Abstract
Background and aims
An integrated understanding of the ecophysiological mechanisms of persistence of buried seeds is essential for understanding plant community dynamics and improving our ability to accurately predict seed persistence. However, little is known about how the interaction of precipitation and microorganisms affects persistence of buried seeds and how well seed traits predict seed persistence, especially in a changing environment.
Methods
Here we determined the combined effect of precipitation and microorganisms on the persistence of buried seeds of 11 species from the Loess Plateau in China, and examined the correlation between seed persistence and seed traits, including seed mass, seed dimensions, seed shape, seed toughness, seed water absorption, total phenolic content, crude protein content and seed longevity.
Results
Seed persistence of all species decreased with increasing precipitation. Fungicide treatment improved seed persistence of all tested species, while the effect size of fungicide treatment on persistence increased with increasing of precipitation. Persistence was positively correlated with seed longevity (P50), seed toughness and total phenolic content in all treatments. Seed water absorption was not correlated with seed persistence. However, the relationship between seed persistence and seed shape, seed mass, crude protein content, seed germination was strongly dependent on environmental conditions.
Conclusions
Changes in precipitation not only affected seed persistence but also the relationship between seed persistence and seed traits. Thus, environmental factors should be fully considered in making predictions about seed persistence based on seed traits.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The ability of mature seeds to remain viable in the habitat either on the mother plant or in/on the soil is referred to as seed persistence, and long-term persistence helps ensure dispersal of seeds through time and space (Long et al. 2015; Ooi 2012; Volis and Dorman 2019). The persistence of seeds is an important adaptive trait that can affect population dynamics and community composition and avoid local extinction of plant species (Fenner and Thompson 2005). Thus, knowledge of seed persistence in the soil is of fundamental importance in understanding the maintenance of biodiversity and genetic diversity in communities (de Jong et al. 2013; Ozinga et al. 2005).
The environmental conditions in which seeds are dispersed affect seed persistence through their influences on seed ageing, dormancy-break and germination (Bekker et al. 1998; Golos and Dixon 2014; Holzel and Otte 2001; Long et al. 2015; Ma et al. 2020; Metzner et al. 2017). For example, low amounts of precipitation enhance longevity of desiccation-tolerant seeds (Burnside et al. 1996), but increase the probability of death of desiccation-sensitive seeds (Tweddle et al. 2003). An et al. (2020) have reported that precipitation rather than temperature directly affected persistence of the seed bank. Other studies showed that groundwater level (Bekker et al. 1998) and flooding regime (Holzel and Otte 2001) also affected seed persistence. In addition, temporally variable environments, such as deserts or arid and semi-arid ecosystems where precipitation trends to be low and unpredictable, are conductive to the persistence of seeds. Seed persistence in arid habitats has been predicted by ecological theory and supported by empirical data (Cohen 1966, 1967; Venable and Brown 1988). Since, changes of precipitation, an important feature of global climate change in some regions, can influence seed ageing, dormancy release, germination and thereby persistence in natural ecosystems (Chen et al. 2020; Cuena-Lombrana et al. 2020; Hu et al. 2018; Walck et al. 2011). Thus, information is needed on the effects of increased or decreased precipitation in arid ecosystems on persistence of buried seeds. Studies on the effect of changes in amount of precipitation on seed persistence, especially in arid and semi-arid ecosystems, would improve prediction with regard to plant regeneration from seeds, migration (move to a more suitable environment by seeds) and coexistence in a scenario of climate change.
Generally, precipitation affects or influences the soil (and seed) moisture content, which could affect seed persistence by promoting or inhibiting seed germination (Abedi et al. 2014; Chen et al. 2020; Hoyle et al. 2008; Hu et al. 2018; Long et al. 2011). For example, Hu et al. (2018) found that soil moisture content had a significant effect on seed dormancy release of four of five widespread weedy species on the semi-arid Loess Plateau of China. Harrington (1960) proposed a rule that seed longevity is halved for each 1% increase in seed moisture content when it is between about 5 and 14%. In general, the moisture content of soil may affect seed longevity by changing the respiration and microbial activity of seeds (Long et al. 2015). On the other hand, numerous studies suggested that microbial activity in the soil could affect seed longevity/persistence (Delgado-Sanchez et al. 2011; Long et al. 2015; Muller-Stover et al. 2016; Nelson 2018; Zhu et al. 2011). Thus, treatment with fungicides increases seed survival in the soil (Wagner and Mitschunas 2008), and the effect of fundicide treatment is stronger in wet than in relatively dry soil (Blaney and Kotanen 2001), suggesting that soil moisture content may have an impact on the effect of microorganisms on seed persistence. However, how the interaction of changes in precipitation and microbial activity affects seed persistence in the soil has rarely been studied (Long et al. 2015).
Seed traits related to dispersal, defense and germination, such as size, shape, dormancy, longevity and phenolic content can affect seed persistence in the soil (Long et al. 2015). Seed size may have negative (Funes et al. 1999; Hodkinson et al. 1998; Thompson et al. 2001; Zhao et al. 2011), positive (Holzel and Otte 2004; Moles et al. 2003) or no effect (Leishman and Westoby 1998; Probert et al. 2009) on seed persistence in the soil. Further, seed persistence due to low predation is promoted by high fiber and phenolic compound content and/or low oil and energy reserves (Davis 2007; Dechaine et al. 2010; Lepiniec et al. 2006; Pourcel et al. 2007; Xiao et al. 2008). However, seed oil content of 195 wild species from 71 families in habitats ranging from tropical forests to cold deserts was not positively correlated with seed longevity (Probert et al. 2009). There could be many reasons for these controversial results, and one possible reason is that different methods were used to describe seed persistence. For example, an accelerated aging test was used to determine the relationship between seed persistence and seed traits in the study by Probert et al. (2009) and Satyanti et al. (2018); however, seed longevity in the soil was used to express seed persistence in the other studies (Davis et al. 2008; Holzel and Otte 2004; Moles et al. 2003; Zhao et al. 2011). Thus the relationship between seed persistence and seed traits may vary with environmental conditions that seeds experienced, but this was not tested in the previous studies.
The broad objective of our research was to determine the effect of interactions between precipitation and microorganisms on seed persistence and examine the correlation between seed persistence and seed traits. Using seeds of 11 species collected from the Loess Plateau in China, we addressed the following questions: (i) Whether the increase or decrease of precipitation affects the persistence of seeds? If so, what is the role of soil moisture content and microbes in persistence of buried seeds? (ii) What is the relationship between seed persistence and seed traits? Does this relationship vary with precipitation and microbial activity?
Materials and methods
Species and seed collection
Freshly matured seeds of the 11 species used in this study were collected in Xifeng (hereafter XF) and Yuzhong (hereafter YZ), Gansu province, China, in July and September 2018, respectively. Seeds of Camelina microcarpa, Capsella bursa-pastoris, Descurainia Sophia, Lepidium apetalum and Ixeris chinensis were collected in XF (40°54′ N, 107°09′ E, 1035 m a.s.l.), where the mean annual temperature and mean annual rainfall are 9.2 °C and 528 mm, respectively, with most of it falling from July to September (Wang et al. 2019). Seeds of Amaranthus retroflexus, Chenopodium album, Setaria glauca, S. viridis, Datura stramonium and Hyoscyamus niger were collected in YZ (35°57′ N, 104°10′ E, 1700 m a.s.l.), where the mean annual temperature and mean annual rainfall are 6.7 °C and 400 mm, respectively, with most of it falling from July to September (Hu et al. 2014). For these 11 species, H. niger and I. chinensis are biennial and perennial, respectively, the other species are annuals. Seeds of five species collected in XF mature in early autumn and seeds of six species collected in YZ mature in late autumn. These 11 species were selected because they are the most common weeds in the Loess Plateau in China and co-exist in the same agricultural system, while they vary in life form and seed dormancy as well as seed germination requirements based on preliminary research findings. Infructescences with ripe seeds were collected from several hundred individual plants of each species and taken to the laboratory, where seeds were separated from other plant material. Seeds were dried at room temperature for 1 week (RH 20–35%, 18–25 °C) and then stored at -20 °C until used in experiments.
Determination of seed viability
Freshly matured seeds of each species were used to determine initial seed viability by staining with 2, 3, 5-triphenyl tetrazolium chloride (TTC, Yantuo, Shanghai, China). Three replicates of 50 seeds were placed in Petri dishes (10 cm) on two sheets of filter paper (Shuangquan, Hangzhou, China) immersed in a solution of 1% TTC. The dishes were wrapped with two layers of aluminum foil and incubated at 30 °C from 3 to 16 h, depending on the species. After staining by TTC solution, seeds were observed under a stereomicroscope without an eyepiece (Vision, England), and seeds that had stained red or pink were considered to be viable, and those without pigmentation were scored as nonviable (Thorogood et al. 2009).
Effect of temperature and light on germination of fresh seeds
To evaluate the effect of temperature and light on germination of mature seeds, freshly collected seeds of all species were tested at 10, 15, 20, 25, 30, 10/20, 15/25 and 20/30 °C in light (12/12 h daily photoperiod, white fluorescent tubes, photon irradiance was 60 μmol m−2 s−1, 400–700 nm) or continuous darkness. For continuous darkness, Petri dishes were covered with two layers of aluminum foil. For each treatment, three replicates of 50 seeds were placed in 10 cm diameter Petri dishes on two sheets of filter paper (Shuangquan, Hangzhou, China) moistened with 6–9 ml distilled water, depending on the species. All Petri dishes were randomly placed in the incubator and distilled water was added daily as needed to keep the filter paper moist. Germination of seeds incubated in light was monitored daily for at least 21 days until no further germination occurred for three consecutive days. Seeds were counted as germinated when the radicle was visible (≥ 2 mm) and any newly germinated seeds were discarded. Seeds incubated in the dark were examined for germination only after 21 days.
Determination of seed traits
Seed traits including seed mass, seed dimensions, seed shape, seed toughness, seed water absorption, total phenolic content, crude protein content and seed longevity were determined for all species used in this study. Seed mass was determined by weighing eight replicates of 100 seeds from each species (ISTA 2014). Thirty seeds of each species were randomly selected to measure seed dimensions (length, width and height) using a vernier caliper (10 μm precision, Chengliang, Chengdu, China). Seed shape was expressed as the variance in seed dimension after dividing each dimension by seed length (Thomas et al. 1993). Seed toughness was defined as the minimum mechanical force required to initiate seed rupture (Zalamea et al. 2018), which was measured by a digital mechanical force gauge (Handpi, Wenzhou, China) using 30 seeds for each species (Dong et al. 2017).
Three replicates of 50 seeds for each species were used to measure seed water absorption. Seeds were weighed and then immersed in distilled water and incubated at 20 °C (Chen et al. 2019). After 24 h, seeds were removed, dried with filter paper and weighed. Water absorption of seeds was calculated as the ration of cumulative water weight to the initial seed weight.
Total phenolic content and crude protein content of seeds for each species were determined as described by Ainsworth and Gillespie (2007) and Mitchell et al. (1974), respectively. In addition, accelerated ageing tests were used to evaluate inherent seed longevity using three replicates of 50 seeds for each species. Each replicate was placed in a 5 cm × 5 cm nylon mesh bag inside a closed 20 cm × 20 cm × 12 cm alloyed box that contained 2500 mL of distilled water, and seeds were suspended above the water. Seeds were aged at 42 °C for different period of time, depending on the species (Table S1), after which a germination test was conducted on all seeds, followed by a TTC test on nongerminated seeds after 21 days to determine seed viability as described above.
Seed burial tests
To determine the effect of precipitation, microorganisms and their interaction on seed persistence, seeds of all species were soaked with or without fungicide and buried under three precipitation treatments (drought (30% precipitation reduction), ambient (natural precipitation) and wet (30% precipitation addition)), after which seeds were retrieved regularly and seed viability determined. The seeds were buried in YZ, one of the seed collection sites, and they were buried in August and October 2018 due to inconsistent collection time of seeds.
A precipitation manipulation experiment was established in July 2018, which included three precipitation levels as described above (Fig. S1). Each treatment had three replicates and 9 plots (3 m × 3 m) that were divided into three blocks. The precipitation treatment was controlled by half of eight transparent acrylic tubes (a cylinder with a diameter of 12.5 cm, Senhuo, Zhejiang, China), which was divided into two parts by a complete acrylic pipe. The drought treatment was controlled by the acrylic pipe with a groove facing up, and 30% of the intercepted rainwater was collected and stored; it was applied to the wet plots within 12 h after each precipitation event with a spray bottle. To account for the effects of shading, eight acrylic tubes with a groove facing down also were installed in the control and wet plots. In addition, to reduce surface runoff and water leakage between adjacent plots, each plot was separated by four layers of plastic film within 1 m of the vertical surface, and a ridge 30 cm wide was set above the surface surrounding each plot.
To evaluate the effect of microbes on seed persistence, carbendazim (Guanlong agrochemical, Hebei, China), which is a systemic fungicide with curative and protection action, used extensively in agriculture, was applied to seeds and soils in this test (Pameri et al. 2016). Fifty percent of seeds were soaked in carbendazim (1 g/Kg, recommended by manufacturer) for 1 h, the other seeds were soaked in distilled water for the same time as a control, after which the seeds were taken out and dried in a cool place prior to burial. Loess soil was collected from YZ and mixed with carbendazim in a ratio of 1 to 250 (recommended by manufacturer) in the laboratory prior to seed burial. Then, 50 seeds were mixed with 20 g of soil treated with carbendazim (nontreated soil as a control) and placed into 5 cm × 5 cm nylon mesh bag. We buried 126 bags per each species (3 precipitation × 2 fungicide × 3 replicates × 7 sampling), for a total of 1386 bags for the 11 study species.
Each plot was divided into two parts with 30 cm left in the middle, which were used to bury seeds soaked with carbendazim and distilled water, respectively. Seventy-seven seed bags (seven bags per species) with 50 seeds were placed at a soil depth of 5 cm, and the spacing between bags was 5 cm. Three replicates of each treatment for all species were retrieved from burial after 1, 3, 5, 7, 9, 11 and 13 months. The numbers of died and germinated seeds were counted immediately and discarded after recovery from the plot. The remaining seeds were put into Petri dishes on two layers of filter paper for germination test at the optimum temperature as described above (Table S1). For all germination tests, seeds that failed to germinate were tested for viability as described above. In this study, seed persistence was expressed by seed survival for 13 months of burial, which refers to the ratio of the total number of germinated and viable seeds in the incubator to the number of initially buried seeds. Physical removal of weeds within plots was applied every week during the experimental period. Soil moisture content of 3.8 cm below the soil surface was measured 1 or 2 times a week during experimental period in each plot using a TDR100 (Campbell Scientific Inc., USA) (Fig. S2-S3).
Data analysis
The effect of precipitation, microorganisms and their interaction on seed persistence of each species was tested by fitting generalized linear mixed models (GLMMs). Precipitation and microorganisms were used as fixed effects, while replicates were included as random effects in each model. Seed germination was a probability ranging from 0 to 1, hence we applied a binomial estimation of the model using a logit link function. Tukey's test was used to compare means when significant difference were found. Correlation analysis was used to determine the relationship between seed persistence and seed traits, including seed longevity in the accelerated aging test, seed shape, seed mass, seed toughness, seed water absorption, total phenolic content, crude protein content and germination percentage under optimum conditions.
Response ratio was chosen to determine the effect of precipitation or fungicide-treatment on seed persistence according to Soltani et al. (2018). Effect size is the natural log of the response ratio (ln R) (Hedges et al. 1999):
\(\mathrm{ln}R=\mathrm{ln}\left({\overline{X} }_{T}-{\overline{X} }_{C}\right)\),
where \({\overline{X} }_{T}\) and \({\overline{X} }_{C}\) are the mean values for survival of treatments (precipitation and fungicide-treated) and control seeds. To avoid the log odds ratio from being infinite due to zero survival in the control and treatment seeds, we added 0.05% to the survival percentage data (Robertson et al. 2006).
A generalized linear model (GLM) with binomial error and probit link function was fitted to the survival data with time of ageing as an explanatory variable. Both species and treatments were included in the GLM as factors, thereby fitting the viability equation (Ellis and Roberts 1980).
\(v={K}_{i} -\left( p / \sigma \right)\),
where \(v\) is the viability (in normal equivalent deviates, NED) of the seed after \(p\) days (or months) in storage, \({K}_{i}\) initial viability (NED) of the seed and \(\sigma\) time for viability to fall by 1 NED (i.e., the standard deviation of the normal distribution of seed deaths over time). P50 in this study is the time (day) when seed viability to decrease 50% in seed accelerated ageing tests. All data were processed with GenStat, vision 18.0 (VSN International, Ltd, Hemel Hempstead, UK).
Results
Effect of temperature and light on germination of fresh seeds
Germination of S. viridis and D. stramonium seeds was null at all temperatures in both light and darkness. Temperature and light had significant effects on seed germination of the remaining species (P < 0.01), except for A. retroflexus (Table S2). Germination percentage of all species increased first and then decreased as temperature increased regardless of the light condition. The optimum germination temperature of seeds in this study varies with the species (Fig. S4, Table S1). Compare with the constant temperature, the alternating temperature was more favorable for seed germination of all species. In addition, seed germination in light was significantly higher than that in darkness, depending on temperature and species. For example, germination percentage of C. album was significantly reduced in darkness compared with that in light at all incubate temperatures except 10 and 15 °C, however, this was only observed at 15/25 °C for D. sophia seeds (P < 0.05) (Fig. S4). Moreover, the interactive effect between temperature and light on seed germination were only found in C. microcarpa, C. album, I. chinensis and S. glauca (Table S1).
Effect of precipitation and microorganisms on seed persistence
Precipitation, microorganisms and their interaction had significant effects on seed persistence of all species, except for the interactive effect on A. retroflexus (P < 0.05) (Table 1). Seed persistence of all tested species decreased significantly as precipitation increased (P < 0.05). Fungicide treatment significantly improved seed persistence of all species (P < 0.05). In addition, the effect size of fungicide treatment on seed persistence increased with increased precipitation, except for seeds of S. glauca. For example, the effect size of fungicide treatment on the persistence of I. chinensis seeds was 0.40, 1.61 and 5.32 under reduction, ambient and additional precipitation, respectively. Moreover, the effect size (absolute value) of precipitation reduction or addition on seed persistence was lower for seeds and soil treated with fungicide compare with the control, except for seeds of S. glauca under precipitation reduction (Fig. 1–2, S5-S6).
Effect of precipitation, microorganisms and their interaction on seed persistence of 11 species. Different letter indicate significant difference at 0.05 level for each species. – W, CK and + W defines as three precipitation levels, 30% precipitation reduction, ambient and 30% precipitation addition, respectively. The same below
Effect size for seed persistence with fungicide versus no fungicide. Error bars are referring to 95% confidence intervals. No overlap of error bars with zero indicate that fungicide-treated significantly affect seed persistence compare with control, the value of effect size is greater or less than zero means a positive and negative effect, respectively
The relationship between seed longevity and seed traits
In the accelerated ageing tests, seed longevity (P50) was positively correlated with seed mass, seed toughness, total phenolic content and crude protein content, however, it was negatively correlated with seed germination (P < 0.05) and not correlated with seed shape or seed water absorption (Table 2).
The relationship between seed persistence and seed traits
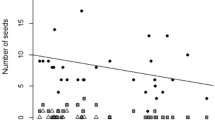
The relationship between seed persistence during burial and seed traits varied with environmental conditions. Seed persistence was positively correlated with P50, seed toughness and total phenolic content in all treatments. Depending on the environmental condition, seed persistence was either positively or not correlated with seed mass and crude protein content. A significant negative relationship was found between seed persistence and seed germination, except for precipitation addition without fungicide treatment (P < 0.05). In addition, seed persistence was not correlated with seed shape, except for precipitation reduction with fungicide treatment. Seed water absorption showed no correlation with seed persistence (Fig. 3).
The relationship between seed persistence and eight traits of seeds of 11 species. N and Y means seeds and soil used in burial tests treated with and without fungicide, respectively. R refers to the correlation coefficient and the number in bold red indicate a significant relationship between seed persistence and seed trait
Discussion
Effect of precipitation and microorganisms on seed persistence
It is clear from our results that seed persistence of all tested species decreased as precipitation increased. These results are consistent with those from a study by Burnside et al. (1996), in which seed persistence was longer with reduced rainfall than that with increased rainfall in a 17-year buried seed study of 41 weedy species in eastern and western Nebraska (USA). Generally, changes in seed moisture content resulting from changes in amount of precipitation play a key role in affecting seed persistence through its influence on seed metabolism, germination and dormancy release (Baskin and Baskin 2014; Long et al. 2015). Firstly, seed moisture content can affect seed metabolism and thus accelerate seed aging, which has been reported in many previous studies (Kibinza et al. 2006; Lee et al. 2019; Zhang et al. 1995). Secondly, especially in the natural state of arid and semi-arid ecosystems, seed moisture content is beneficial to seed germination when it increases to a certain degree, especially for seeds at shallow soil depths (Baskin and Baskin 2014; Fenner and Thompson 2005; Wagner et al. 2011). Consistent with this, our results also clearly showed that germination percentage of all species in the field increased with increased precipitation. Furthermore, seed moisture content has been found to have a significant effect on seed dormancy release (Chen et al. 2020; Hu et al. 2018), and thus it consequently affects seed germination and seed persistence.
On the other hand, soil microbial communities (e.g., soil fungi) could increase with increasing precipitation, which promotes seed decay and death, thereby decreasing seed persistence (He et al. 2017; Leishman et al. 2000). Thus, as expected, fungicide treatment promoted survival of burial seeds of several grass species Schafer and Kotanen (2004), Mimosa pigra in northern Australia (Lonsdale 1993), pioneer trees in Panama (Dalling et al. 1998) and old field species in southern Ontario (Blaney and Kotanen 2001).
In addition, soil microbes can promote seed germination by degrading allelochemicals that inhibit germination (Zhu et al. 2011). We found that without fungicide treatment germination percentage of all species in the field were improved significantly, consequently reduced seed persistence. On the other hand, some soil microbes can suppress the growth of pathogenic microbes, thereby protecting seeds from being killed (Nelson 2004). These controversial results may be due to the diversity of soil microbial and their many interactions with seeds (Chee-Sanford et al. 2006; Wagner and Mitschunas 2008). In the present study, although fungicide treatment improved seed persistence of all species, the effect size of fungicide on seed persistence varied a lot with species. The difference among species may be due to differences in seed traits and microbial communities they carries, but this has not been investigated.
In addition, a significantly interactive effect between precipitation and fungicide on seed persistence was found in ten of 11 species in our study, in which the effect size of fungicide on seed persistence increased with the increase of precipitation, one possible reason is that the fungicide works better at high than low moisture levels. Indeed, there is evidence that fungal pathogens generally are more prevalent in moist soil than in dry soil (Blaney and Kotanen 2001). Blaney and Kotanen (2001) also reported that fungicide addition improved seed survival more strongly in wetland soils than drier upland soils. Therefore, these results indicated that levels of seed mortality due to fungal decay may depend on soil moisture content (Wagner and Mitschunas 2008), and there is some evidence for the activity of microbial being highest at intermediate soil moisture levels that promote fungal growth (Kiewnick 1964). However, supplement watering did not promoted morality of Convolvulus arvensis, Lotus corniculatus, Medicago lupulina and Rubus fruticosus seeds treated with fungicide (Leishman et al. 2000). Moreover, our results showed that the effect size of fungicide treatment on seed persistence of different species was different, and it increased with increasing precipitation. Thus, this difference between species may be one reason for the controversial results in the previous studies (Burnside et al. 1996; Nelson 2004; Tweddle et al. 2003; Zhu et al. 2011).
Our study clearly showed that a change in precipitation will affect seed persistence, however, the response is complex and varies greatly in direction, magnitude and seasonality, especially in the arid and semi-arid ecosystems (Burrows et al. 2011; Long et al. 2015). Ma et al. (2020) used a structural equation model to explore underlying mechanistic basis of climate change on seed bank along an elevation gradient, they have found that rainfall not only affects soil moisture content and soil microbial communities, but it also affects soil chemical factors (pH, total nitrogen, total phosphorus, available nitrogen, available phosphorus, soil organic matter), and thus consequently affecting seed persistence. Thus, more biotic and abiotic factors should be considered when quantifying how changes in precipitation affect seed persistence in future studies. Moreover, changes in precipitation also affect soil temperature, which in turn affects the persistence of burial seeds. Generally, higher temperature experienced by seed can accelerate seed aging (Ellis and Roberts 1981) and alleviate dormancy of physical (Baskin 2003) and physiological (Iglesias-Fernandez et al. 2011), and thus decreasing seed persistence. However, our results showed that changes in precipitation do not affect the soil temperature (Fig. S7), a possible reason is that soil moisture content would not remain in a high level for a long time even for precipitation increasing treatment on the study area (semi-arid region), and consequently show less effect on soil temperature.
The relationship between seed persistence and seed traits
It generally has been assumed that total phenolic compounds in seeds can enhance their longevity via their antimicrobial, antioxidant and anti-predation characteristics (Davis et al. 2008; Lepiniec et al. 2006; Pourcel et al. 2007; Xiao et al. 2008). We found a significant relationship between seed persistence (or P50) and total phenolic compound content, both in the burial tests and accelerated ageing tests, suggesting that these compounds can play a crucial role in determining the susceptibility of seeds to ageing and microbial degradation. In addition, seed persistence was positively correlated with seed toughness both in the burial tests and in the accelerated ageing tests. High seed toughness would make it difficult for predators to eat them (Blate et al. 1998; Lundgren and Rosentrater 2007) and for microorganisms to destroy them (Gardarin et al. 2010). In general, our results indicate that total phenolic contents and seed toughness are perhaps the two most reliable predictors of seed persistence, even in a changing environment (Zalamea et al. 2018).
By comparison of seed persistence and seed longevity under rapid ageing conditions (45 °C and 60% RH) for 27 northwest European species, Long et al. (2008) found a significantly positive correlation. However, given that seed persistence is a continuous variable and the natural environment is complex, further studies are needed to test the applicability of using seed longevity to predict seed persistence under changing environment, especially in the arid and semi-arid ecosystems (Long et al. 2008). Our study clearly showed that seed persistence was positively correlated with P50 in all treatments. Thus, P50 is a reliable predictor of seed persistence, even in a changing environment, which complements the study of Long et al. (2008).
Numerous studies have reported other potential correlates of P50 in accelerated ageing tests, including seed mass, crude protein content and seed germination (Davis et al. 2008; Gardarin et al. 2010; Lepiniec et al. 2006; Merritt et al. 2014; Satyanti et al. 2018; Waterworth et al. 2010). Consistent with this, our results clearly found a significant positive relationship between P50 and seed mass and crude protein content, but a negative relationship with seed germination. Seed mass is often a significant factor in seed longevity studies with longevity generally increasing with seed mass across 172 Australian species (Merritt et al. 2014). However, seed longevity and seed mass may be negatively related (Satyanti et al. 2018) or not relatedd at all (Probert et al. 2009; Walters et al. 2005). Clearly presence of proteins for repair and protection against oxidative help determine seed longevity (Hundertmark et al. 2011; Waterworth et al. 2010) and Rajjou and Debeaujon (2008) have described some of the seed longevity proteins. Furthermore, higher germination percentages are not conducive to seed survival, leading to low seed longevity (Baskin and Baskin 2014; Long et al. 2015; Soltani et al. 2018; Wagner and Mitschunas 2008).
Although seed mass, crude protein content and seed germination were key factors for seed longevity in accelerated ageing tests, seed persistence was not necessarily correlated with these traits in all treatments. Seed persistence was negatively correlated with seed germination, except for precipitation addition without fungicide treatment. One possible reason is that germination percentage of all species in the field was significantly increased under precipitation addition and without fungicide treatment. Thus, these two positive effects may weaken the relationship between seed persistence and seed germination. This result indicated that environmental factors are as important as innate seed traits in determining the relationships between seed persistence and seed traits. The persistent soil seed bank tends to have smaller seeds than the transient seed bank (Funes et al. 1999; Hodkinson et al. 1998; Thompson et al. 2001; Zhao et al. 2011). However, there are exceptions to this size-persistence relationship in which large seeds live longer than small seeds (Holzel and Otte 2004; Moles et al. 2003) and where there is no correlation between persistence and seed mass (Leishman and Westoby 1998; Probert et al. 2009). Since environmental conditions such as rainfall characteristics and wind speed and species composition of the vegetation, have been suggested to have an effect on the relationship between seed persistence and seed traits (Yu et al. 2007), a primary reason for the difference between studies may be that seeds came from different ecosystems. Thus, the factors determining seed persistence depend on the species, biomes, the scale of study, as well as the combinations of variables included (Satyanti et al. 2018). According to Long et al. (2015), “Contradictory results do not necessarily discount the contribution of seed traits to seed persistence, as the influence biota and abiotic factors may determine whether their contribution is significant or not.” In any case, our results clearly showed that P50, seed toughness and total phenolic content could be used to predict the persistence of seeds well, even in a changing natural environment.
Seed persistence (or P50) was not correlated with seed shape in either the burial or the accelerated ageing tests. This result is different from the previous studies (Funes et al. 1999; Gomez-Gonzalez et al. 2011; Thompson et al. 2001), in which rounded seeds tended to persist in the soil for a longer time than elongated or flattened seeds. A possible reason may be that there were only 11 species used in our study and ten of them are roughly rounded in shape. In addition, seed moisture content is closely related to seed persistence as described above. Generally, seeds persist longer if seeds does not absorb water or if water absorption is low. However, no correlation between seed persistence (or P50) and seed water absorption was found in our study, suggesting that the variation of seed persistence (or P50) is independent of this trait, at least in our study. One possible reason is that seeds of all species may reach a water balance within a short period of time, although seed water absorption differs among the species.
Conclusions
In summary, seed persistence decreased as precipitation increase. Fungicide treatment improved seed persistence of all tested species, while the effect size of fungicide treatment on seed persistence increased with precipitation. Further, P50, seed toughness and total phenolic content could be used to predict the persistence of seeds, even in a changing environment. These results indicated that environmental factors, such as precipitation and microbial activity, not only affect seed persistence but also the relationship between seed persistence and seed traits. Therefore, environmental factors should be considered in studies using seed traits to predict seed persistence.
References
Abedi M, Bartelheimer M, Poschlod P (2014) Effects of substrate type, moisture and its interactions on soil seed survival of three Rumex species. Plant Soil 374:485–495. https://doi.org/10.1007/s11104-013-1903-x
Ainsworth EA, Gillespie KM (2007) Estimation of total phenolic content and other oxidation substrates in plant tissues using Folin-Ciocalteu reagent. Nat Protoc 2:875–877. https://doi.org/10.1038/nprot.2007.102
An H, Zhao Y, Ma M (2020) Precipitation controls seed bank size and its role in alpine meadow community regeneration with increasing altitude. Global Change Biol 26:5767–5777. https://doi.org/10.1111/gcb.15260
Baskin CC (2003) Breaking physical dormancy in seeds-focussing on the lens. New Phytol 158: 229–232. https://doi.org/10.1046/j.1469-8137.2003.00751.x
Baskin CC, Baskin JM (2014) Seeds: ecology, biogeography, and evolution dormancy and germination, 2nd edn. Academic Press, San Diego
Bekker RM, Oomes MJM, Bakker JP (1998) The impact of groundwater level on soil seed bank survival. Seed Sci Res 8:399–404. https://doi.org/10.1017/s0960258500004323
Blaney CS, Kotanen PM (2001) Effects of fungal pathogens on seeds of native and exotic plants: a test using congeneric pairs. J Appl Ecol 38:1104–1113. https://doi.org/10.1046/j.1365-2664.2001.00663.x
Blate GM, Peart DR, Leighton M (1998) Post-dispersal predation on isolated seeds: a comparative study of 40 tree species in a Southeast Asian rainforest. Oikos 82:522–538. https://doi.org/10.2307/3546373
Burnside OC, Wilson RG, Weisberg S, Hubbard KG (1996) Seed longevity of 41 weed species buried 17 years in eastern and western Nebraska. Weed Sci 44:74–86. https://doi.org/10.1017/s0043174500093589
Burrows MT, Schoeman DS, Buckley LB, Moore P, Poloczanska ES, Brander KM, Brown C, Bruno JF, Duarte CM, Halpern BS, Holding J, Kappel CV, Kiessling W, O’Connor MI, Pandolfi JM, Parmesan C, Schwing FB, Sydeman WJ, Richardson AJ (2011) The Pace of Shifting Climate in Marine and Terrestrial Ecosystems. Science 334:652–655. https://doi.org/10.1126/science.1210288
Chee-Sanford JC, Williams MM, Davis AS, Sims GK (2006) Do microorganisms influence seed-bank dynamics? Weed Sci 54:575–587. https://doi.org/10.1614/ws-05-055r.1
Chen D, Zhang R, Baskin CC, Hu XW (2019) Water permeability/impermeability in seeds of 15 species of Caragana (Fabaceae). PeerJ 7:e6870. https://doi.org/10.7717/peerj.6870
Chen DL, Luo XP, Yuan Z, Bai MJ, Hu XW (2020) Seed dormancy release of Halenia elliptica in response to stratification temperature, duration and soil moisture content. Bmc Plant Biol 20:1–8. https://doi.org/10.1186/s12870-020-02560-8
Cohen DT (1966) Optimizing reproduction in a randomly varying environment. J Theor Biol 12:119–129. https://doi.org/10.1016/0022-5193(66)90188-3
Cohen DT (1967) Optimizing reproduction in a randomly varying environment when a correlation may exist between the conditions at the time a choice has to be made and the subsequent outcome. J Theor Biol 16:1–14. https://doi.org/10.1016/0022-5193(67)90050-1
Cuena-Lombrana A, Porceddu M, Dettori CA, Bacchetta G (2020) Predicting the consequences of global warming on Gentiana lutea germination at the edge of its distributional and ecological range. PeerJ 8:e8894. https://doi.org/10.7717/peerj.8894
Dalling JW, Swaine MD, Garwood NC (1998) Dispersal patterns and seed bank dynamics of pioneer trees in moist tropical forest. Ecology 79:564–578. https://doi.org/10.2307/176953
Davis AS (2007) Nitrogen fertilizer and crop residue effects on seed mortality and germination of eight annual weed species. Weed Sci 55:123–128. https://doi.org/10.1614/ws-06-133.1
Davis AS, Schutte BJ, Iannuzzi J, Renner KA (2008) Chemical and physical defense of weed seeds in relation to soil seedbank persistence. Weed Sci 56:676–684. https://doi.org/10.1614/ws-07-196.1
de Jong TJ, Tudela Isanta M, Hesse E (2013) Comparison of the crop species Brassica napus and wild B. rapa: characteristics relevant for building up a persistent seed bank in the soil. Seed Sci Res 23:169–179. https://doi.org/10.1017/s0960258513000159
Dechaine JM, Burger JC, Burke JM (2010) Ecological patterns and genetic analysis of post-dispersal seed predation in sunflower (Helianthus annuus) crop-wild hybrids. Mol Ecol 19:3477–3488. https://doi.org/10.1111/j.1365-294X.2010.04740.x
Delgado-Sanchez P, Ortega-Amaro MA, Jimenez-Bremont JF, Flores J (2011) Are fungi important for breaking seed dormancy in desert species? Experimental evidence in Opuntia streptacantha (Cactaceae). Plant Biol 13:154–159. https://doi.org/10.1111/j.1438-8677.2010.00333.x
Dong DK, Yan LF, Dong R, Liu WX, Wang YR, Liu ZP (2017) Evaluation and Analysis of Pod Dehiscence Factors in Shatter-Susceptible and Shatter-Resistant Common Vetch. Crop Sci 57:2770–2776. https://doi.org/10.2135/cropsci2017.03.0191
Ellis RH, Roberts EH (1981) The quantification of aging and survival in orthodox seeds. Seed Sci and Technol 9:373–409
Ellis RH, Roberts EH (1980) Improved equations for the prediction of seed longevity. Ann Bot-London 45:13–30. https://doi.org/10.1093/oxfordjournals.aob.a085797
Fenner M, Thompson K (2005) The ecology of seeds. Cambridge University Press, Cambridge
Funes G, Basconcelo S, Diaz S, Cabido M (1999) Seed size and shape are good predictors of seed persistence in soil in temperate mountain grasslands of Argentina. Seed Sci Res 9:341–345. https://doi.org/10.1017/s0960258599000355
Gardarin A, Dürr C, Mannino MR, Busset H, Colbach N (2010) Seed mortality in the soil is related to seed coat thickness. Seed Scie Res 20:243–256. https://doi.org/10.1017/s0960258510000255
Golos PJ, Dixon KW (2014) Waterproofing Topsoil Stockpiles Minimizes Viability Decline in the Soil Seed Bank in an Arid Environment. Restor Ecol 22:495–501. https://doi.org/10.1111/rec.12090
Gomez-Gonzalez S, Torres-Diaz C, Bustos-Schindler C, Gianoli E (2011) Anthropogenic fire drives the evolution of seed traits. P Natl Acad Sci USA 108:18743–18747. https://doi.org/10.1073/pnas.1108863108
Harrington JF (1960) Drying, storing and packaging seed to maintain germination. Seed Man’s Digest 1:16–18
He D, Shen W, Eberwein J, Zhao Q, Ren L, Wu QL (2017) Diversity and co-occurrence network of soil fungi are more responsive than those of bacteria to shifts in precipitation seasonality in a subtropical forest. Soil Biol Biochem 115:499–510. https://doi.org/10.1016/j.soilbio.2017.09.023
Hedges LV, Gurevitch J, Curtis PS (1999) The meta-analysis of response ratios in experimental ecology. Ecology 80:1150–1156. https://doi.org/10.2307/177062
Hodkinson DJ, Askew AP, Thompson K, Hodgson JG, Bakker JP, Bekker RM (1998) Ecological correlates of seed size in the British flora. Func Ecol 12:762–766. https://doi.org/10.1046/j.1365-2435.1998.00256.x
Holzel N, Otte A (2001) The impact of flooding regime on the soil seed bank of flood-meadows. J Veg Sci 12:209–218. https://doi.org/10.2307/3236605
Holzel N, Otte A (2004) Assessing soil seed bank persistence in flood-meadows: The search for reliable traits. J Veg Sci 15:93–100. https://doi.org/10.1111/j.1654-1103.2004.tb02241.x
Hoyle GL, Daws MI, Steadman KJ, Adkins SW (2008) Mimicking a semi-arid tropical environment achieves dormancy alleviation for seeds of Australian native Goodeniaceae and Asteraceae. Ann Bot-London 101:701–708. https://doi.org/10.1093/aob/mcn009
Hu XW, Ding XY, Baskin CC, Wang YR, Albrecht H (2018) Effect of soil moisture during stratification on dormancy release in seeds of five common weed species. Weed Res 58:210–220. https://doi.org/10.1111/wre.12297
Hu XW, Wu YP, Ding XY, Zhang R, Wang YR, Baskin JM, Baskin CC (2014) Seed dormancy, seedling establishment and dynamics of the soil seed bank of Stipa bungeana (Poaceae) on the Loess Plateau of northwestern China. PLoS ONE 9:e112579. https://doi.org/10.1371/journal.pone.0112579
Hundertmark M, Buitink J, Leprince O, Hincha DK (2011) The reduction of seed-specific dehydrins reduces seed longevity in Arabidopsis thaliana. Seed Sci Res 21:165–173. https://doi.org/10.1017/s0960258511000079
Iglesias-Fernandez R, del Carmen R-G, Matilla AJ (2011) Progress in research on dry afterripening. Seed Sci Res 21:69–80. https://doi.org/10.1017/S096025851000036X
ISTA (2014) International rules for seed testing, Edition 2014. International Seed Testing Association, Bassersdorf, Switzerland.
Kibinza S, Vinel D, Come D, Bailly C, Corbineau F (2006) Sunflower seed deterioration as related to moisture content during ageing, energy metabolism and active oxygen species scavenging. Physiol Plantarum 128:496–506. https://doi.org/10.1111/j.1399-3054.2006.00771.x
Kiewnick L (1964) Untersuchungen Über den einfluss der samen-und bodenmikroflora auf die lebensdauer der spelzfrÜchte des flughafers (Avena fatua L.) ii. Zum einfluss der mikroflora auf die lebensdauer der samen im boden. Weed Res 4:31–43
Lee JS, Velasco-Punzalan M, Pacleb M, Valdez R, Kretzschmar T, McNally KL, Ismail AM, Cruz PCS, Hamilton NRS, Hay FR (2019) Variation in seed longevity among diverse Indica rice varieties. Ann Bot-London 124:447–460. https://doi.org/10.1093/aob/mcz093
Leishman MR, Masters GJ, Clarke IP, Brown VK (2000) Seed bank dynamics: the role of fungal pathogens and climate change. Func Ecol 14:293–299. https://doi.org/10.1046/j.1365-2435.2000.00425.x
Leishman MR, Westoby M (1998) Seed size and shape are not related to persistence in soil in Australia in the same way as in Britain. Func Ecol 12:480–485
Lepiniec L, Debeaujon I, Routaboul JM, Baudry A, Pourcel L, Nesi N, Caboche M (2006) Genetics and biochemistry of seed flavonoids. Annu Rev Plant Biol 57:405–430. https://doi.org/10.1146/annurev.arplant.57.032905.105252
Long RL, Gorecki MJ, Renton M, Scott JK, Colville L, Goggin DE, Commander LE, Westcott DA, Cherry H, Finch-Savage WE (2015) The ecophysiology of seed persistence: a mechanistic view of the journey to germination or demise. Biol Rev 90:31–59. https://doi.org/10.1111/brv.12095
Long RL, Kranner I, Panetta FD, Birtic S, Adkins SW, Steadman KJ (2011) Wet-dry cycling extends seed persistence by re-instating antioxidant capacity. Plant Soil 338:511–519. https://doi.org/10.1007/s11104-010-0564-2
Long RL, Panetta FD, Steadman KJ, Probert R, Bekker RM, Brooks S, Adkins SW (2008) Seed persistence in the field may be predicted by laboratory-controlled aging. Weed Sci 56:523–528. https://doi.org/10.1614/ws-07-189.1
Lonsdale WM (1993) Losses from the seed bank of Mimosa pigra: soil-microorganisms vs. temperature fluctuations. J Appl Ecol 30:654–660. https://doi.org/10.2307/2404244
Lundgren JG, Rosentrater KA (2007) The strength of seeds and their destruction by granivorous insects. Arthropod-Plant Inte 1:93–99. https://doi.org/10.1007/s11829-007-9008-1
Ma M, Collins SL, Du G (2020) Direct and indirect effects of temperature and precipitation on alpine seed banks in the Tibetan Plateau. Ecol Appl 30:e02096. https://doi.org/10.1002/eap.2096
Metzner K, Gachet S, Rocarpin P, Saatkamp A (2017) Seed bank, seed size and dispersal in moisture gradients of temporary pools in Southern France. Basic Appl Ecol 21:13–22. https://doi.org/10.1016/j.baae.2017.06.003
Merritt DJ, Martyn AJ, Ainsley P, Young RE, Seed LU, Thorpe M, Hay FR, Commander LE, Shackelford N, Offord CA, Dixon KW, Probert RJ (2014) A continental-scale study of seed lifespan in experimental storage examining seed, plant, and environmental traits associated with longevity. Biodivers Conserv 23:1081–1104. https://doi.org/10.1007/s10531-014-0641-6
Mitchell GA, Bingham FT, Yermanos DM (1974) Growth, mineral composition and seed characteristics of sesame as affected by nitrogen, phosphorus and potassium nutrition. Soil Sci Soc Am J 38:925–931
Moles AT, Warton DI, Westoby M (2003) Seed size and survival in the soil in arid Australia. Austral Ecol 28:575–585. https://doi.org/10.1046/j.1442-9993.2003.01314.x
Muller-Stover D, Nybroe O, Baraibar B, Loddo D, Eizenberg H, French K, Sonderskov M, Neve P, Peltzer DA, Maczey N, Christensen S (2016) Contribution of the seed microbiome to weed management. Weed Res 56:335–339. https://doi.org/10.1111/wre.12218
Nelson EB (2004) Microbial dynamics and interactions in the spermosphere. Annu Rev Phytopathol 42:271–309. https://doi.org/10.1146/annurev.phyto.42.121603.131041
Nelson EB (2018) The seed microbiome: Origins, interactions, and impacts. Plant Soil 422:7–34. https://doi.org/10.1007/s11104-017-3289-7
Ooi MKJ (2012) Seed bank persistence and climate change. Seed Sci Res 22:S53–S60. https://doi.org/10.1017/s0960258511000407
Ozinga WA, Schaminee JHJ, Bekker RM, Bonn S, Poschlod P, Tackenberg O, Bakker J, van Groenendael JM (2005) Predictability of plant species composition from environmental conditions is constrained by dispersal limitation. Oikos 108:555–561. https://doi.org/10.1111/j.0030-1299.2005.13632.x
Pameri MS, Chaurasia AK, Ahmad JP (2016) Effect of seed treatments on storability of different varieties of paddy (Oryza sativa L.). Int J Multidiscip Res Dev 3:142–145
Pourcel L, Routaboul JM, Cheynier V, Lepiniec L, Debeaujon I (2007) Flavonoid oxidation in plants: from biochemical properties to physiological functions. Trends Plant Sci 12:29–36. https://doi.org/10.1016/j.tplants.2006.11.006
Probert RJ, Daws MI, Hay FR (2009) Ecological correlates of ex situ seed longevity: a comparative study on 195 species. Ann Bot-London 104:57–69. https://doi.org/10.1093/aob/mcp082
Rajjou L, Debeaujon I (2008) Seed longevity: Survival and maintenance of high germination ability of dry seeds. CR Biol 331:796–805. https://doi.org/10.1016/j.crvi.2008.07.021
Robertson AW, Trass A, Ladley JJ, Kelly D (2006) Assessing the benefits of frugivory for seed germination: the importance of the deinhibition effect. Func Ecol 20:58–66. https://doi.org/10.1111/j.1365-2435.2005.01057.x
Satyanti A, Nicotra AB, Merkling T, Guja LK (2018) Seed mass and elevation explain variation in seed longevity of Australian alpine species. Seed Sci Res 28:319–331. https://doi.org/10.1017/s0960258518000090
Schafer M, Kotanen PM (2004) Impacts of naturally-occurring soil fungi on seeds of meadow plants. Plant Ecol 175:19–35. https://doi.org/10.1023/b:Vege.0000048096.00772.23
Soltani E, Baskin CC, Baskin JM, Heshmati S, Mirfazeli MS (2018) A meta-analysis of the effects of frugivory (endozoochory) on seed germination: role of seed size and kind of dormancy. Plant Ecol 219:1283–1294. https://doi.org/10.1007/s11258-018-0878-3
Thomas K, Band SR, Hodgson JG (1993) Seed size and shape predict persistence in soil. Func Ecol 7:236–241. https://doi.org/10.2307/2389893
Thompson K, Jalili A, Hodgson JG, Hamzeh’ee B, Asri Y, Shaw S, Shirvany A, Yazdani S, Khoshnevis M, Zarrinkamar F, Ghahramani MA, Safavi R (2001) Seed size, shape and persistence in the soil in an Iranian flora. Seed Sci Res 11:345–355. https://doi.org/10.1079/SSR2002105
Thorogood CJ, Rumsey FJ, Hiscock SJ (2009) Seed viability determination in parasitic broomrapes (Orobanche and Phelipanche) using fluorescein diacetate staining. Weed Res 49:461–468. https://doi.org/10.1111/j.1365-3180.2009.00716.x
Tweddle JC, Dickie JB, Baskin CC, Baskin JM (2003) Ecological aspects of seed desiccation sensitivity. J Ecol 91:294–304. https://doi.org/10.1046/j.1365-2745.2003.00760.x
Venable DL, Brown JS (1988) The selective interactions of dispersal, dormancy, and seed size as adaptations for reducing risk in variable environments. Am Nat 131:360–384. https://doi.org/10.1086/284795
Volis S, Dorman M (2019) Effects of soil type, period of burial and moisture levels on the germination of Oncocyclus iris seeds. Plant Ecol 220:1021–1028. https://doi.org/10.1007/s11258-019-00971-8
Wagner M, Mitschunas N (2008) Fungal effects on seed bank persistence and potential applications in weed biocontrol: A review. Basic Appl Ecol 9:191–203. https://doi.org/10.1016/j.baae.2007.02.003
Wagner M, Pywell RF, Knopp T, Bullock JM, Heard MS (2011) The germination niches of grassland species targeted for restoration: effects of seed pre-treatments. Seed Sci Res 21:117–131. https://doi.org/10.1017/s0960258510000450
Walck JL, Hidayati SN, Dixon KW, Thompson K, Poschlod P (2011) Climate change and plant regeneration from seed. Global Change Biol 17:2145–2161. https://doi.org/10.1111/j.1365-2486.2010.02368.x
Walters C, Wheeler LM, Grotenhuis JM (2005) Longevity of seeds stored in a genebank: species characteristics. Seed Sci Res 15:1–20. https://doi.org/10.1079/ssr2004195
Wang ZK, Cao Q, Shen YY (2019) Modeling light availability for crop strips planted within apple orchard. Agr Syst 170:28–38. https://doi.org/10.1016/j.agsy.2018.12.010
Waterworth WM, Masnavi G, Bhardwaj RM, Jiang Q, Bray CM, West CE (2010) A plant DNA ligase is an important determinant of seed longevity. Plant J 63:848–860. https://doi.org/10.1111/j.1365-313X.2010.04285.x
Xiao ZS, Chang G, Zhang ZB (2008) Testing the high-tannin hypothesis with scatter-hoarding rodents: experimental and field evidence. Anim Behav 75:1235–1241. https://doi.org/10.1016/j.anbehav.2007.08.017
Yu S, Sternberg M, Kutiel P, Chen H (2007) Seed mass, shape, and persistence in the soil seed bank of Israeli coastal sand dune flora. Evol Ecol Res 9:325–340
Zalamea PC, Dalling JW, Sarmiento C, Arnold AE, Delevich C, Berhow MA, Ndobegang A, Gripenberg S, Davis AS (2018) Dormancy-defense syndromes and tradeoffs between physical and chemical defenses in seeds of pioneer species. Ecology 99:1988–1998. https://doi.org/10.1002/ecy.2419
Zhang M, Yoshiyama M, Nagashima T, Nakagawa Y, Yoshioka T, Esashi Y (1995) Aging of soybean seeds in relation to metabolism at different relative humidities. Plant Cell Physiol 36:1189–1195
Zhao LP, Wu GL, Cheng JM (2011) Seed mass and shape are related to persistence in a sandy soil in northern China. Seed Sci Res 21:47–53. https://doi.org/10.1017/s0960258510000358
Zhu X, Zhang J, Ma K (2011) Soil biota reduce allelopathic effects of the invasive eupatorium adenophorum. PLoS ONE 6:e25393. https://doi.org/10.1371/journal.pone.0025393
Acknowledgements
We are grateful to Professor Carol Baskin for her critical review and constructive suggestions on this manuscript. This study was supported by Gansu Provincial Science and Technology Projects (18JR2TA023), State Key Laboratory of Grassland Agro-Ecosystems (Lanzhou University) (SKLGAE-2020-05) and Fundamental Research Funds for the Central Universities (lzujbky-2020-26, lzujbky-2021-it03).
Author information
Authors and Affiliations
Contributions
Xiaowen Hu conceived the topic. Dali Chen, Xianglai Chen, Cunzhi Jia, Yan Wang and Lingjie Yang performed the experiments. Dali Chen analyzed all statistical data. Dali Chen and Xiaowen Hu wrote the manuscript. All authors have read and approved the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Additional information
Responsible Editor: Jeffrey Walck.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
11104_2021_4990_MOESM4_ESM.tif
Supplementary file4 (TIF 1853 KB) Effect of temperature and light on germination of fresh seeds of 11 species. Different uppercase and lowercase letters are indicate significant difference in light and dark, respectively, at 0.05 level for each species. *, ** and *** indicate a significant difference between light and dark at 0.05, 0.01 and 0.001 levels, respectively. The same below
11104_2021_4990_MOESM5_ESM.tif
Supplementary file5 (TIF 296 KB) Effect size for seed persistence with precipitation reduction versus control. Error bars are referring to 95% confidence intervals. No overlap of error bars with zero indicate that precipitation reduction significantly affect seed persistence compare with control, the value of effect size is greater or less than zero means a positive and negative effect, respectively
11104_2021_4990_MOESM6_ESM.tif
Supplementary file6 (TIF 301 KB) Effect size for seed persistence with precipitation addition versus control. Error bars are referring to 95% confidence intervals. No overlap of error bars with zero indicate that precipitation addition significantly affect seed persistence compare with control, the value of effect size is greater or less than zero means a positive and negative effect, respectively
11104_2021_4990_MOESM7_ESM.tif
Supplementary file7 (TIF 564 KB) Mean daily soil temperature under three levels of precipitation conditions from 2020 to 2021
Rights and permissions
About this article
Cite this article
Chen, D., Chen, X., Jia, C. et al. Effects of precipitation and microorganisms on persistence of buried seeds: a case study of 11 species from the Loess Plateau of China. Plant Soil 467, 181–195 (2021). https://doi.org/10.1007/s11104-021-04990-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11104-021-04990-1