrogen application rates were used for each treatment: 0 kg N ha−1 (0 N), 75 kg N ha−1 (75 N), 150 kg N ha−1 (150 N), 225 kg N ha−1 (225 N). Each treatment was repeated thrice, yielding a total of 60 test micro areas wherein the 15 N isotope experiment was conducted. 15 N-labeled urea produced by Shanghai Research Institute of Chemical Industry was used as the nitrogen fertilizer, and the 15 N abundance was 10.28%. Base fertilizer and tiller fertilizer containing nitrogen were applied at a 1:1 ratio. After the surface water in the RC fields receded, but before rice crops were planted, a round PVC pipe with a diameter of 0.4 m and height of 0.4 m was inserted 0.4 m underground. Further, 0.5 m of the pipe was kept above the ground to prevent the loss of 15 N and the entry of external N. A 0.5-cm hole was drilled in the PVC pipe 20 cm above the surface of the field. When the water level in the micro area dropped to the field surface, the field surface water flowed into the micro area as replenishment. While fertilizer was added, the hole was blocked with mud to prevent fertilizer loss in the micro area.
In the non-rice season, straw was returned to the field using a harvester such that a low stubble remained. The straw was then crushed on the surface to cover the field. For the no-straw treatment, the straw was manually removed from the field after the rice harvest in 2017. In the cultivation system, we also used crayfish (Procambarus clarkii). Feed was added every afternoon from March 12 to June 1, but for the no-feed treatment, no additional feed was added. Crayfish were fed with commercial formula feed containing 4.6% nitrogen per day at about 17:00 throughout the experiment. The daily amount of feed added was about 1.5% of the fresh body mass of the crayfish in each plot, and this amount increased as the crayfish grew. Crayfish were harvested daily between March and June; adult crayfish were picked, and the juveniles were returned to the original community. The amount of straw returned to the field and the amount of feed added are shown in Table 1.
In the rice season, we used the rice variety Taiyou 390 (Oryza sativa L). Three weeks after germination, rice seedlings were transferred to the test plots, with 25-cm gaps between rows and 26-cm gaps between the hills (four seedlings per hill) within each row. During the growth period, other management measures such as pest control were implemented uniformly across treatment plots.
Plant, soil and crayfish sampling and analysis
0–20 cm of the cultivated soil layer were collected and then air dried for chemical analysis. For the experiment, 1 g of 0.15 mm dry soil was obtained and concentrated sulfuric acid was added for digestion and measured soil total nitrogen. The determination of soil bulk density was performed using the ring knife method (Bao 2000). In the experiment, all the above-ground rice plants in the microcosms experiment were collected after rice growth was complete. The stems, leaves, and panicles were separated, heated at 105 °C for 0.5 h, and then dried in an oven at 80 °C until they reached constant weight. Rice plant samples that were dried to a constant weight and ground with 0.15 mm diameter. The soil samples and dried plants were ground with a ball mill and wrapped separately into balls with tin foil. The isotopic abundance was measured using a stable isotope mass spectrometer (IsoPrime 100 IRMS, Isoprime Ltd, Stockport, UK).
During the feeding period, 15 crayfish were randomly selected from among the crayfish caught from each plot and dried at 80 °C until they reached constant weight. The TN content of the rice plants, crayfish feed, and straw were obtained separately using a C/N analyzer (Vario MAX-CN elemental analyzer, Germany).
Data analysis
Analysis of nitrogen balance
The nitrogen balance with the RC field was calculated by subtracting the TN output (nitrogen present in harvested plants and crayfish) from the total input (nitrogen present in fertilizers, crayfish feed, and straw). A positive value indicated that some portion of the input nitrogen had not been used by rice and crayfish and remained in the field plot (as nitrogen in the soil or water) or had moved into the surrounding environment (as nitrogen in the atmosphere or drainage water).
Analysis of the fate of the nitrogen derived from crayfish feed and straw
In fields subjected to NN treatment, the nitrogen in crayfish (crayfish N) was derived from the environment only and the nitrogen in rice plants (rice N) was derived from fertilizers and the environment. In fields subjected to NF treatment, crayfish N was derived from crayfish feed and the environment, and rice N was derived from fish feed, fertilizers, and the environment. In fields subjected to SN treatment, crayfish N was derived from straw and the environment, and rice N was derived from straw, fertilizers, and the environment. To understand the fate of the crayfish- and straw-derived nitrogen in the RC system, we performed eight calculations, which included the following variables:
-
XSN, nitrogen input through straw under the SN treatment;
-
XNF, nitrogen input through feed under the NF treatment;
-
crayfishSN-N, crayfish N under the SN treatment;
-
riceSN-N, rice N under the SN treatment;
-
crayfishNF-N, crayfish N under the NF treatment;
-
riceNF-N, rice N under the NF treatment;
-
fishNN-N, crayfish N under the NN treatment;
-
riceNN-N, rice N under the NN treatment;
-
FXNF, crayfish N derived from crayfish feed under the NF treatment;
-
RXNF, rice N derived from crayfish feed under the NF treatment;
-
FXSN, crayfish N derived from straw under the SN treatment;
-
RXSN, rice N derived from straw under the SN treatment;
-
(%) FXNF, percentage of total crayfish feed-derived nitrogen incorporated into the crayfish biomass under the NF treatment;
-
(%) RXNF, percentage of total crayfish feed-derived nitrogen incorporated into the rice biomass under the NF treatment;
-
(%) environment NF, percentage of total crayfish feed-derived nitrogen remaining in the environment under the NF treatment;
-
(%) FXSN, percentage of total straw-derived nitrogen incorporated into the crayfish biomass under the NF treatment;
-
(%) RXSN, percentage of total straw-derived nitrogen incorporated into the rice biomass under the NF treatment;
-
(%) environment SN, percentage of total straw-derived nitrogen remaining in the environment under the NF treatment.
The calculations were performed as follows:
Stable isotope analysis
Stable isotope values were reported in δ notation:
where R is 15 N/14 N, and the standard sample used for determining 15 N concentration is N2 from the air (15 N = 0.003676).
The percentage of nitrogen from the fertilizer in the total rice biomass-N or total soil nitrogen was calculated as follows:
where Ndff (%) represents the proportion of rice N derived from the fertilizer. 15Nsample, 15N0, 15Nfertilizer, and 15Nnature represent the plant sample, 0 kg N ha−1 treatment sample, fertilizer, and natural abundance value in the mesocosms, respectively.
We estimated the percentage of fertilizer-derived nitrogen that was used by the rice as follows:
where Nrice is the total rice biomass-N and Nfertilizer is total fertilizer-N in the mesocosms experiment.
We estimated the percentage of fertilizer-derived nitrogen that was stored by the soil as follows:
where Ndff is the percentage of fertilizer-derived nitrogen in the soil. The H value is 20, representing the thickness of the soil layer (cm). BD is soil bulk density (g cm-3), and TNsoil is the TN content of the soil (g kg−1). Nfertilizer is 15 N-labeled urea nitrogen content (kg ha−1) and the 10 is for unit conversion.
where Rloss (%) represents the percentage of fertilizer-derived nitrogen lost to the environment.
Statistical analysis
All statistical analyses were performed using SPSS 22.0 (SPSS, Inc., USA). One-way analysis of variance (ANOVA) at α = 0.05 probability was conducted to test the significance of differences across different treatments, and if significance was observed via ANOVA, an LSD test was used to compare means. OriginPro 9.0 was used to prepare all graphs.
Results
Rice aboveground biomass, yield, 15 N natural abundance, and nitrogen content of the rice fractions
In the NF treatment, the rice stem biomass under 225 N was significantly higher than that under 0 N (p < 0.05), but there was no significant difference between the values at 75 N and 150 N (Table 2). Among NN, SF, and NF treatments, the rice yield under 75 N and 150 N application was significantly higher than that under 225 N and 0 N application (p < 0.05) (Table 2). There was no significant difference in rice yield between 75 and 150 N application (Table 2).
Under different nitrogen rates, the natural abundance of 15 N (δ15N) in the fractions of rice was significantly different (p < 0.05); it was the highest under 225 N, followed by 150 N, 75 N, and 0 N (Table 3). During NN, SF, and SN treatment, there was no significant difference in nitrogen content in the fraction of rice at different nitrogen rates (Table 3). During NF treatment, at 225 N, the nitrogen content in the leaves was significantly higher than that at 150 N, 75 N, and 0 N (p < 0.05). However, there was no significant difference in grain nitrogen content under different nitrogen rates (Table 3).
Crayfish yield and nitrogen content
The yield of crayfish under SF and NF treatments was significantly higher than that under NN and SN treatments (Table 4) (p < 0.05). The yield of crayfish in SN was significantly higher than that in NN (p < 0.05). Although there was no significant difference in crayfish yield between SF and NF, the crayfish yield with SF treatment was 9% higher than that with NF treatment (Table 4). The nitrogen accumulation in crayfish under SF and NF treatments was also significantly higher than that under NN and SN treatments (p < 0.05) (Table 4). The crayfish yield was therefore mainly affected by feed input.
Soil nitrogen and 15 N content
After one year of RC co-culturing, the total nitrogen content of the soil did not differ significantly under different nitrogen rates in the NN, SF, SN, and NF treatments (Table 5). However, the 15 N content of the soil in each treatment increased significantly with an increase in nitrogen rate (225 N > 150 N > 75 N) (p < 0.05). Under 75 N, 150 N, and 225 N nitrogen rates, the total nitrogen content of the soil during SF, SN, and NF treatments was significantly higher than that during NN treatment (Table 5) (p < 0.05).
Nitrogen use efficiency in RC
Efficiency of straw- and feed-derived nitrogen use
We calculated the use rate of straw and feed nitrogen in the RC system using the subtraction method (Xie et al. 2011). Under the 0 N, 150 N, and 225 N treatments, 13.66% of feed nitrogen was absorbed by the crayfish and the remaining 86.34% was lost to the environment (Fig. 2a, c, d). However, under a nitrogen application rate of 75 N, 1.86% of the feed nitrogen was absorbed by the rice, and the loss of feed nitrogen to the environment was reduced to 84.48% (Fig. 2b).
Pie charts showing the proportion of nitrogen derived from crayfish feed in harvested rice, harvested crayfish, and the environment (e.g., 13.6% of the N supplied by crayfish feed was estimated to be present in the RC system) and the proportion of nitrogen derived from straw in harvested rice, harvested crayfish, and the environment in the RC system (e.g., 4.3% of the nitrogen supplied by straw was estimated to be contained in the RC system) under different nitrogen application conditions. (a) 0 kg N ha−1 level, (b) 75 kg N ha−1 level, (c) 150 kg N ha−1 level, and (d) 225 kg N ha−1 level. The calculations for the balance of nitrogen output and input within the RC system are described in the Materials and methods “Analysis of nitrogen balance” section. The calculations used to determine the fate of feed-N and straw-N are described in the Materials and methods “Analysis of the fate of the nitrogen derived from crayfish feed and straw” section
Under different rates of nitrogen application, 4.3% of straw nitrogen was absorbed by crayfish (Fig. 2). However, under the 0 N, 75 N, 150 N, and 225 N treatment, 10.33%, 8.78%, 8.15%, and 0% of straw nitrogen was absorbed by the rice, respectively (Fig. 2a, b, c, d). With the increase in nitrogen rates, the amount of straw nitrogen lost to the environment increased (Fig. 2).
Efficiency of fertilizer-derived nitrogen use
Under NN, SF, NF, and SN treatments, the percentage of fertilizer-derived nitrogen in rice stems, leaves, and grains (Ndff) increased significantly with the increase in nitrogen application rates (p < 0.05) (Table 6). As shown by the nitrogen distribution pattern (Table 6), the nitrogen uptake by grains was significantly higher than that by rice stems and leaves. Under SF treatment, panicles accounted for 65.1%, 66.8%, and 62.4% of the total nitrogen uptake at application rates of 75 N, 150 N, and 225 N, respectively.
There was no significant difference in the NUE of rice stems and leaves across nitrogen levels under each treatment (Table 6). Under NN and SF treatments, the NUE of rice panicles at application rates of 75 N and 150 N was significantly higher than that at 225 N (p < 0.05) (Table 6). However, there was no significant difference in NUE between the 75 N and 150 N rates. Although the NUE of rice panicles at 150 N was significantly higher than that at 75 N and 225 N under the SN treatment (p < 0.05), there was no significant difference between the values at 75 N and 150 N under the SF treatment.
The application of chemical nitrogen fertilizers significantly increases food production. However, not all the nitrogen fertilizer added to agricultural fields remains available for crop nourishment — some is absorbed by the crop, some is stored in the soil, some is discharged into the atmosphere in gaseous form, and some is lost to water bodies due to rainfall and irrigation. Nitrogen gas is discharged into the atmosphere, intensifying the greenhouse effect and accelerating global warming. The addition of nitrogen to surface water bodies, especially those receiving return flow from the agricultural farmland in the irrigation region, can lead to eutrophication.
The flow of nitrogen at different nitrogen fertilizer application rates under different straw and feed treatments in RC fields is shown in Fig. 3. The storage of fertilizer nitrogen in the soil ( Rsoil(%)) was calculated using formula 9 and the soil bulk density value (Table 7) (Fig. 3). In the SF and NF treatments, the Rsoil(%) value under the 75 N nitrogen rate was significantly higher than that under the 150 N and 225 N treatments (p < 0.05). Under SF treatment, the amount of nitrogen fertilizer stored in the soil and rice plants at application rates of 75 N, 150 N, and 225 N was 44 kg ha−1, 72 kg ha−1, and 88 kg ha−1, respectively. The nitrogen didn’t accounted for in the rice plant and soil was presumably lost; therefore, under SF treatment, the rates at which 15 N was lost were 41%, 52%, and 61% at application rates of 75 N, 150 N, and 225 N, respectively. The amount of nitrogen recovered increased and the rate of loss increased when more nitrogen fertilizer was added to the fields under 150 N and 225 N treatments. When fertilizer application was reduced to 75 N, nitrogen uptake by rice plants and soil reduced to 43 kg ha−1, 44 kg ha−1, and 40 kg ha−1 under NN, SF, and NF treatments, respectively, but the loss rate (Rloss(%)) significantly reduced too, reaching 43%, 41%, and 46% (p < 0.05), respectively. Although, Rloss (%) at application rates of 75 N, 150 N, and 225 N under the SN treatment was 66%, 66%, and 62%, respectively, there was no significant difference in the nitrogen levels across microcosms treatment with these application rates.
Flow direction of fertilizer nitrogen. Different capital letters represent significant differences (p < 0.05) between different treatments under the same nitrogen fertilizer application rates; different lowercase letters represent significant differences (p < 0.05) under the same treatment but different nitrogen fertilizer application rates. The labels 75, 150, and 225 on the X-axis represent the nitrogen application rates of 75 kg ha−1, 150 kg ha−1, and 225 kg ha−1, respectively. NN, no straw with no feed; SF, straw with feed; SN, straw with no feed; NF, no straw with feed. Error bars represent the standard deviation. NUE (%), the percentage of the nitrogen in the fertilizer absorbed and utilized by rice plants; Rsoil (%), the percentage of the nitrogen in the fertilizer stored in the soil; Rloss (%), the percentage of the nitrogen in the fertilizer lost to the environment
Nitrogen storage in the RC system
With the increase in the nitrogen rate, the nitrogen storage in rice grains increased slightly under the NN, SF, SN, and NF treatments, but the differences were not significant (Fig. 4). Under different nitrogen rates, the nitrogen accumulation in crayfish was significantly higher under SF and NF treatments than under NN and SN treatments (p < 0.05). Under 0 N, the rice and crayfish in the RC system could absorb 2 and 1.5 kg N ha−1 from the environment at NN and SN treatment, respectively (Fig. 4a). However, under SF and NF treatment, 60 kg ha−1 and 30 kg ha−1 of nitrogen was released into the environment, respectively. With the increase in the amount of nitrogen fertilizer, the nitrogen released into the environment in the RC system under treatments of returning straw to the field and feeding increased. Under different nitrogen fertilizer rates, the storage capacity for nitrogen was significantly higher in the SF and NF treatments than in the NN and SN treatments (Fig. 4) (p < 0.04).
Nitrogen storage in rice grains, leaves, and stems; crayfish; and the environment in a field experiment in which the rice-crayfish (RC) system was subjected to different nitrogen application conditions. (a) 0 kg N ha−1 level, (b) 75 kg N ha−1 level, (c) 150 kg N ha−1 level, and (d) 225 kg N ha−1 level. Values are means ± SD. A negative mean for environmental nitrogen indicates that rice and/or crayfish, in addition to obtaining N from feed, obtained N from environmental sources. A positive value for environmental N indicates that N was lost to the environment
Discussion
Our results showed that the yield of crayfish under feeding conditions (SF and NF) was significantly higher than that under no feeding conditions (SN and NN). As the direct food source for crayfish, feed can effectively promote their growth (Mukherjee et al. 2010; Naseem et al. 2020). Interestingly, our research found that the yield of crayfish in the SF condition was 13% higher than that in the NF condition, and the value in the SN condition was 2.3% higher than that in the NN condition (Table 4). Returning straw to the field increases the production of crayfish. During the non-rice season, crayfish feed on rotten straw and microorganisms in order to obtain nutrients (Chien and Avault 1983; Brown et al. 1990). Crayfish feed on straw, which accelerates the decomposition of owing to swallowing the straw effect, promotes the accumulation of organic matter in the paddy soil, and improves the fertility of the paddy soil (Yuan et al. 2020).
Under SF and NF conditions, 75 N and 150 N treatments provided the highest rice yield, and there was no significant difference in rice yield between 75 and 150 N treatment (Table 2). However, in the SN condition, the Rloss(%) under 150 N and 75 N nitrogen application rates was 41% and 52%, respectively, with a significant difference between the values (Fig. 3). Although under the NF condition, the Rloss(%) was not significantly different between 75 and 150 N, the Rloss(%) at 150 N was 15% higher than that at 75 N (Fig. 3). Under the SF condition, although the dry matter accumulation in rice stems and leaves was the highest at an application rate of 225 N, the dry matter in the grains was the lowest (Table 2). Further, the total nitrogen accumulation in the stems, leaves, and grains was the lowest under SF treatment (Fig. 4). The excessive use of nitrogen fertilizers can make rice stems and leaves flourish but reduce the overall efficiency, resulting in insufficient grains and poor maturity of rice plants (Yang et al. 2018). The application of high-nitrogen fertilizers results in an increase in pests and diseases in rice fields and reduces rice yields (Bowling 1963; Prabhu et al. 1996; Slaton et al. 2003). In the paddy field, rice plants can use the feed nitrogen that is not consumed by animals, as well as the ammonia that is excreted by fish and prawns (Mirhaj et al. 2014). In the RC system, feed nitrogen is a supplementary nitrogen source that can be used by rice, and therefore, the application of nitrogen fertilizer can be reduced. Our research shows that rice plants can use 13.66% and 4.3% of feed-N and straw-N in the RC system, respectively (Fig. 2). In the rice-fish system, rice plants can absorb 31.8% of feed-N, and the rice yield increases (Xie et al. 2011). Therefore, rice yield can be maintained with the application rate of 75 N, and we suggest that nitrogen fertilizer application should be reduced in the RC system.
Aquaculture provides protein for human nutrition (Nissa et al. 2021), but the eutrophication and environmental pollution caused by intensive aquaculture production are widespread (Herbeck et al. 2013; Bian et al. 2012). The eutrophication of water bodies during intensive aquaculture is mainly caused by the nitrogen and phosphorus from unconsumed feed (Cantas and Yildirim 2019). Combining the breeding of aquatic animals with the cultivation of rice can effectively promote the recycling and utilization of materials in the paddy field (Xie et al. 2011; Zhang et al. 2016). In the RC system, feed-N and straw-N can not only be absorbed by crayfish but can also be absorbed and utilized by rice, effectively promoting the nitrogen use efficiency.
Straw is an important renewable organic resource, which contains organic matter, nitrogen, phosphorus, potassium, calcium, sulfur and other nutrients required for crop growth and development (Ponnamperuma 1984). Straw returning to the field can improve soil physical properties, increase soil organic quality, improve soil water storage and moisture retention capacity, promote the growth of crop roots, and effectively improve the absorption and use of nitrogen in the soil by crops, thereby increasing rice yield (Huang et al. 2021; Wang et al. 2020a, b; Zhang et al. 2021). Studies have shown that the ratio C/N of straw is high, and it is difficult to be decomposed by microorganisms (Thippayarugs et al. 2008). The straw directly applied to the soil will stimulate the vigorous activity of microorganisms and retain part of the available nitrogen, resulting in the phenomenon of nitrogen competition with crops, thus affecting the growth of crops (Moritsuka et al. 2004). Therefore, The combination of straw returning and suitable nitrogen fertilizer management can alleviate the phenomenon of "nitrogen competition" caused by the growth and development of rice in the process of straw decomposition (Singh and Rengel 2007). With the decomposition of microorganisms, the nitrogen in the straw will be slowly released for crop growth (Guan et al. 2020). Research shows that straw incorporation coupled with deep placement of nitrogen fertilizer could be a feasible technique with the increase of grain yield and nitrogen use efficiency in direct-seeded rice in South China (Chen et al. 2020). It is important for the supply of optimum N to the crop and to maintain grain production with straw incorporation and adjusting the ratio of basal and topdressing nitrogen fertilizer (Tian et al. 2019). Combined straw organic nitrogen and fertilizer inorganic nitrogen application of can effectively improve crop nitrogen use efficiency and crop yield. In the RC system, due to the replenishment of feed-N and straw-N, the threshold of fertilizer nitrogen application during rice planting should be lower than the conventional application rate used by local farmers. Compared with the nitrogen application levels of 150 N and 225 N, the nitrogen content of rice plants and crayfish under the 75 N treatment was higher. With the increased of the nitrogen application rate, the nitrogen content in rice plants and crayfish did not increase significantly, but the nitrogen levels in the environment did (Fig. 4). The unreasonable application of nitrogen fertilizer in the RC system causes economic losses and also increases the risk of non-point source pollution in the rice field (Hu et al. 2021). A reasonable amount of nitrogen fertilizer should be used in conjunction with straw return to the field and feeding treatment to ensure a high yield of rice, while improving the nitrogen use efficiency in the RC system, effectively reducing environmental pollution. This can provide a win–win situation in RC culture during the production process.
Conclusion
Integrating intensive crayfish culture with rice culture can allow for the procurement of substantial crayfish yields and stable rice yields. Straw and feed addition increase the nitrogen input in the RC system. The part of the feed-derived nitrogen that is not consumed by the crayfish can be absorbed by rice plants, and part of the straw-derived nitrogen can also be absorbed by rice plants and crayfish. Our research shows that under the RC model, with field management measures such as straw return to the field and feeding, the amount of nitrogen fertilizer used in the rice season should be controlled and maintained under 75 kg ha−1. This will not only ensure a good rice yield but also reduce the accumulation of feed-N, straw-N, and fertilizer-N in the environment, thus reducing the potential for RC culture-induced environmental pollution during the production process.
Data availability
The datasets generated during and/or analysed during the current study are available from the corresponding author on reasonable request.
Abbreviations
- ORN:
-
Optimizing the rate of nitrogen fertilizer application
- SF:
-
Straw with feed
- SN:
-
Straw with no feed
- NF:
-
No straw with feed
- NN:
-
No straw with no feed
- 0N:
-
0 kg nitrogen ha−1
- 75N:
-
75 kg nitrogen ha−1
- 150N:
-
150 kg nitrogen ha−1
- 225N:
-
225 kg nitrogen ha−1
- Ndff (%):
-
The proportion of rice N derived from the fertilizer
- NUE(%):
-
Percentage of fertilizer-derived N that was used by the rice
- Rsoil(%):
-
The percentage of fertilizer-derived N that was stored by the soil
- Rloss (%):
-
Represents the percentage of fertilizer-derived N lost to the environment
References
Bao SD (2000) Soil Agrochemical Analysis. China Agricultural Press, Beijing, p 2000
Bian W, Wang LG, Zhang HZ, Wang JF, Tian ZF, Chen XY (2012) Study on phosphorus pollution load of aquaculture in baiyangdian lake. Adv Mater Res 518–523:1406–1411. https://doi.org/10.4028/www.scientific.net/AMR.518-523.1406
Bowling CC (1963) Effect of nitrogen levels on rice water weevil populations. J Econ Entomol 56(6):826–827. https://doi.org/10.1093/jee/56.6.826
Brown PB, Hooe ML, Blythe WG (1990) Preliminary evaluation of production systems and forages for culture of orconectes virilis, the northern or fantail crayfish. J World Aquac Soc 21(1). https://doi.org/10.1111/j.1749-7345.1990.tb00953.x
Cantas IB, Yildirim O (2019) Reducing the impact of feeds on the environment in sustainable aquaculture. Ege J Fish Aquat Sci 36(1):87–97. https://doi.org/10.12714/egejfas.2019.36.1.12
Chen SK, Jang CS, Chen SM, Chen KH (2013) Effect of N-fertilizer application on return flow water quality from a terraced paddy field in Northern Taiwan. Paddy Water Environ 11(1):123–133. https://doi.org/10.1007/s10333-011-0298-7
Chen YY, Fan PS, Li L, Tian H, Pan S, Ashraf U, Mo ZW, Duan MY, Wu QT, Zhang Z, Tang XR, Pan SG (2020) Straw incorporation coupled with deep placement of nitrogen fertilizer improved grain yield and nitrogen use efficiency in direct-seeded rice. J Soil Sci Plant Nutr 20(1):20. https://doi.org/10.1007/s42729-020-00301-2 (2338–2347)
Chien YH, Avault W (1983) Effects of flooding dates and disposals of rice straw on crayfish, Procambarus clarkii (Girard), culture in rice fields. Aquaculture 31(2–4):339–359. https://doi.org/10.1016/0044-8486(83)90324-1
China National Statistics Bureau (2018) China Population & Employment Statistic Yearbooks (China Statistics Press, Beijing, China, 2000–2016)
Cui K, Shoemaker SP (2018) A look at food security in china. npj Sci Food 2(1). https://doi.org/10.1038/s41538-018-0012-x
Davidson EA (2009) The contribution of manure and fertilizer nitrogen to atmospheric nitrous oxide since 1860. Nat Geosci 2(9):659–662. https://doi.org/10.1038/NGEO608
Erisman JW, Galloway JN, Seitzinger S, Bleeker A, Dise NB, Petrescu AMR, Leach AM, Devries W (2013) Consequences of human modification of the global nitrogen cycle. Phil Trans R Soc B 368:20130116. https://doi.org/10.1098/rstb.2013.0116
Guo JH, Liu XJ, Zhang Y, Shen JL, Han WX, Zhang WF, Christie P, Goulding KWT, Vitousek PM, Zhang FS (2010) Significant acidification in major Chinese croplands. Science 327:1008–1010. https://doi.org/10.1126/science.1182570
Guan XK, Wei L, Turner NC, Ma SC, Yang MD, Wang TC (2020) Improved straw management practices promote in situ straw decomposition and nutrient release, and increase crop production. J Clean Prod 250:119514. https://doi.org/10.1016/j.jclepro.2019.119514
Herbeck LS, Unger D, Wu Y, Jennerjahn TC (2013) Effluent, nutrient and organic matter export from shrimp and fish ponds causing eutrophication in coastal and back-reef waters of ne hainan, tropical china. Cont Shelf Res 57:92–104. https://doi.org/10.1016/j.csr.2012.05.006
Hu LL, Ren WZ, Tang JJ, Li NN, Zhang J, Chen X (2013) The productivity of traditional rice–fish co-culture can be increased without increasing nitrogen loss to the environment. Agr Ecosyst Environ 177:28–34. https://doi.org/10.1016/j.agee.2013.05.023
Hu LL, Zhang J, Ren WZ, Guo L, Cheng YX, Li JY, Li KX, Zhu ZW, Zhang JE, Luo SM, Cheng L, Tang JJ, Chen X (2016) Can the co-cultivation of rice and fish help sustain rice production? Sci Rep 6:28728. https://doi.org/10.1038/srep28728
Hu NJ, Liu CH, Chen Q, Zhu LQ (2021) Life cycle environmental impact assessment of rice-crayfish integrated system: a case study. J Clean Prod 280:102444. https://doi.org/10.1016/j.jclepro.2020.124440
Huang S, Zeng YJ, Wu JF, Shi QH, Pan XH (2013) Effect of crop residue retention on rice yield in China: a meta-analysis. Field Crop Res 154:188–194. https://doi.org/10.1016/j.fcr.2013.08.013
Huang TT, Yang N, Lu C, Qin XL, Siddique KHM (2021) Soil organic carbon, total nitrogen, available nutrients, and yield under different straw returning methods. Soil Tillage Res 214:105171. https://doi.org/10.1016/j.still.2021.105171
John C, Anthonie MB (2021) Comparison of Nutrient Costs from Fish Feeds and Inorganic Fertilizers for aquaponics systems. Aquacult Eng 95:102205. https://doi.org/10.1016/j.aquaeng.2021.102205
Li CF, Cao CG, Wang JP, Zhan M, Yuan WL, Ahmad S (2008) Nitrogen losses from integrated rice-duck and rice-fish ecosystems in southern China. 307(1-2):207-217. https://doi.org/10.1007/s11104-008-9597-1
Matson PA, Parton WJ, Power AG, Swift MJ (1997) Agricultural intensification and ecosystem properties. Science 277(5325):504–509. https://doi.org/10.1126/science.277.5325.504
Matsuyama N, Saigusa M, Sakaiya E, Tamakawa K, Oyamada Z, Kudo K (2005) Acidification and soil productivity of allophanic Andosols affected by heavy application of fertilizers. Soil Sci Plant Nutr 51(1):117–123. https://doi.org/10.1111/j.1747-0765.2005.tb00014.x
Mirhaj M, Razzak MA, Wahab MA (2014) Comparison of nitrogen balances and efficiencies in rice cum prawn vs. rice cum fish cultures in Mymensingh, North-Eastern Bangladesh. Agric Syst 125:54–62. https://doi.org/10.1016/j.agsy.2013.12.004
Mukherjee AK, Kalita P, Unni BG, Wann SB, Saika D, Mukhpadhyay PK (2010) Fatty acid composition of four potential aquatic weeds and their possible use as fish-feed nutraceuticals. Food Chem 123:1252–1254. https://doi.org/10.1016/j.foodchem.2010.05.057
Moritsuka N, Yanai J, Mori K, Kosaki T (2004) Biotic and abiotic processes of nitrogen immobilization in the soil-residue interface. Soil Biol Biochem 36(7):1141–1148. https://doi.org/10.1016/j.soilbio.2004.02.024
Naseem S, Bhat SU, Gani A, Bhat FA (2020) Perspectives on utilization of macrophytes as feed ingredient for fish in future aquaculture. Rev Aquac: 1–19.https://doi.org/10.1111/raq.12475
Nissa MU, Pinto N, Parkar H, Goswami M (2021) Proteomics in fisheries and aquaculture: an approach for food security. Food Control 127:108125. https://doi.org/10.1016/j.foodcont.2021.108125
Prabhu AS, Filippi MC, Zimmerman F (1996) Genetic control of blast in relation to nitrogen fertilization in upland rice. Pesqui Agrop Bras 31(5):339–347. https://doi.org/10.1002/(SICI)1097-0010(199605)71:1%3c137::AID-JSFA563%3e3.0.CO;2-Y
Ponnamperuma FN (1984) Straw as source of nutrients for wetland rice. In Organic matter and rice. Eds. S Barta and C V Mondeza. pp. 117–136. IRRI, Los Banos, Philippines
Powlson DS, Glendining MJ, Coleman K, Whitmore AP (2011) Implications for soil properties of removing cereal straw: results from long-term studies. Agron J 103(1):279. https://doi.org/10.2134/agronj2010.0146s
Si GH, Peng CL, Yuan JF, Xu XY, Zhao SJ, Xu DB, Wu JS (2017) Changes in soil microbial community composition and organic carbon fractions in an integrated rice–crayfish farming system in subtropical China. Sci Rep 7:2856. https://doi.org/10.1038/s41598-017-02984-7
Singh B, Rengel Z (2007) The role of crop residues in improving soil fertility. In: Marschner P, Rengel Z (eds) Nutrient Cycling in Terrestrial Ecosystems. Soil Biology. Springer, Berlin, Heidelberg, vol 10. https://doi.org/10.1007/978-3-540-68027-7_7
Slaton NA, Cartwright RD, Meng J, Gbur EE, Norman RJ (2003) Sheath blight severity and rice yield as affected by nitrogen fertilizer rate, application method, and fungicide. Agron J 95(6):1489–1496. https://doi.org/10.2134/agronj2003.1489
Thippayarugs S, Toomsan B, Vityakon P, Limpinuntana V, Patanothai A, Cadisch G (2008) Interactions in decomposition and n mineralization between tropical legume residue components. Agrofor Syst 72(2):137–148. https://doi.org/10.1007/s10457-007-9062-9
Tian ZW, Ge YX, Zhu Q, Yu JH, Zhou Q, Cai J, Jiang D, Cao WX, Dai TB (2019) Soil nitrogen balance and nitrogen utilization of winter wheat affected by straw management and nitrogen application in the Yangtze river basin of China. Arch Agron Soil Sci 65:1–15. https://doi.org/10.1080/03650340.2018.1479743
Xie J, Hu LL, Tang JJ, Wu X, Li NN, Yuan YG, Yang HS, Zhang JE, Luo SM, Chen X (2011) Ecological mechanisms underlying the sustainability of the agricultural heritage rice-fish coculture system. Proc Natl Acad Sci USA 108(50):1381–1387. https://doi.org/10.1073/pnas.1111043108
Wang QX, Li ML, Chen XJ, Su Y, Yu M, Shen AL (2020a) Effects of nitrogen management in soil microbial community structure at different growth stage under straw returning in paddy soils. Chin J Appl Ecol 31(03):935–944. https://doi.org/10.13287/j.1001-9332.202003.027
Wang W, Lai DYF, Wang C, Pan T, Zeng C (2015) Effects of rice straw incorporation on active soil organic carbon pools in a subtropical paddy field. Soil Tillage Res 152:8–16. https://doi.org/10.1016/j.still.2015.03.011
Wang HN, Yang GT, Wang XC, Zhao CK, Muhammad N, Chen YJ, Hu YG (2020b) Oilseed rape straw returning changes soil reducibility and affects the root and yield of rice in the rape-rice rotation field at Sichuan Basin area of China. Agron J 112(6):4681–4692. https://doi.org/10.1002/agj2.20408
Xu GH, Fan XR, Anthony JM (2012) Plant nitrogen assimilation and use efficiency. Annu Rev Plant Biol 63(1):153–182. https://doi.org/10.1146/annurev-arplant-042811-105532
Xu YL, Chen ZM, Ding WX, Fan JL (2017) Responses of manure decomposition to nitrogen addition: role of chemical composition. Sci Total Environ, 587–588, 11-21. http://doi.org/10.1016/j.scitotenv.2017.02.033
Yang GT, Wang XC, Peng YL, Rasul F, Zou T, Hu YG (2018) Different micro-climate response of indica rice population to nitrogen fertilizer. Plant Soil Environ 64(No. 9):407–412. https://doi.org/10.17221/422/2018-pse
Yi J, Gao JP, Zhang WZ, Zhao C, Wang Y, Zhen XX (2019) Differential uptake and utilization of two forms of nitrogen in japonica rice cultivars from north-eastern china. Front Plant Sci 10:1061. https://doi.org/10.3389/fpls.2019.01061
Yuan PL, Wang JP, Li CF, Cao CG (2020a) Long-term rice-crayfish farming aggravate soil gleying and induced changes of soil iron morphology. Soil Use Manag: 1–14.https://doi.org/10.1111/sum.12688
Yuan PL, Wang JP, Li CF, Xiao QQ, Liu QJ, Sun ZC, Wang JH, Cao CG (2020b) Soil quality indicators of integrated rice-crayfish farming in the jianghan plain, china using a minimum data set. Soil Tillage Res 204:104732. https://doi.org/10.1016/j.still.2020.104732
Yuan PL, Wang JP, Guo C, Guo ZY, Guo Y, Cao CG (2022) Sustainability of the rice–crayfish farming model in waterlogged land: A case study in Qianjiang County, Hubei Province, China. J Integr Agr, 21(4): 1203–1214. https://doi.org/10.1016/S2095-3119(21)63787-5.
Zhao GC, Hou LG, Cao Z, Zhao FJ, Sui PJ (2005) Study on the technology of rice-duck co-cultivation. J Jilin Agric Sci 30(1):13–15. https://doi.org/10.16423/j.cnki.1003-8701.2005.01.004 (in Chinese)
Zhang J, Hu LL, Ren WZ, Guo L, Tang JJ, Shu MA, Chen X (2016) Rice-soft shell turtle coculture effects on yield and its environment. Agr Ecosyst Environ 224:116–122. https://doi.org/10.1016/j.agee.2016.03.045
Zhang LH, Zhao HX, Xu C, Yan WP, Sun N, Tan GB, Yu J, Meng XM, Li F, Bian SF (2021) Effects of straw returning on soil moisture and maize yield in semi-humid area. Cereal Res Commun: 1-9. https://doi.org/10.1007/s42976-021-00194-6
Acknowledgements
This research was special fund for basics cientific research operation fees of central universities (2662019FW010 and 266202KPY014).
Funding
The authors declare that no funds, grants, or other support were received during the preparation of this manuscript.
Author information
Authors and Affiliations
Contributions
Pengli Yuan: Conceptualization, design, data collection, Writing, Revision. Xinhao Li: Visualization, revision. Mingli Ni: Material preparation. Cougui Cao: Supervision. Ligeng Jiang: Writing – review. Anas Iqbal: Editing. Jinping Wang: Experiment design.
Corresponding author
Ethics declarations
Competing interests
The authors have no relevant financial or non-financial interests to disclose.
Additional information
Responsible Editor: Al Imran Malik.
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Yuan, P., Li, X., Ni, M. et al. Effects of straw return and feed addition on the environment and nitrogen use efficiency under different nitrogen application rates in the rice–crayfish system. Plant Soil 475, 411–426 (2022). https://doi.org/10.1007/s11104-022-05376-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11104-022-05376-7